Short-Term Results of Operative Treatment of Primary Ileocecal Crohn’s Disease: Retrospective, Comparative Analysis between Early (Luminal) and Complicated Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Design and Setting
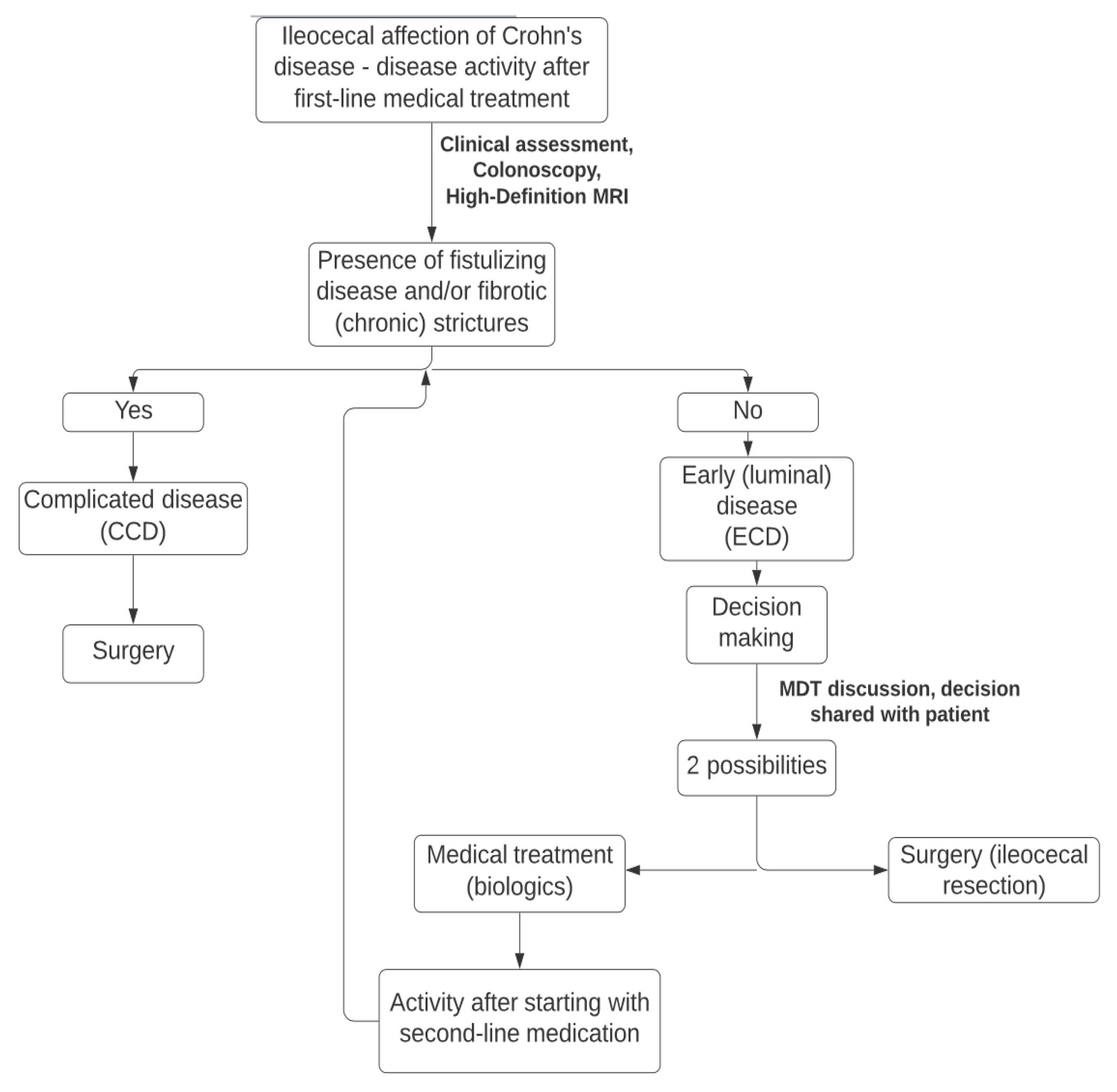
2.3. Patient Management Strategy
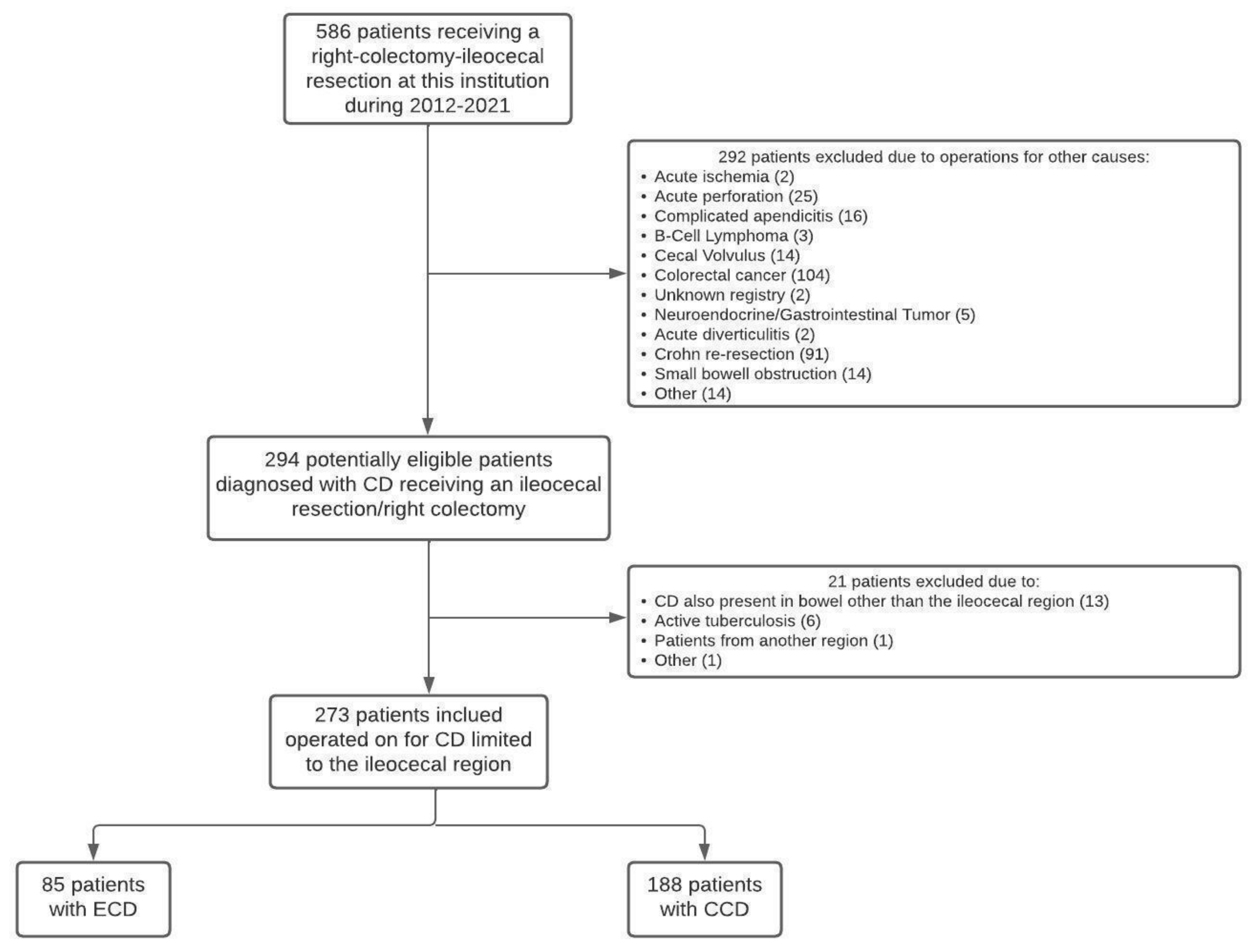
2.4. Inclusion and Exclusion Criteria
2.5. Data Collection and Management
2.6. Variables Analyzed
2.7. Outcomes
2.8. Statistical Analysis
3. Results
3.1. Main Outcome
3.2. Preoperative Characteristics
3.3. Intraoperative Variables
3.4. Postoperative Results
3.5. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Algorithm of Management Used at Our Institution for Management of Patient with Ileocecal CD
References
- Thoreson, R.; Cullen, J. Pathophysiology of inflammatory bowel disease: An overview. Surg. Clin. N. Am. 2007, 87, 575–585. [Google Scholar] [CrossRef]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: Diagnosis and medical management. J. Crohn’s Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Yamamoto, T.; Lightner, A.L.; Spinelli, A.; Kotze, P.G. Perioperative management of ileocecal Crohn’s disease in the current era. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Iesalnieks, I.; Kilger, A.; Glaß, H.; Obermeier, F.; Agha, A.; Schlitt, H.J. Perforating Crohn’s ileitis: Delay of surgery is associated with inferior postoperative outcome. Inflamm. Bowel Dis. 2010, 16, 2125–2130. [Google Scholar] [CrossRef]
- Kotze, P.; Magro, D.O.; Martinez, C.A.R.; Spinelli, A.; Yamamoto, T.; Warusavitarne, J.; Coy, C.S.R. Long Time from Diagnosis to Surgery May Increase Postoperative Complication Rates in Elective CD Intestinal Resections: An Observational Study. Gastroenterol. Res. Pract. 2018, 2018, 4703281. [Google Scholar] [CrossRef]
- Surgical IBD LATAM Consortium. Earlier surgery is associated to reduced postoperative morbidity in ileocaecal Crohn’s disease: Results from SURGICROHN—LATAM study. Dig. Liver Dis. 2022, in press. [Google Scholar]
