Abstract
Cardiogenic shock is a complex syndrome manifesting with distinct phenotypes depending on the severity of the primary cardiac insult and the underlying status. As long as therapeutic interventions fail to divert its unopposed rapid evolution, poor outcomes will continue challenging health care systems. Thus, early recognition in the emergency setting is a priority, in order to avoid delays in appropriate management and to ensure immediate initial stabilization. Since advanced therapeutic strategies and specialized shock centers may provide beneficial support, it seems that directing patients towards the recently described shock network may improve survival rates. A multidisciplinary approach strategy commands the interconnections between the strategic role of the ED in affiliation with cardiac shock centers. This review outlines critical features of early recognition and initial therapeutic management, as well as the utility of diagnostic tools and risk stratification models regarding the facilitation of patient trajectories through the shock network. Further, it proposes the implementation of precise criteria for shock team activation and the establishment of definite exclusion criteria for streaming the right patient to the right place at the right time.
Keywords:
cardiogenic shock; risk stratification; risk scores; management; shock team; shock network 1. Introduction
Cardiogenic shock (CS) represents a life-threatening condition equated to a dismal prognosis [1]. Since the introduction of the fundamental mechanisms of shock in 1972 [2], CS has been universally defined as a state of severe end-organ hypoperfusion and tissue hypoxia resulting primarily from cardiac pump failure [1,3,4].
Epidemiological data over the last decade report a significant increase in the incidence rate of hospitalizations, while in-hospital mortality has not shown any significant improvement [5,6,7]. Traditionally, acute coronary syndrome (ACS) has been the leading cause of CS, accounting for approximately 80% of cases [8]. However, recent observational studies report a declining incidence of ACS-CS, ranging from 30 to 55.4% [6,7,9,10], with a concurrent increase in the incidence of CS of other etiologies, mainly decompensated heart failure (AHF-CS). With the advent of the early revascularization strategy [11,12] established by the SHOCK trial [11], mortality rates significantly decreased from 80% [13] to 40–50% [14] in the late 1990s and early 2000s. However, in the last 15 years, CS mortality has reached a plateau even in the most leading-edge cardiac centers, with estimates of approximately 30–40% [6,10,15]. The proven efficiency of standardized protocols through organized structures such as catheterization laboratories and trauma centers for the management of ST elevation myocardial infarction (STEMI) [16] and polytrauma [17] patients, respectively, suggested that the development of cardiac shock centers and the establishment of a prespecified patient pathway could ideally contribute to an improvement in CS survival [11]. Considering the significant percentage of patients presenting with CS in the emergency department (ED) (15% of patients with shock) [18] along with the high ED mortality of severe heart failure patients [12], emergency physicians play a pivotal role in the chain of survival of patients presenting with cardiogenic shock in the ED. A prompt and structured approach in the ED can maximize the likelihood of a favorable outcome for those patients. The ED’s contribution is focused on early detection as well as timely referral to advanced cardiac care centers.
Apart from the optimization of care systems, a thorough insight into the complexity of the syndrome is essential to overcome challenging issues in the management of CS patients. The intricate relationships among various etiologies, different hemodynamic phenotypes and heterogeneous patient populations underscore the need for a more concrete classification and risk stratification in order to improve outcomes [1]. In addition, study design protocols mandate the establishment of uniform criteria, applicable at bedside, to tailor pharmacological and mechanical interventions and assess patient response.
The aim of this review is to focus on the initial approach to CS, pointing out the pivotal role of the ED for the early identification and optimal initial management of CS, and to summarize current evidence on practical handling issues. Additionally, it highlights the importance of a multidisciplinary approach through the shock team, and it defines the criteria for shock team activation as well as criteria of exclusion for advanced treatment.
