Abstract
Myocardial native T1 is a known cardiovascular magnetic resonance (CMR) imaging biomarker to quantify diffuse myocardial fibrosis in valvular cardiomyopathy. We hypothesized that diffuse myocardial fibrosis assessed by preoperative T1 mapping might correlate with LV reverse remodeling after valvular surgery. A prospective monocentric cohort study was conducted including 79 consecutive patients with valvular cardiomyopathy referred for surgical treatment of severe aortic or severe functional mitral regurgitation. Native T1 values were assessed by CMR before surgery. LV geometry parameters (i.e., LVEDV, LVESV) were obtained by 2D transthoracic echocardiography before and six months after surgery. Postoperative change of LV geometry parameters was calculated as delta (∆) variable (i.e., six months value minus baseline value). Mean native T1 was 1047 ± 39 ms, mean ∆LVEDV was −33 ± 42 mL, and mean ∆LVESV was −15 ± 27 mL. Native T1 values correlated with ∆LVEDV (Pearson r = 0.29; p = 0.009) and ∆LVESV (Pearson r = 0.29; p = 0.015). Native T1 values < 1073 ms were identified as independent predictor of postoperative reduction of LVEDV (HR 3.0; 95%-CI: 1.1–8.0; p = 0.03) and LVESV (HR 2.9; 95%-CI: 1.1–7.4; p = 0.03). Diffuse myocardial fibrosis assessed by myocardial native T1 correlates with LV reverse remodeling at six months after valvular surgery. T1 mapping may be a valuable tool to predict LV reverse remodeling in valvular heart disease.
1. Introduction
Heart valve regurgitation is a common disease in the Western world and its prevalence increases with an advancing age [1]. Even severe aortic or mitral regurgitation may remain asymptomatic for many years, although volume overload of the left ventricle (LV) leads to progressive LV remodeling. According to recent ESC/EACTS guidelines for the management of valvular heart disease, the indication for surgery of aortic regurgitation (AR) exists in symptomatic patients and in those with a progressive LV disease (i.e., LVEF < 50% or left ventricular end-systolic diameter (LVESD) > 50 mm) [2]. In case of functional mitral regurgitation (FMR), the indications for surgery are even more restrictive and are reserved for patients who remain severely symptomatic despite guideline-directed medical therapy and cardiac resynchronization therapy (CRT) [2].
Currently, the extent to which LV function and dimensions recover after successful valve repair is unknown. In theory, the myocardial damage should recede following correction of heart valve regurgitation. Practically, not all patients achieve postoperative LV reverse remodeling, depending on the degree of myocardial fibrosis [3,4]. In addition, persistence of postoperative LV dysfunction predicts a poor long-term prognosis [5].
The question of major clinical relevance is whether a more expeditious valvular surgery in case of severe asymptomatic aortic or mitral valve regurgitation may prevent the progression of LV remodeling at an early stage. Therefore, more sensitive biomarkers would be required to better and earlier quantify the ongoing myocardial damage that may precede a significant LV enlargement.
Current cardiac magnetic resonance (CMR) quantitative mapping techniques show a strong correlation between native T1 and histologic collagen volume fraction, which determines the degree of myocardial fibrosis [6]. A previous consensus statement underlined the value of native T1 for visualization and quantification of diffuse myocardial fibrosis [7]. However, myocardial tissue analysis by CMR currently has no impact in the decision-making process regarding the timing of valvular surgery.
We hypothesized that diffuse myocardial fibrosis defined by T1 mapping in the preoperative CMR may correlate with LV reverse remodeling defined by a reduction of LVEDV and LVESV at six months after valvular surgery.
2. Materials and Methods
2.1. Study Design
The institutional ethics committee approved this prospective study, and all subjects gave written informed consent (Ethical Committee of Medical Council, Hamburg, Germany; PV5382).