- Sebastian, S.; Segal, J.P.; Hedin, C.; Pellino, G.; Kotze, P.G.; Adamina, M.; Campmans-Kuijpers, M.; Davies, J.; de Vries, A.C.; Casbas, A.G.; et al. ECCO Topical Review: Roadmap to optimal peri-operative care in IBD. J. Crohn’s Colitis 2022, 17, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Ponsioen, C.Y.; de Groof, E.J.; Eshuis, E.J.; Gardenbroek, T.J.; Bossuyt, P.M.; Hart, A.; Warusavitarne, J.; Buskens, C.J.; van Bodegraven, A.A.; Brink, M.A.; et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: A randomized controlled, open-label, multicentre trial. Lancet 2017, 2, 785–792. [Google Scholar] [CrossRef] [PubMed]
- De Groof, E.J.; Stevens, T.W.; Eshuis, E.J.; Gardenbroek, T.J.; Bosmans, J.E.; van Dongen, J.M.; Mol, B.; Buskens, C.J.; Stokkers, P.C.; Hart, A.; et al. Cost-effectiveness of laparoscopic ileocaecal resection versus infliximab treatment of terminal ileitis in Crohn’s disease: The LIR!C Trial. Gut 2019, 68, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.W.; Haasnoot, M.L.; D’Haens, G.R.; Buskens, C.J.; de Groof, E.J.; Eshuis, E.J.; Gardenbroek, T.J.; Mol, B.; Stokkers, P.C.F.; Bemelman, W.A.; et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: Retrospective long-term follow-up of the LIR!C trial. Lancet Gastroenterol. Hepatol. 2020, 5, 900–907. [Google Scholar] [CrossRef]
- Cosnes, J.; Cattan, S.; Blain, A.; Beaugerie, L.; Carbonnel, F.; Parc, R.; Gendre, J.P. Long-term evolution of disease behavior of Crohn’s disease. Inflamm. Bowel Dis. 2002, 8, 244–250. [Google Scholar] [CrossRef]
- Pellino, G.; Sampietro, G.M. Defining the role of abdominal surgery and its impact on the disease course in patients with Crohn’s disease: Unsolved issues and novel insights. Dig. Liver Dis. 2023, S1590–865800190-1. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, B.Y.; Ma, C.; Panaccione, R.; Kotze, P.G. Early Laparoscopic Ileal Resection for Localized Ileocecal Crohn’s Disease: Hard Sell or a Revolutionary New Norm? Inflamm. Intest. Dis. 2022, 7, 13–20. [Google Scholar] [CrossRef]