2. Early Recognition
Cardiogenic Shock Definition and Severity Classification
Clearly defined criteria are usually the mainstay of early recognition in clinical medicine. Traditionally, in clinical trials, cardiogenic shock diagnosis has been based on the presence of hypotension (systolic blood pressure (SBP) < 90 mmHg for >30 min or the need for pharmacological or mechanical support to maintain SPB > 90 mmHg), along with end-organ hypoperfusion (altered mental status or cool extremities or lactate > 2.0 mmol/L or urine output < 30 mL/h). In addition, pulmonary congestion, or invasive hemodynamic criteria [Cardiac Index (CI) ≤ 2.2 L/min/m2 and pulmonary capillary wedge pressure (PCWP) ≥ 15 mmHg] have been used [19]. The latest guidelines of the European Society of Cardiology (ESC) for the diagnosis and treatment of acute and chronic heart failure [4] focus on the presence of hypoperfusion based on clinical signs (such as cold and sweaty extremities, oliguria, altered mental status, dizziness, narrow pulse pressure) and on addition laboratory parameters (such as elevated creatinine, metabolic acidosis, and elevated serum lactate). Importantly, it is acknowledged that shock may be present even without hypotension [20]. Normotensive shock may be manifested due to increased vascular resistance as a compensatory mechanism of counterbalancing reduced cardiac output. Recent studies highlight the importance of identifying CS in the initial stages, given the increased mortality of patients with hypoperfusion in the absence of hypotension, compared to patients with isolated hypotension [21].
The principal limitation regarding the aforementioned criteria is that CS is approached as a binary condition. However, CS in clinical practice does not represent a static state but comprises a dynamic process with a spectrum of clinical presentations, alternating through various degrees of severity, time progression or response to therapy. Thus, in 2019, the Society for Cardiovascular Angiography and Interventions (SCAI) proposed a shock severity classification which covered the relevant practical gap in the formerly used CS criteria and bedside clinical assessment. CS patients were categorized into five clinical stages: A (at risk) for developing CS, but hemodynamically stable; B (beginning), clinical evidence of hemodynamic instability determined by tachycardia (heart rate > 100 beats per minute) or hypotension (SBP < 90 mmHg, MAP < 60 mmHg or >30 mmHg drop from baseline), but without hypoperfusion; C (classic), clinical and biochemical evidence of hypoperfusion that requires pharmacological or mechanical support, usually, but not always, accompanied by hypotension; D (deteriorating), clinical evidence of shock (as in stage C) and failure of the initial support strategy to restore perfusion as evidenced by worsening hemodynamics or rising lactate; E (extremis), refractory shock or circulatory collapse with highly deranged biochemical markers (lactate > 8 mmol/L, pH < 7.2, base deficit > 10 mEq/L). Cardiac arrest is cited as the “A modifier” that aggravates clinical outcomes, regardless of stage classification [22].
Since its publication, several observational studies have validated the SCAI SHOCK classification in various populations and clinical settings [23]: patients with AMI-CS [24,25,26] and HF-CS [25,26,27,28,29], and patients with out-of-hospital cardiac arrest (OHCA) [25,26,30,31]. All studies found a correlation between SCAI SHOCK stage and mortality, revealing increasing mortality rates in more advanced stages of shock severity. Successively, the SCAI expert consensus writing group proposed a three-axis model of risk stratification, considering shock severity, phenotype and risk modifiers, and recommended a more individualized approach [23]. Lately, the SCAI CS working group presented a novel algorithm to enhance the application of the SCAI SHOCK classification on the basis of distinct clinical parameters, with defined cut-off values for each SCAI stage; parameters included OHCA, serum lactate (≤2, 2–5, or >10 mmol/L), ALT (≤200, 200–500, or >500 IU/L), SBP(<60, 60–90, >90 mmHg) and pH (≤7.2 or >7.2). In addition, the study group identified a significant association between baseline stage and in-hospital mortality and poorer prognosis for AMI-CS with respect to HF-CS patients and for those experiencing out-of-hospital cardiac arrest (OHCA) or receiving intensified treatment [32].
Table 1 outlines the definitions most widely used.

Table 1.
Definition of Cardiogenic Shock in various clinical trials and guidelines.
3. Initial Diagnostic Approach
CS management mandates a short timeframe, widely referred to as the “golden hour” [33]. Prompt identification following a comprehensive assessment will allow timely resuscitation and early management/disposition decisions [4]. For patients not already hospitalized, the emergency physician plays the critical role of within-hospital first medical contact, and her/his actions will determine patient outcomes to a significant degree. For patients with CS, the “door to balloon” time used for patients with STEMI requiring primary percutaneous coronary intervention (PCI) has been translated into “door to support” [34].