Consecutive patients with severe AR or severe FMR referred to our institution for elective valvular surgery between July 2017 and August 2019 were prospectively enrolled in this study. Study exclusion criteria were as follows: predominant aortic or mitral valve stenosis, valvular redo procedures, common contraindications for MRI such as severe obesity (250 kg CMR table weight limit), and presence of a metallic foreign bodies.
The severity of valve regurgitation was defined by established diagnostic criteria, including transthoracic echocardiography and clinical aspects [2].
Study protocol imaging consisted of preoperative CMR with myocardial native T1 mapping and transthoracic 2D-echocardiography with measurement of LV size parameters: LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and LV ejection fraction (LVEF). Follow-up echocardiographic re-evaluation was performed at 6-months after surgery.
In addition, laboratory values of serum N-terminal prohormone of brain natriuretic peptide (NT-proBNP; pg/mL) and serum creatinine (mg/dL) were preoperatively assessed.
2.2. Assessment of Cardiac Magnetic Resonance Imaging
Preoperative CMR was routinely performed within one week prior to surgery. All cardiac examinations were conducted by a 1.5-T MR scanner (Achieva; Philips Medical Systems, Best, The Netherlands). CMR was performed using a standardized protocol including an electrocardiogram-gated, standard steady-state, free-precession cine MR sequence, acquired during breath holds in standard long-axis views (4-, 3-, and 2-chamber view) and short-axis slices covering the entire left ventricle [8]. Native T1 mapping was acquired using a 5 s (3 s) 3 s modified Look-Locker inversion-recovery (MOLLI) sequence on three short-axis sections (apical, middle, and basal). Each CMR set was independently analyzed by two investigators. Mapping values were measured by drawing a single region of interest (ROI) in the septum on mid-cavity short-axis maps.
Native T1 measurements showed good inter-observer reliability (intraclass correlation coefficient: absolute value 0.895; 95% confidence interval 0.825–0.937).
2.3. Assessment of Echocardiographic Left Ventricular Geometry Parameters
Baseline and follow-up 2D transthoracic echocardiography images were routinely recorded and analyzed in our echocardiographic core laboratory by two independent investigators. Baseline echocardiographic examination was conducted within one week prior to surgery. Follow-up echocardiographic examination was conducted six months after surgery.
Using a standardized protocol, LV geometry parameters, including LVEF, LVEDD, LVESD, LVEDV, and LVESV, were measured in the 4- and 2-chamber view during end-systole or end-diastole according to the Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults from the American Society of Echocardiography and the European Association of Cardiovascular Imaging [9]. The method for 2D echocardiographic volume calculations was the biplane method of disks summation (modified Simpson’s rule) [9]. Missing follow-up values of LVEDV and LVESV were calculated using Teichholz formula based on LVEDD and LVESD [10].
To generate high accuracy and reproducibility of biplane two-dimensional echocardiographic measurements, the same investigators followed the same patients at baseline and follow-up as recommended by Otterstad et al. [11] Our study followed a very similar echocardiographic measurement protocol as compared to Baron et al. [12]; therefore, we expected comparable high test-retest reliability values for the echocardiographic LV measurements.
2.4. Surgical Technique
Depending on the underlying disease (i.e., AR or FMR) surgical strategies differed. Surgical approach for AR cases were partial sternotomy or median sternotomy depending on the need of concomitant cardiac procedures and patients’ comorbidities. Treatment strategies for AR patients ranged from aortic valve repair to biological or mechanical aortic valve replacement.
Surgical approach to FMR was either median sternotomy or right antero-lateral mini-thoracotomy. FMR was treated using a standard rigid complete annuloplasty ring, which was downsized by one size, according to the length of the anterior mitral leaflet. In case of severe leaflet tenting, subannular repair with repositioning of both papillary muscles was performed [13]. There were no major variations in the surgical or perioperative management during the study period.
2.5. Statistical Analysis
Normally distributed continuous variables are presented as mean ± standard deviation and categorical variables are expressed as percentages throughout the manuscript. Comparison of normally distributed continuous variables was performed by unpaired t-test. Non-normally distributed, unpaired data was compared using the Mann–Whitney-U test. Fisher’s exact test was used for univariable comparisons of categorical variables. A Pearson correlation coefficient was used for correlation analyses.