- Bemelman, W.A.; Warusavitarne, J.; Sampietro, G.M.; Serclova, Z.; Zmora, O.; Luglio, G.; Overstraeten, A.D.B.V.; Burke, J.P.; Buskens, C.J.; Francesco, C.; et al. ECCO-ESCP consensus on surgery for Crohn’s Disease. J. Crohn’s Colitis 2017, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Hoffmann, H.; Clavien, P.A.; Bucher, H.C.; Dell-Kuster, S. Definition and Classification of Intraoperative Complications (CLASSIC): Delphi Study and Pilot Evaluation. World J. Surg. 2015, 39, 1663–1671. [Google Scholar] [CrossRef]
- Coffey, C.J.; Kiernan, M.G.; Sahebally, S.M.; Jarrar, A.; Burke, J.P.; Kiely, P.A.; Shen, B.; Waldron, D.; Peirce, C.; Moloney, M.; et al. Inclusion of the mesentery in ileocolic resection for Crohn’s disease is associated with reduced surgical recurrence. J. Crohn’s Colitis 2018, 12, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2019, 14, 4–22. [Google Scholar] [CrossRef]
- Pellino, G.; Siccr, T.I.S.O.C.S.; Keller, D.S.; Sampietro, G.M.; Angriman, I.; Carvello, M.; Celentano, V.; Colombo, F.; Di Candido, F.; Laureti, S.; et al. Italian Society of Colorectal Surgery SICCR. Inflammatory bowel disease position statement of the Italian Society of Colorectal Surgery (SICCR): Crohn’s disease. Tech. Coloproctology 2020, 24, 421–448. [Google Scholar] [CrossRef]
- Pellino, G.; Keller, D.S.; Sampietro, G.M.; Annese, V.; Carvello, M.; Celentano, V.; Coco, C.; Colombo, F.; Cracco, N.; di Candido, F.; et al. The Italian Society of Colorectal Surgery (SICCR). Inflammatory bowel disease (IBD) position statement of the Italian Society of Colorectal Surgery (SICCR): General principles of IBD management. Tech. Coloproctology 2020, 24, 105–126. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, M.; Zhao, M.; Lo, B.; Bendtsen, F.; Burisch, J. Disease course and treatment outcomes of Crohn’s disease patients with early or late surgery—A Danish nationwide cohort study from 1997 to 2015. Dig. Liver Dis. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- An, V.; Cohen, L.; Lawrence, M.; Thomas, M.; Andrews, J.; Moore, J. Early surgery in Crohn’s disease a benefit in selected cases. World J. Gastrointest. Surg. 2016, 8, 492–500. [Google Scholar] [CrossRef]
- Punwani, S.; Rodriguez-Justo, M.; Bainbridge, A.; Greenhalgh, R.; De Vita, E.; Bloom, S.; Cohen, R.; Windsor, A.; Obichere, A.; Hansmann, A.; et al. Mural inflammation in Crohn disease: Location-matched histologic validation of MR imaging features. Radiology 2009, 252, 712–720. [Google Scholar] [CrossRef]
- Zappa, M.; Stefanescu, C.; Cazals-Hatem, D.; Bretagnol, F.; Deschamps, L.; Attar, A.; Larroque, B.; Tréton, X.; Panis, Y.; Vilgrain, V.; et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm. Bowel Dis. 2011, 17, 984–993. [Google Scholar] [CrossRef]
- Bruining, D.H.; Zimmermann, E.M.; Loftus, E.V., Jr.; Sandborn, W.J.; Sauer, C.G.; Strong, S.A. Consensus Recommendations for Evaluation, Interpretation, and Utilization of Computed Tomography and Magnetic Resonance Enterography in Patients With Small Bowel Crohn’s Disease. Gastroenterology 2018, 154, 1172–1194. [Google Scholar] [CrossRef] [PubMed]

| Variables | All Patients N = 273 (100%) | ECD N = 85 (31.14) | CCD N = 188 (68.86) | p Value | Missing Values |
|---|---|---|---|---|---|
| Sex, female (n, %) | 160 (59.04) | 55 (64.71) | 105 (55.85) | 0.169 | 0 |
| Age (mean, range) | 37.49 (10–79) | 38.76 (10–76) | 36.92 (10–79) | 0.430 | 1 |
| Smoking | 75 (28.25) | 27 (32.14) | 49 (26.49) | 0.340 | 4 |
| BMI (mean, range) | 24.60 (11.4–42.6) | 24.73 (11.4–42.6) | 24.54 (14–41) | 0.784 | 3 |
| Low BMI (<20) | 52 (19.26) | 12 (14.29) | 40 (21.51) | 0.164 | |
| High BMI (>30) | 49 (18.15) | 14 (16.67) | 35 (18.82) | 0.671 | |