3.1. Detection of CS
An initial systematic Airway, Breathing, Circulation, Disability, Exposure (ABCDE) approach ensures prompt and holistic evaluation of patients with suspected CS [35]. Close monitoring of blood pressure, heart and respiratory rate and oxygen saturation is required for continuous assessment of clinical status, disease severity and response to treatment [1,3,4]. The main goals in the primary investigation are the identification of signs of hemodynamic compromise and the assessment of end-organ perfusion and volume status. Hemodynamic instability can manifest as hypotension ((SBP) < 90 mmHg for >30 min or the need for pharmacological or mechanical support to maintain SPB > 90 mmHg) or tachycardia (HR > 100 bpm). As shock is characterized by overt catecholamine activation with subsequent vasoconstriction, relative hypotension and narrow pulse pressure are high risk signs that demand meticulous evaluation [1,3,36]. Additionally, the diagnosis of shock demands the presence of hypoperfusion, which needs to be identified early by the determination of critical signs such as altered mental status, cold and sweaty extremities, prolonged capillary refill time (>2 s) and oliguria (<30 mL/h).
In parallel, the respiratory status necessitates early evaluation and management and characterizes the patient phenotypic profile. Physical findings of orthopnea, tachypnea, hypoxemia, cyanosis, lung crackles and respiratory wheezing are signs of respiratory distress and volume overload.
The Forrester classification [37] of patients as “cold and wet” (classic CS), “cold and dry” (euvolemic CS), and “warm and wet” (vasodilatory CS) [3] using the aforementioned clinical criteria may assist in the identification of cardiac involvement (left- versus right-sided or biventricular failure) and in the guidance of initial therapy and development of a treatment plan through the cardiac shock network. Routine invasive hemodynamic evaluation with a pulmonary artery catheter (PAC), measuring CI and PCWP, has an ambiguous contribution to mortality [38,39], and due to its difficult applicability in the ED environment, it is only recommended in patients with CS of unknown etiology or with refractory CS, who need mechanical circulatory support [40,41].
Cardinal signs and findings that may aid in the diagnosis and phenotyping of CS are diaphoresis, lower limb edema, hepatomegaly, jugular distension and the presence of heart murmurs.
3.2. Detection of CS Etiology
The prompt comprehensive diagnostic approach facilitates the identification of the CS cause. Identifying the underlying cause early is critical, as the patient’s subsequent management should be targeted and can be significantly different depending on etiology. The principal element of the primary evaluation is the awareness and investigation of potential causes of circulatory compromise [1,3,4]. Practitioners should follow a focused algorithm denoted by the CHAMPIT acronym (C indicating ACS; H, Hypertension emergency; A, Arrhythmia; M, Mechanical cause; P, Pulmonary embolism; I, Infection; and T, cardiac Tamponade) [4]. Reversible causes of CS necessitate prompt identification and management, particularly ACS whose prognosis is significantly correlated with an immediate reperfusion strategy [13]. Similarly, patients should be assigned for surgical repair in cases of mechanical complications of ACS, valvular causes or aortic dissection, and optimal medical management should be provided early in the course of HF-CS of alternative etiologies (acute or decompensated HF, arrhythmias, myocarditis, Takotsubo cardiomyopathy, infection) [4]. Furthermore, appropriate MCS should be considered early for patient stabilization before definite treatment, as a bridge to decision, bridge to bridge or bridge to recovery, in relation to the etiological trigger [1]. Valuable bedside diagnostic examinations for the immediate detection of the CS cause include electrocardiograms (ECG) and ultrasound, while blood gas analysis and biochemical studies may contribute to the holistic assessment of CS patients.
3.2.1. ECG
Upon patient presentation, a 12-lead electrocardiogram is mandatory due to its diagnostic role in the identification of tachy- or bradyarrhythmias and ACS (ST or non-ST- segment elevation myocardial infraction) and emergent activation of the cardiac catheterization laboratory when needed [1,3,42]. In patients presenting with chest pain, identification of AMI should be undertaken without delay in order to activate their direct transfer to PCI-capable centers. Therefore, ECG should be carried out in the pre-hospital setting, at fist medical contact by the emergency medical system (EMS). Furthermore, ECG interpretation may reveal characteristic features of other causes of CS such as myocarditis, Takotsubo cardiomyopathy, pulmonary embolism, cardiac tamponade and electrolyte abnormalities [3].