The difference of LVEF, LVEDD, LVESD, LVEDV, and LVESV at six months’ follow-up and at baseline were calculated as delta (∆) variable (i.e., follow-up value minus baseline value). In line with this, negative ∆ values of LVEDD, LVESD, LVEDV, and LVESV indicated postoperative reduction of LV geometry parameters and presence of LV reverse remodeling. While positive ∆ values of LVEDD, LVESD, LVEDV, and LVESV indicated increase of postoperative LV geometry parameters and absence of LV reverse remodeling. For ∆LVEF applied the opposite, negative ∆LVEF values indicated postoperative decrease of LVEF and absence of LV reverse remodeling, while positive ∆LVEF indicated presence of LV reverse remodeling.
We calculated receiver operating characteristic (ROC) curves to determine optimal cut-off points of myocardial native T1 values to predict postoperative reduction in LV geometry parameters. The optimal cut-off value was defined by Youden index. Predictors of reduction in LV geometry parameters were subsequently assessed by multivariable Cox regression analysis. All p-values < 0.05 were considered statistically significant. All statistical analyses were performed using the SPSS version 26.0 statistical package (IBM Corp., Markham, ON, Canada).
2.6. Study Endpoint
Primary study endpoint was the correlation between baseline T1 values and LV reverse remodeling defined by reduction of LVEDV and LVESV at six months postoperatively after valvular surgery.
3. Results
3.1. Demographics and Baseline Characteristics
A total of 79 consecutive patients (mean age 56 ± 14 years) with severe AR (n = 46) or severe FMR (n = 33) were included in our study (see Table 1).

Table 1.
Demographics and baseline characteristics.
Overall, there were 58/79 male (73%) and 36/79 female (27%) participants. The original aortic valve pathology included unicuspid aortic valve disease in five cases (11%) and bicuspid aortic valve disease in 16 cases (35%). All other AR patients presented with tricuspid aortic valves (n = 25; 54%). Concomitant root enlargement was found in 11 cases (24%).
Depending on the underlying disease, operative procedures consisted of aortic valve repair (n = 27), or aortic valve replacement (n = 19), or mitral valve repair using ring annuloplasty combined with subannular repair (n = 20) or isolated ring annuloplasty (n = 13). In the FMR cohort, concomitant surgeries included coronary artery bypass surgery (n = 13) and tricuspid valve repair (n = 5). None of the thirteen patients who underwent simultaneously CABG showed a regional myocardial fibrosis corresponding to the diseased coronary artery territory in CMR.
During the follow-up period, four re-operations were required, including endocarditis following aortic valve replacement at six months follow-up (n = 1), recurrence of AR after aortic valve repair due to suture dehiscence at eight months follow-up (n = 1), and recurrent severe FMR following isolated ring annuloplasty at one week follow-up and one month follow-up (n = 2). Echocardiographic grading of recurrent valve regurgitation at six months follow-up are depicted in Table 1. There were no deaths registered during the follow-up period.
3.2. Imaging Data and Correlation between T1 Values and Echocardiographic LV Measurements
The comparison of echocardiographic LV measurements at 6 months postoperatively vs. baseline revealed mean ∆LVEF of 1 ± 8%, mean ∆LVEDD −6 ±7 mm, mean ∆LVESD −5 ±7 mm, mean ∆LVEDV of −33 ± 42 mL, and mean ∆LVESV of −15 ± 27 mL. A reduction of diastolic and systolic LV volumes was found in 58 patients (73%) and in 56 patients (71%), respectively.
The myocardial native T1 values of the study cohort were normally distributed with a mean baseline native T1 of 1047 ± 39 ms (see Figure 1).