| WHO performance status >2 | 6 (2.20) | 1 (1.18) | 5 (2.66) | 0.439 | 0 |
| Charlson comorbidity score | 0.471 | 0 | |||
| 0–2 | 227 (83.15) | 68 (80) | 159 (84.57) | ||
| 2–3 | 35 (12.82) | 14 (16.47) | 21 (11.17) | ||
| >3 | 11 (4.03) | 3 (3.53) | 8 (4.26) | ||
| Anemia | 48 (17.58) | 6 (7.06) | 42 (22.34) | 0.002 | 0 |
| Preoperative albumin (median, range) | 3.56 (1.7–4.9) | 3.73 (3–4.6) | 3.47 (1.7–4.9) | <0.001 | 0 |
| Low preoperative albumin (<3) | 31 (11.44) | 2 (2.35) | 29 (15.59) | 0.001 | |
| Weight loss | 79 (28.94) | 18 (21.18) | 61 (32.45) | 0.057 | 0 |
| Kilos (mean, range) | 8.98 (1–30) | 9.32 (3.23) | 8.87 (1–30) | 0.785 | 5 |
| Previous abdominal surgery | 60 (21.98) | 16 (18.82) | 44 (23.40) | 0.397 | 0 |
| Time from diagnosis to surgery (months, median, range) | 52 (0–298) | 52 (1–298) | 53 (0–284) | 0.943 | 0 |
| More than 2 years | 144 (53.14) | 40 (47.62) | 104 (55.61) | 0.222 | |
| More than 5 years | 90 (33.21) | 28 (33.33) | 62 (33.16) | 0.255 | |
| Emergency surgical procedure | 57 (20.88) | 7 (8.24) | 50 (26.60) | <0.001 | 0 |
| Montreal classification | 1 | ||||
| A1 | 20 (7.35) | 8 (9.52) | 12 (6.38) | 0.438 | |
| A2 | 168 (61.76) | 44 (52.38) | 124 (65.96) | 0.036 | |
| A3 | 84 (30.51) | 32 (38.10) | 52 (27.81) | 0.090 | |
| B2 | 93 (35.07) | ||||
| B3 | 95 (34.80) | ||||
| Previous perianal disease | 39 (14.29) | 7 (8.24) | 32 (17.02) | 0.055 | 0 |
| Chronic steroids at the time of surgery | 39 (14.29) | 9 (10.59) | 30 (15.96) | 0.240 | 0 |
| Previous exposure to biologics | 133 (48.72) | 40 (47.06) | 93 (49.47) | 0.712 | 0 |
| Number of biologics received before surgery | 0.242 | 0 | |||
| 1 | 79 (28.78) | 22 (26.19) | 57 (30.48) | 0.486 | |
| 2 | 41 (15.50) | 17 (20.24) | 24 (13.37) | 0.145 | |
| 3 | 10 (3.69) | 1 (1.19) | 9 (4.81) | 0.145 | |
| >3 | 3 (2.24) | 0 | 3 (1.60) | 0.253 | |
| Time from first biologic to surgery (months, mean, range) | 39 (1–152) | 32 (1–112) | 43(1–152) | 0.111 | 5 |