3.2.2. Ultrasound
Echocardiography is highly recommended as part of the initial approach for CS patients. Prior to a comprehensive assessment of cardiac performance, point-of-care ultrasound (POCUS) should be performed, applying standardized and restricted protocols primarily in order to distinguish CS from other shock types, assess cardiac contractility, check for the presence of congestion, and identify CS etiology [1,43]. In a systematic review, POCUS enhanced the diagnostic performance of patients with undifferentiated shock and improved etiological diagnostic certainty of CS patients, compared to clinical examination without the use of POCUS [44]. Various POCUS protocols, such as the RUSH (Rapid Ultrasound in Shock) protocol, are used widely in order to differentiate shock types (obstructive, distributive, hypovolemic or cardiogenic) and to identify shock etiology and physiopathology. Focused evaluation involves estimation of “the pump” (left and right ventricular function and sizes, accumulation of pericardial fluid) and “the tank” (inferior vena cava (IVC) dimension and collapsibility, lung congestion indicated by the presence of B-lines and pleural effusion, free abdominal fluid and signs of tension pneumothorax) and finally, assessment of “the pipes” (vascular system: aorta, femoral and popliteal veins) [45]. Recently, another protocol has been proposed for the early identification of CS in patients presenting in the ED with undifferentiated hypotension, based on semi-automated imaging software (SAIS). ED physicians were able to obtain advanced measurements of hemodynamic compromise, such as LVOT VTI (left ventricular outflow tract velocity time integral) in relation to IVC collapsibility and B-line pattern, and identify patients at risk for CS [46].
As soon as CS is identified as the most plausible type of shock, a more thorough echocardiographic investigation should be conducted with the aim to confirm the diagnosis and to investigate the presence of mechanical complications of AMI or severe valve dysfunction. Moreover, it may aid in the identification of candidates for MCS and subsequent device positioning [1,47].
Further, bedside echocardiographic monitoring may provide essential information regarding hemodynamic status, response to fluid challenge, and pharmacological and mechanical interventions (IVC respiratory variation and collapsibility index; B-line lung pattern; LV and RV contractility and diastolic diameters; LV filling pressure (e/e’) and RV filling pressure). Echocardiography should be combined with venous ultrasound (hepatic, portal and intrarenal veins) so as to assess venous congestion as a marker of organ congestion and damage [47,48].
3.2.3. Blood Gas Analysis
Serum lactate measurement on admission is recommended as a marker of hypoperfusion and tissue hypoxia [49,50]. A cut-off level of 2 mmol/L has been suggested by the SCAI SHOCK workgroup [22] to support CS diagnosis. Additionally, serial lactate measurements are a useful tool in order to estimate a patient’s response to therapy [51] and prognosis [21]. Recent studies underline the importance of lactate clearance (LC) as a prognostic marker, superior to baseline lactate, since higher LC has been significantly associated with better prognosis [52,53]. Lactate clearance is the reduction in lactate concentrations at two different timepoints and can be measured by calculating the percentage of the exact time difference (Δt) between lactate concentration on admission (L1) and lactate concentration after initial resuscitation (L2) [53].
(L1 and L2 are prespecified time points).
In the context of established CS, arterial blood gas analysis may result in findings of metabolic acidosis with hyperlactatemia and increased base deficit [54]. pH and base deficit have been used as markers of shock severity in various risk/severity models [19]. Furthermore, ABG is used to assess features of respiratory failure with low partial oxygen pressure or respiratory acidosis [1].
3.2.4. Laboratory Evaluation
Troponin and natriuretic peptides are additional biomarkers of diagnostic and prognostic importance and are useful in the identification of etiology (AMI versus HF), as they may reflect myocardial necrosis and cardiac wall stress, respectively; however, pending results should not delay initiation of pharmacological and revascularization therapy [1,3,55]. Point-of-care troponin tests may offer valuable information in the emergency setting, allow the early identification of ACS and differentiate CS from other causes of shock [56]. Several novel biomarkers seem to have a promising prognostic role in patients with acute heart failure and may contribute to patient risk stratification. Bioactive adrenomedullin (bio-ADM) may aid in the early detection and severity assessment of congestion [57], and proenkephalin A may be helpful in predicting worsening renal function in acute heart failure patients [58]. Both have a recognized role as prognostic markers of mortality [59,60]. Additionally, growth differentiation factor-15 (GDF-15) [61], associated with upregulation of inflammatory pathways, and angiopoietin-2 [62], related to vascular instability, were independently associated with poor short- [61] and long-term mortality and reperfusion success [62] in AMI-CS patients, in two subanalyses of the IABP-SHOCK II Trial.