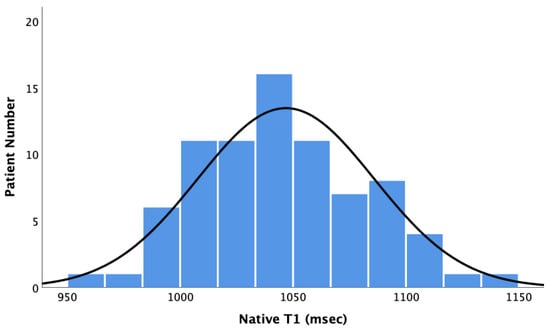
Figure 1.
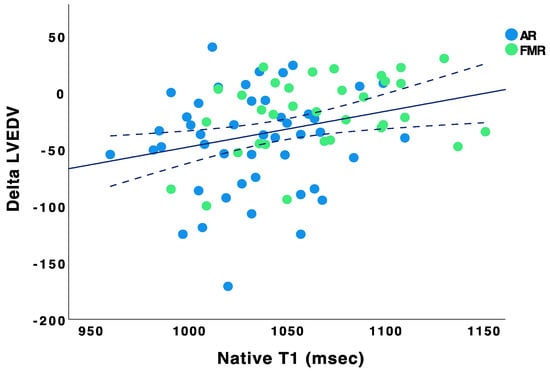
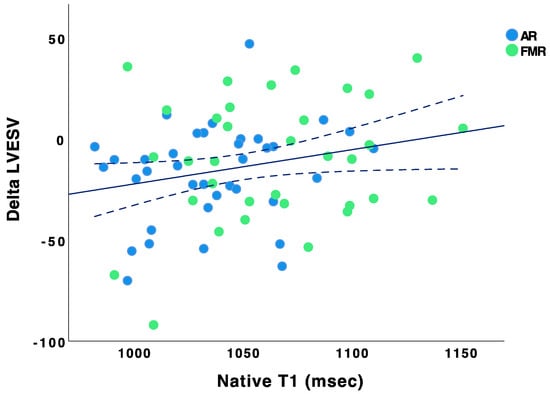
Bar Chart showing the distribution of native T1 values. Baseline native T1 values did not correlate with ∆LVEF (Pearson r = −0.002, p = 0.98), ∆LVEDD (Pearson r = 0.02, p = 0.84), and ∆LVESD (Pearson r = −0.05, p = 0.71). However, baseline native T1 values correlated with ∆LVEDV (Pearson r = 0.291, p = 0.009) and ∆LVESV (Pearson r = 0.292, p = 0.015) (see Figure 2 and Figure 3).
ROC curve analysis revealed an optimal cut-off point of myocardial native T1 of 1073 ms to predict reduction of LVEDV (sensitivity 85%, 95%-CI: 74–95; specificity 57%, 95%-CI: 29–85; area under the curve (AUC) 0.64, 95%-CI: 0.51–0.78) and LVESV (sensitivity 80%, 95%-CI: 67–83; specificity 60%, 95%-CI: 32–88; AUC 0.64, 95%-CI: 0.51–0.78). Calculated in a Cox regression model using covariables age, gender, and level of creatinine, native T1 < 1073 ms was identified as an independent predictor for reduction of LVEDV (HR 3.0; 95%-CI: 1.1–8.0; p = 0.03) and LVESV (HR 2.9; 95%-CI: 1.1–7.4; p = 0.03) (Table 2).

Table 2.
Predictors of LV reverse remodeling.
3.3. Post-Hoc Subgroup Analysis by Valve Pathology
Comparison of the baseline characteristics between AR patients (n = 46) vs. FMR patients (n = 33) revealed significantly younger age (AR: 51.4 ± 14.2 years vs. FMR: 61.6 ± 12.1 years; p = 0.001), lower values of preoperative NT-proBNP (AR: 801 ± 1633 pg/mL vs. FMR: 3715 ± 3483 pg/mL; p < 0.001), and serum creatinine (AR: 1.0 ± 0.4 vs. FMR: 1.3 ± 0.6 mg/dL; p = 0.02) in the AR subgroup (Table 1). In line with this, preoperative LVEF was significantly higher in AR patients vs. FMR patients (AR: 57 ± 8% vs. FMR: 37 ± 11%; p < 0.001). Similar, preoperative CMR revealed significantly lower native T1 values in AR vs. FMR patients (AR: 1032 ± 32 ms vs. FMR: 1066 ± 39 ms; p < 0.001).