| Previous complications associated to biologics | 42 (15.44) | 15 (17.65) | 27 (14.44) | 0.497 | 1 |
| Exposure to biologics within 12 weeks before surgery | 95 (34.80) | 26 (30.59) | 69 (36.70) | 0.282 | 0 |
| Infliximab | 44 (46.32) | 15 (57.69) | 29 (42.03) | ||
| Adalimumab | 38 (40) | 9 (34.62) | 29 (42.03) | ||
| Vedolizumab | 5 (5.26) | 1 (3.85) | 4 (5.80) | ||
| Ustekinumab | 5 (5.26) | 1 (3.85) | 4 (5.80) | ||
| Other | 3 (3.16) | 0 | 3 (4.35) | ||
| Requirement of preoperative nutritional optimization | 18 (6.59) | 1 (1.18) | 17 (9.04) | 0.015 | 0 |
| ASA | 0.450 | 0 | |||
| I | 40 (14.65) | 12 (14.12) | 28 (14.89) | ||
| II | 218 (79.85) | 71 (83.53) | 147 (78.19) | ||
| III | 13 (4.76) | 2 (2.35) | 11 (5.85) | ||
| IV | 2 (0.73) | 0 | 2 (1.06) |
| Variables | All Patients N = 273 (100%) | ECD N = 85 (31.14) | CCD N = 188 (68.86) | p Value | OR | Missing Variables |
|---|---|---|---|---|---|---|
| Operating time (median, range) | 121 (28–305) | 115 (49–286) | 126 (57–305) | 0.012 | 0 | |
| Prolonged surgery (>150 min) | 39 (14.29) | 7 (8.24) | 32 (17.02) | 0.055 | 2.36 | |
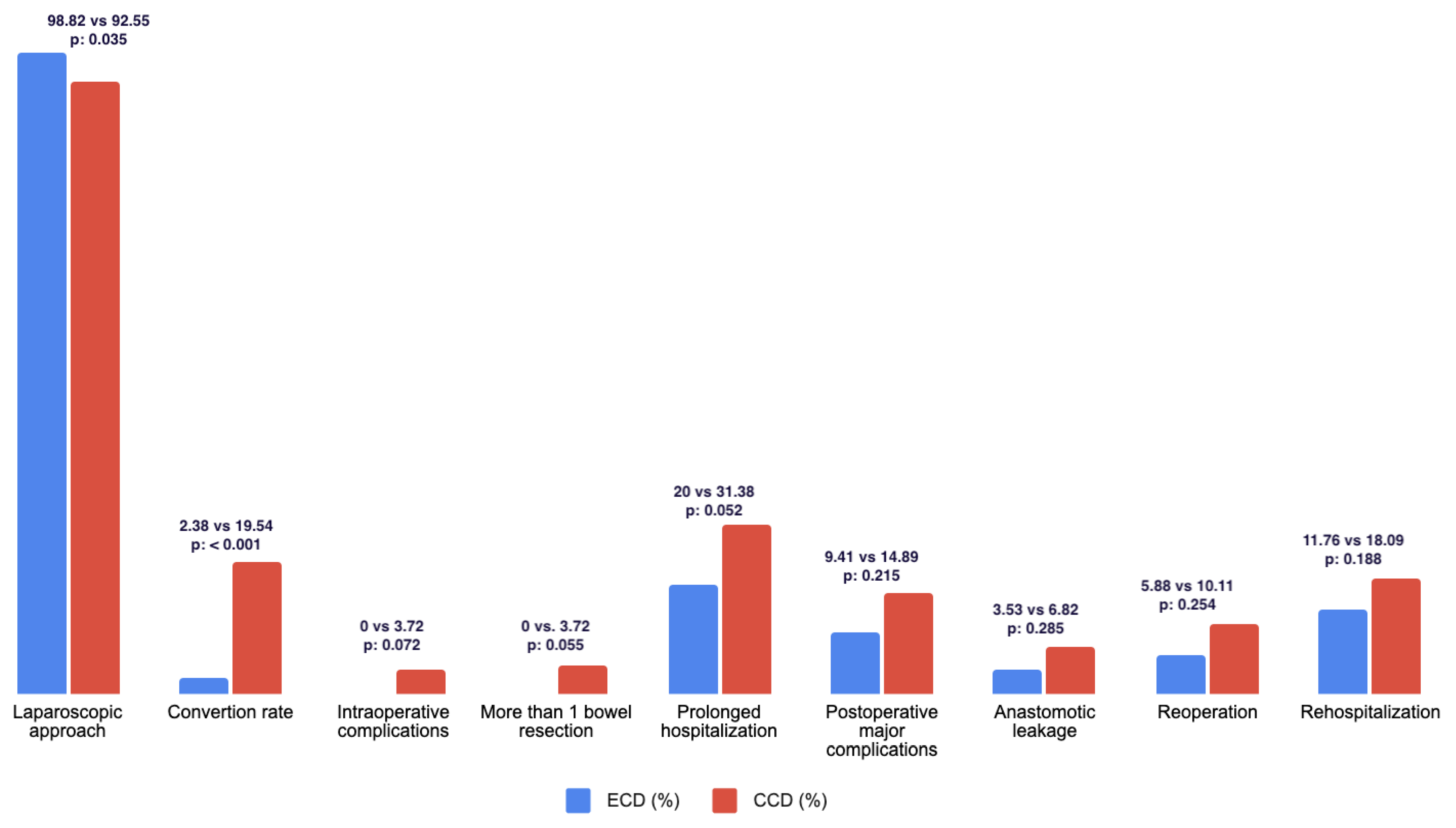
| Laparoscopic approach | 258 (94.51) | 84 (98.82) | 174 (92.55) | 0.035 | 6.72 | 0 |
| Conversion rate | 36 (13.19) | 2 (2.35) | 34 (18.62) | <0.001 | 10.27 | 0 |
| Extension of surgery | 0.175 | N/A | 0 | |||
| Ileocecal resection | 269 (98.53) | 85 (100) | 184 (97.87) | |||
| Right colectomy | 4 (1.47) | 0 | 4 (2.13) | |||
| Mesenteric resection | 0.501 | N/A | 0 | |||
| Mesenteric sparing | 272 (99.63) | 85 (100) | 187 (99.47) | |||
| Wide mesenteric resection | 1 (0.37) | 0 | 1 (0.53) | |||
| Associated surgery | 37 (13.55) | 1 (1.18) | 36 (19.15) | <0.001 | 21.00 | 0 |
| Number of patients requiring more than 1 bowel resection | 7 (2.56) | 0 | 7 (3.72) | 0.055 | N/A | 0 |
| Intraoperative complications | 7 (2.56) | 0 | 7 (3.72) | 0.072 | N/A | 0 |