Other important laboratory investigations include renal and liver function tests, coagulation studies and serum electrolytes [1,3]. Acute kidney injury complicates 15–55% of patients with CS and is strongly correlated with increased mortality [63,64]. Serum creatinine and urinary output are useful indicators of renal perfusion and escalation of therapy with renal replacement therapy (RRT). Moreover, levels of alanine transaminase (ALT), aspartase transaminase (AST), alkaline phosphatase (AP), serum bilirubin and lactate dehydrogenase should be monitored for evidence of hepatic injury and attributed to either liver congestion or ischemia [65]. A cut-off level of ALT > 200 U/L has been lately proposed as a clinical criterion of shock severity according to the SCAI SHOCK staging [32].
4. CS Risk Stratification and Risk Scores
As already discussed, CS extends not only across a wide range of causes, but also across a wide spectrum of clinical presentation, severity, and prognosis. This is one of the reasons why clinical trials that treat CS patients as homogenous cohorts often come back negative. The use of temporary mechanical circulatory support (t-MCS) is such an example. International guidelines suggest that t-MCS should be considered, but the patients who will benefit are not clearly described, nor are the cases in which escalating care would be futile. The task of patient selection for therapy escalation becomes even more difficult due to the time-sensitive course of CS; decision should be taken early, and interventions should take place ideally before irreversible damage is established. Continuous clinical examination and serial review of shock severity stage allows for early identification of deterioration implying the need for escalation of therapy [22]. The challenge with CS management is individualizing care as much as possible, as the strategy of “one size fits all” (e.g., IABP for all, or no use of IABP at all) has failed. Therefore, the estimation of an individual patient’s prognosis is essential in order to expedite decision making: patient at risk of deterioration, so extra monitoring/management care needed; need for escalation to MCS; futility of further escalation; decision to de-escalate. Furthermore, standardized risk prognostication facilitates communication among physicians and medical groups who are responsible for patient management (e.g., ED physician and interventional cardiologist, ED team and ICU). Additionally, it allows communication with the patients’ next-of-kin/family on a realistic and honest basis. Finally, it is required to conduct clinical studies with tailored therapies and increase comparability of different studies. Several scores for patient risk stratification have been suggested to reinforce physicians’ decision making with tools for outcome prognostication and therapeutic plan organization. However, despite the abundancy of available risk scores, their use remains limited in the acute setting, as they derive from intensive care unit (ICU)/cardiac ICU registries or from AMI-CS populations. Moreover, they entail differences in the definition of CS (hemodynamic versus clinical criteria) and in therapies provided (pharmacological versus mechanical support).
The majority of risk scores stem from AMI-CS registries [66,67,68]: the ORBI score [66], SHOCK score [67] and IABP-SHOCK-II score [68] have high predictive value for mortality [67,68] or predict the probability for CS after PCI following AMI [66]. The growing need to address mixed populations of CS has been appraised in the CardShock [8] and the INOVA [69] scores. Although the variables in the CardShock score are easy to estimate on presentation, the score does not incorporate the potential need for MCS or provide prognosis for short-term mortality [8]. On the other hand, the INOVA score is applicable only for cardiac intensive care unit patients invasively monitored with PAC [69]. The IABP-SHOCK-II and CardShock scores have received external validation, with modest predictive value for non-AMI-CS patients [70]. The SCAI SHOCK classification is a valuable score for evaluating disease severity of various CS etiologies and through serial time points and clinical care settings, but it has only been validated retrospectively [32]. However, its use is appealing due to its feasibility in the ED setting and its ability to estimate clinical status at bedside. A thorough review of the abundancy of risk scores developed over the last five decades underscores the necessity of a novel risk score applicable at bedside and on admission, so as not only to predict mortality but also to promptly identify patients who will probably benefit from advanced therapies and to contribute to decision making [71].