Systolic echocardiographic LV geometry indexes including LVESD (AR: 41 ± 9 mm vs. FMR 50 ± 9 mm; p < 0.001) and LVESV (AR: 74 ± 31 mL vs. FMR: 110 ± 40 mL; p < 0.001) were significant lower in AR patients vs. FMR patients at baseline. After six months, reduction of LVEDV and LVESV was found in 37 (80%) and 35 (75%) AR patients vs. 21 (64%) and 21 (64%) FMR patients, respectively. Therefore, contribution of the primary study endpoint was comparable between both study cohorts (reduction of LVEDV: AR: 80% vs. FMR: 64%, p = 0.43; reduction of LVESV: AR: 75% vs. FMR: 64%, p = 0.12). Although, the mean ∆LVEDV improved more extensively in AR vs. FMR patients (as indicated by postoperative LVEDV reduction of −44 ± 45 mL in AR patients vs. −19 ± 34 mL in FMR patients, p = 0.01). Of note, there were no statistically significant difference between AR and FMR patients regarding the postoperative reduction of LVESV (i.e., −17 ± 24 mL in the AR cohort vs. −13 ± 30 mL in the FMR cohort; p = 0.5).
4. Discussion
In our study, we aimed to evaluate the relationship between preoperative T1 values as defined by CMR, and LV reverse remodeling in valvular cardiomyopathy. We were able to demonstrate that native T1 values may quantify a diffuse myocardial fibrosis and correlate with the change in LVEDV and LVESV between baseline and 6-months follow-up after valvular surgery. Particularly, shorter preoperative native T1 times (i.e., <1073 ms) were associated with an increased prevalence of LV reverse remodeling following valvular surgery. Based on these findings, we conclude that preoperative native T1 values might be a valuable tool to predict reverse LV remodeling after surgery for aortic or mitral regurgitation. Furthermore, shorter native T1 times may indicate a reversible myocardial dysfunction, which improves after valve surgery, while higher T1 values could imply a rather fixed defect.
Post-hoc subgroup analysis revealed significantly longer T1 times in FMR patients indicating a more severe LV disease in FMR patients in comparison to AR patients. Accordingly, FMR patients showed lower degree of postoperative LV reverse remodeling. These findings confirm our clinical observation, that FMR patients present mostly with a more advanced LV disease as compared to AR patients.
Native T1 mapping has previously been proposed to be a valuable diagnostic marker for several cardiac pathologies [14,15,16,17,18]. However, native T1 measurements vary between CMR sites due to several technical factors and values are not standardized. In line with this, the identified cut-off point of native T1 of 1073 ms of our study can be used as rough orientation. In line with this, it should not be generalized as reference value for other CMR sites.
Currently, there is a lack of robust evidence from adequately powered clinical trials on the role of fibrosis variables in informing clinical decisions for surgical or catheter-based interventions. If confirmed by future studies, our findings may have important clinical implications. By means of native T1 mapping, risk stratification and treatment selection in patients with valvular cardiomyopathy may be further improved. Especially, timing of surgical intervention may be optimized before severe myocardial damage occurs. In line with this, patients with valvular heart and an irreversible myocardial defect may benefit from less invasive treatment strategies (i.e., transcatheter procedures).