| CLASSIC Minor | 7 (100) | 0 | 7 (100) | |||
| CLASSIC Major | 0 | 0 | 0 | |||
| Primary anastomosis | 261 (95.60) | 85 (100) | 176 (93.62) | 0.017 | N/A | 0 |
| Type of anastomosis | 0.597 | N/A | 0 | |||
| Hand-sewn | 2 (0.77) | 1 (1.18) | 1 (0.57) | |||
| Stapled | 259 (99.23) | 84 (98.82) | 175 (99.43) | |||
| Anastomosis orientation | 0.488 | N/A | ||||
| End to end | 1 (0.38) | 0 | 1 (0.57) | |||
| Side to side | 260 (99.62) | 85 (100) | 175 (99.43) | |||
| Anastomosis orientation | 0.281 | N/A | ||||
| Isoperistaltic | 8 (3.07) | 5 (5.88) | 3 (1.70) | |||
| Antiperistaltic | 253 (96.93) | 80 (94.12) | 173 (98.30) |
| Variables | All Patients N = 273 (100%) | ECD N = 85 (31.14) | CCD N = 188 (68.86) | p Value | OR | Missing Variables |
|---|---|---|---|---|---|---|
| Hospitalization days (mean, range) | 6.80 (1–96) | 5.45 (1–39) | 7.40 (2–96) | 0.093 | 0 | |
| Prolonged hospitalization (more than 7 days) | 76 (27.84) | 17 (20) | 59 (31.38) | 0.052 | 1.83 | |
| Requirement of prolonged postoperative ICU | 8 (2.93) | 0 | 8 (4.26) | 0.054 | N/A | 0 |
| Postoperative major complications | 36 (13.19) | 8 (9.41) | 28 (14.89) | 0.215 | 1.74 | 0 |
| Abdominal abscess | 15 (5.49) | 3 (3.53) | 12 (6.38) | 0.348 | 1.84 | 0 |
| Fascial rupture | 7 (2.56) | 1 (1.33) | 6 (3.19) | 0.489 | 2.74 | 0 |
| Postoperative gastrointestinal bleeding | 7 (2.56) | 1 (1.19) | 6 (3.21) | 0.441 | 2.74 | 0 |
| Reoperation for bleeding | 4 (57.14) | 0 | 4 (66.67) | |||
| Surgical site infection | 5 (1.85) | 1 (1.19) | 4 (2.14) | 0.592 | 0 | |
| Anastomotic leakage | 15 (5.75) | 3 (3.53) | 12 (6.82) | 0.285 | 2.00 | 0 |
| Minor leakage | 4 (26.67) | 1 (33.33) | 3 (25) | |||
| Major leakage | 11 (73.33) | 2 (66.67) | 9 (75) | |||
| Readmission | 44 (16.12) | 10 (11.76) | 34 (18.09) | 0.188 | 1.69 | 0 |
| Reoperation | 24 (8.79) | 5 (5.88) | 19 (10.11) | 0.254 | 1.88 | 0 |
| Mortality | 1 (0.37) | 0 | 1 (0.53) | 0.501 | N/A | 0 |