Recently, a novel risk score has been published, the CSP (Cardiogenic Shock Prognosis), based on retrospective data from patients presenting at the ED with CS of various etiologies. A nomogram was established incorporating information from a patient’s medical history, point-of-care and laboratory testing and medical interventions made within 72 h of admission. The score proved to be a reliable predictive tool for in-hospital mortality, but external validation studies are needed [72].
A novel risk model that could anticipate the diagnosis of CS two hours before its development has been proposed lately by Chang et al. The authors proposed an algorithm based on a machine learning model that identified older age, male gender, higher troponin level, lower pulse pressure, medium level of immature granulocytes, higher O2 saturation, and lower bicarbonate as risk factors that, in correlation with the clinical picture, could alert physicians to the increased probability of a patient’s entering the lethal spiral of CS, or likewise stages A or B of the SCAI SHOCK classification [73].
Interestingly, a simple score for the identification of cardiogenic hypotension in the ED has been described, evaluating findings integrated in an emergency assessment. High troponin levels, ECG signs of ischemia, shortness of breath, absence of fever and history of heart failure were independent predictive factors of cardiogenic hypotension. A score ≥ 2 has shown 82% sensitivity and 75% specificity in identifying CS in the ED [74].
A different approach in the evolution of risk stratification models is based on biomarker criteria. The CLIP score, designated in a population of AMI-CS patients, considers the complexity of CS irrespectively of hemodynamic measurements. With a view of the deranged systemic pathways following the primary cardiac insult, it evaluates levels of cystatin C, lactate, interleukin-6 (IL-6) and N-terminal pro-B-type natriuretic peptide as markers of impaired renal function, hypoperfusion, inflammation and congestion, respectively. The score was reliably predictive of mortality and externally validated but only in AMI-CS patients [75].
Table 2 summarizes the most useful risk stratification scores.

Table 2.
Useful risk scores for the risk stratification of cardiogenic shock (CS) patients. Risk scores highlighted with * could be used in the Emergency setting.
5. Initial Management
Current guidelines [1,4] set a number of goals at first medical contact: symptom alleviation, organ perfusion and congestion improvement, oxygenation support and organ damage limitation.
5.1. Symptom Relief
Pain and anxiety should be cautiously managed with the administration of morphine in selected patients with intense insisting pain not resolving with supportive treatment. Routine use of opiates is not recommended [4], as morphine has been associated with increased need for mechanical ventilation support, prolonged hospitalization and worse prognosis [76].
5.2. Fluid Resuscitation
In the absence of signs of congestion and in patients with preload-dependent phenotypes, fluid resuscitation should be considered with boluses of normal saline or Ringer’s lactate, 250 mL over 15–30 min and under close monitoring with POCUS [1]. POCUS is a dynamic tool for the real-time and serial assessment of volume status and systematic congestion in order detect volume overload and to guide fluid administration. Valuable parameters for optimizing and monitoring fluid resuscitation include the IVC respiratory variation and collapsibility index, the presence of a B-line profile in the lungs, LV and RV diastolic diameters, LV filling pressure (e/e’) and RV filling pressure [1,43], as well as VExUS score [48]. Passive leg raising (PLR) could be reliably used in order to assess fluid responsiveness [77,78]. Fluid responsiveness is the increase in cardiac output of greater than 10% following a 500 mL fluid bolus [79]. PLR, acting as an endogenous fluid challenge, augments venous return, central venous pressure and biventricular preload, and the eventual rise in cardiac output indicates the need for volume expansion. Performance of the test in the ED setting could be aided by the use of echocardiography, estimating changes in the CO through the measurement of the left ventricular outflow tract velocity time integral (LVOT VTI) [80,81].
Currently, data on crystalloid type remain controversial for use in sepsis and shock. There is lack of robust data in the literature specifying the appropriate type of crystalloid fluid for CS patient resuscitation. There is a relative concern regarding the use of saline solutions, as they may cause hyperchloremic metabolic acidosis and acute kidney injury (AKI) [82], and since cardiorenal syndrome is a common complication of CS [83], selection of the least harmful fluid retains a significant importance. Studies on critically ill ICU patients have shown a favorable effect of balanced crystalloid (plasmalyte or Ringer’s lactate) versus saline administration with respect to the need for renal replacement therapy (RRT), AKI and mortality [84,85]. Hammond et al. showed in a metanalysis that administration of balanced crystalloids resulted in a relative reduction in the risk of death at 90 days, ranging from a 9% to 1% relative increase [86]. However, two other metanalyses concluded that there was no statistically significant difference between administration of balanced crystalloid solutions and saline in terms of mortality, incidence of AKI and RRT [87,88]. Given the rather contradictory results, and as critically ill ICU patients represent fairly heterogeneous populations, more studies are needed in order to elucidate which is the appropriate fluid therapy for such high-risk patients, especially those with CS.