Unfortunately, the current treatment recommendations for AR or FMR of the ESC/EACTS guidelines of management valvular heart disease do only refer to LVEF < 50% and LVESD > 50 mm (LVESDi > 25 mm/m2) as a trigger for valvular intervention [2]. In the guidelines, neither the impact of diffuse myocardial fibrosis diagnosed by T1 mapping during CMR nor the effects of LV reverse remodeling on the outcomes following valvular surgery are further discussed [2]. Repeated measurements of native T1 values in asymptomatic patients with left-sided heart valve insufficiency may support individual decision making in appropriate timing of valvular intervention. Such strategy could prevent irreversible myocardial damage.
Our study possesses all the limitations of a single-center experience with a relatively low-numbered study cohort.
- (1)
- Our study cohort consisted of patients with different valve pathologies including tricuspid (TAV) and bicuspid (BAV) aortic valve disease and functional mitral valve disease. This could be a confounder to our analysis. However, the development of diffuse myocardial fibrosis and valvular cardiomyopathy has the same pathophysiological background in both TAV and BAV patients. Chronic volume overload results in eccentric LV hypertrophy and LV remodeling, which is independent of valve morphology. Due to the common pathophysiological pathway in TAV and BAV, we analyzed both valve pathologies as a uniform entity, the AR subgroup. Furthermore, we aimed to demonstrate that the correlation between baseline T1 and LV reverse remodeling after valvular surgery is independent of the type of valvular disease (i.e., AR vs. FMR). To evaluate this assumption, we included valve type (i.e., AR vs. FMR) into our Cox regression model. Cox regression model confirmed the correlation between native dichotomized T1 and LV reverse remodeling to be independent of the type of valvular disease (i.e., AR vs. FMR). In other words, the correlation between native T1 mapping and post-surgical LV reverse remodeling was comparable between AR and FMR patients.
- (2)
- Our study cohort included thirteen study patients, who underwent simultaneous CABG surgery. Even though the presence of CAD was the main pathophysiological mechanism leading to cardiomyopathy, none of them had a regional myocardial fibrosis corresponding to the diseased coronary artery territory. Therefore, this patient cohort with CAD represents a very selective patient subgroup with predominantly diffuse myocardial fibrosis.
- (3)
- Four patients underwent redo surgery during the study period, which might have influenced the LV reverse remodeling course as defined by reduction of LVEDV and LVESV. However, the redo surgeries were performed at the first month or after the 6 months follow-up echocardiographic examination. Therefore, in all four cases, there was presumably enough time for the LV to remodel.
- (4)
- The ROC analyses of our study, which we performed to determine the cut-off point of the prediction variable native T1, revealed only acceptable AUC values for reduction of LVEDV and LVESV. In addition, the confidence intervals of AOCs showed a wide range of values due to the low number of study patients.
- (5)
- Analysis and interpretation of echocardiographic measurements of LV remodeling parameters relied on the test-retest variability published by Baron et al. [12].
In summary, our current findings need to be confirmed by a consecutive prospective large multicenter study.
5. Conclusions
Diffuse myocardial fibrosis assessed by myocardial native T1 correlates with LV reverse remodeling at six months after valvular surgery. T1 mapping may be a valuable tool to predict LV reverse remodeling in valvular heart disease; however, this should be validated in a larger prospective multicenter study.