| Length of resected bowel (cm, mean, range) | 26 (5–158) | 24 (7–140) | 27 (5–158) | 0.143 | 4 |
| Variable | OR | Standard Error | p Value | 95% CI |
|---|---|---|---|---|
| Prolonged hospitalization (>1 week) | 2.69 | 2.24 | 0.24 | 0.53–13.71 |
| Major postoperative complications | 0.77 | 0.57 | 0.72 | 0.18–3.33 |
| Anastomotic leakage | 1.14 | 1.18 | 0.90 | 0.15–8.74 |
| Reoperation | 1.86 | 1.80 | 0.52 | 0.28–12.51 |
| Re-hospitalization | 2.65 | 1.62 | 0.11 | 0.80–8.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avellaneda, N.; Haug, T.; Worm Ørntoft, M.-B.; Harsløf, S.; Skovgaard Larsen, L.P.; Tøttrup, A. Short-Term Results of Operative Treatment of Primary Ileocecal Crohn’s Disease: Retrospective, Comparative Analysis between Early (Luminal) and Complicated Disease. J. Clin. Med. 2023, 12, 2644. https://doi.org/10.3390/jcm12072644
Avellaneda N, Haug T, Worm Ørntoft M-B, Harsløf S, Skovgaard Larsen LP, Tøttrup A. Short-Term Results of Operative Treatment of Primary Ileocecal Crohn’s Disease: Retrospective, Comparative Analysis between Early (Luminal) and Complicated Disease. Journal of Clinical Medicine. 2023; 12(7):2644. https://doi.org/10.3390/jcm12072644
Chicago/Turabian StyleAvellaneda, Nicolas, Tora Haug, Mai-Britt Worm Ørntoft, Sanne Harsløf, Lars Peter Skovgaard Larsen, and Anders Tøttrup. 2023. "Short-Term Results of Operative Treatment of Primary Ileocecal Crohn’s Disease: Retrospective, Comparative Analysis between Early (Luminal) and Complicated Disease" Journal of Clinical Medicine 12, no. 7: 2644. https://doi.org/10.3390/jcm12072644
APA StyleAvellaneda, N., Haug, T., Worm Ørntoft, M.-B., Harsløf, S., Skovgaard Larsen, L. P., & Tøttrup, A. (2023). Short-Term Results of Operative Treatment of Primary Ileocecal Crohn’s Disease: Retrospective, Comparative Analysis between Early (Luminal) and Complicated Disease. Journal of Clinical Medicine, 12(7), 2644. https://doi.org/10.3390/jcm12072644