Author Contributions
M.v.S. and E.G. contributed to conception and design of the study. M.v.S., J.P. (Johannes Petersen), T.H., T.M.S.-G., L.M. and J.P. (Jonas Pausch) organized the database. M.v.S. and E.G. performed the statistical analysis. M.v.S. wrote the first draft of the manuscript. M.S. and P.B. wrote sections of the manuscript. G.A. and H.R. revised thoroughly the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The institutional ethics committee approved the prospective study, and all subjects gave written informed consent (Ethical Committee of Medical Council, Hamburg, Germany; PV5382). All authors had full control of the data and the material submitted for publication. This prospective observational study complies with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Iung, B.; Vahanian, A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011, 8, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Beyersdorf, F.; Vahanian, A.; Milojevic, M.; Praz, F.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardio-Thoracic Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.F.; Nigri, M.; Higuchi, M.D.L.; Pomerantzeff, P.M.; Spina, G.S.; Sampaio, R.O.; Tarasoutchi, F.; Grinberg, M.; Rochitte, C.E. Prognostic Significance of Myocardial Fibrosis Quantification by Histopathology and Magnetic Resonance Imaging in Patients with Severe Aortic Valve Disease. J. Am. Coll. Cardiol. 2010, 56, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Murashita, T.; Schaff, H.V.; Suri, R.M.; Daly, R.C.; Li, Z.; Dearani, J.A.; Greason, K.L.; Nishimura, R.A. Impact of Left Ventricular Systolic Function on Outcome of Correction of Chronic Severe Aortic Valve Regurgitation: Implications for Timing of Surgical Intervention. Ann. Thorac. Surg. 2016, 103, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Neumann, N.; Naito, S.; Gross, T.S.; Massel, R.; Reichenspurner, H.; Girdauskas, E. Persistence of Reduced Left Ventricular Function after Aortic Valve Surgery for Aortic Valve Regurgitation: Bicuspid versus Tricuspid. Thorac. Cardiovasc. Surg. 2019, 69, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, S.; Dohi, K.; Ishida, M.; Goto, Y.; Imanaka-Yoshida, K.; Omori, T.; Goto, I.; Kumagai, N.; Fujimoto, N.; Ichikawa, Y.; et al. Native T1 Mapping and Extracellular Volume Mapping for the Assessment of Diffuse Myocardial Fibrosis in Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 1–24. [Google Scholar] [CrossRef]
- Radunski, U.K.; Kluwe, J.; Klein, M.; Galante, A.; Lund, G.K.; Sinning, C.; Bohnen, S.; Tahir, E.; Starekova, J.; Bannas, P.; et al. Cardiovascular magnetic resonance demonstrates structural cardiac changes following transjugular intrahepatic portosystemic shunt. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Teichholz, L.E.; Kreulen, T.; Herman, M.V.; Gorlin, R. Problems in echocardiographic volume determinations: Echocardiographic-angiographic correlations in the presence or absence of asynergy. Am. J. Cardiol. 1976, 37, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Otterstad, J.E.; Froeland, G.; Sutton, M.S.J.; Holme, I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur. Hear. J. 1997, 18, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Baron, T.; Berglund, L.; Hedin, E.-M.; Flachskampf, F.A. Test–retest reliability of new and conventional echocardiographic parameters of left ventricular systolic function. Clin. Res. Cardiol. 2018, 108, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Conradi, L.; Harmel, E.K.; Reichenspurner, H. Minimally Invasive Mitral Valve Annuloplasty with Realignment of Both Papillary Muscles for Correction of Type IIIb Functional Mitral Regurgitation. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2017, 12, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-P.; Lee, W.; Lee, J.M.; Park, E.-A.; Kim, H.-K.; Kim, Y.-J.; Sohn, D.-W. Assessment of Diffuse Myocardial Fibrosis by Using MR Imaging in Asymptomatic Patients with Aortic Stenosis. Radiology 2015, 274, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, J.-B.; Yoon, Y.E.; Park, E.-A.; Kim, H.-K.; Lee, W.; Kim, Y.-J.; Cho, G.-Y.; Sohn, D.-W.; Greiser, A.; et al. Noncontrast Myocardial T1 Mapping by Cardiac Magnetic Resonance Predicts Outcome in Patients with Aortic Stenosis. JACC Cardiovasc. Imaging 2017, 11, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, P.; Messroghli, D.R.; Reid, S.; Ridgway, J.P.; Bainbridge, G.; Sivananthan, M.U. Myocardial T1 Mapping for Detection of Left Ventricular Myocardial Fibrosis in Chronic Aortic Regurgitation: Pilot Study. Am. J. Roentgenol. 2006, 187, W630–W635. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Gutman, S.J. Myocardial T1 Mapping in Heart Disease. JACC Cardiovasc. Imaging 2019, 13, 55–57. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).