5.3. Oxygenation Support
The need for immediate intubation and mechanical ventilatory support must be addressed on arrival on a patient-to-patient basis [4]. Clinical presentation and point-of-care-acquired data will define the choice of noninvasive ventilatory support (NIV) versus invasive mechanical ventilatory support (IMV) [89]. The majority of patients with signs of congestion and without signs of acute RV failure will benefit from positive pressure ventilation both in respiratory and hemodynamic features, since the mechanical-ventilation-related decrease in left ventricular (LV) preload and afterload can reduce the workload of the failing LV [90,91]. Special caution must be taken with hemodynamically unstable patients who are not responding to initial therapy with vasopressors and inotropes and with those with signs of acute RV failure, where the reduction in the preload of the RV and increase in pulmonary vascular resistance caused by positive intrathoracic pressures may lead to further hemodynamic deterioration [8,91]. In this context, in most of the patients with CS, an early NIV trial of 30 min to 1 h will be beneficial, along with initial hemodynamic stabilization, and may help to avoid endotracheal-intubation-related risks such as airway complications, further destabilization due to anesthesia induction and the need for ongoing sedation [90]. Furthermore, IMV is associated with complications such as ventilator-acquired pneumonia, increased length of ICU stays, and increased in-hospital mortality [92]. Major contraindications to NIV are altered mental status on presentation, refractory hypotension, acute RV failure, facial deformities, secretions and vomiting, as well as an uncooperative patient [8,91,93]. The choice of NIV mode (C-PAP vs. Bi-PAP) depends mainly on the presence of hypercapnia, which outlines the need to support both oxygenation and ventilation [89]. Nevertheless, in patients scheduled for primary coronary intervention, the choice of IMV with early intubation and stabilization during the ED stay may be preferential, considering patient control, safety and positioning in the cath-lab. The indications and initial settings for both NIV and IMV are presented in Table 3.

Table 3.
Indications and initial settings for both noninvasive ventilation (NIV) and invasive mechanical ventilation (IMV) [89,90].
5.4. Vasoactive Agents
Inotropes and vasopressors represent a necessary evil in the initial management of patients on the verge of circulatory compromise. They are indispensable and promptly available pharmacological agents, but they should be used at the lowest possible dose and for the lowest possible duration [94,95,96], since prolonged administration is associated with increased oxygen demand (further aggravating myocardial ischemia), increased afterload, impaired microcirculation, arrhythmogenesis [97] and mortality [98]. Their hemodynamic effects may vary, and selection of the appropriate agent should be based on CS etiology, hemodynamic profile and shock severity [22,97,99].
Use of vasopressors and inotropes should be individualized based on patient fluid status and CS etiology and should be adjusted based on clinical judgement. In fluid-responsive patients with signs of hypoperfusion (tachycardia and vasoconstriction) but without hypotension (compensated CS), inotropes (dobutamine or levosimendan) may be cautiously started after first the bolus of fluids has failed to restore peripheral organ perfusion. Attention is required in patients with RV dysfunction who may not tolerate fluid administration or in patients with signs of congestion. When hypotension is also present, concomitant administration of an inotropic agent and a vasopressor (preferably norepinephrine) should be initiated and titrated until perfusion is restored. In patients with refractory shock, escalation of therapy is required with the addition of a second vasopressor (vasopressin) or MCS [99].
Norepinephrine is recommended as a first-line vasopressor (Class IIb/B recommendation) [4] to restore end-organ perfusion and maintain systolic blood pressure [1,97]. Additionally, through cardiac β1 adrenergic stimulation, it enhances cardiac contractility and ventricular–arterial coupling [100]. The exact target of SBP is not fully clarified, but an initial goal of SBP > 90 mmHg and/or MAP of 55–75 mmHg in conjunction with other clinical markers of end-organ perfusion is advised [54]. Compared to epinephrine, norepinephrine had similar hemodynamic effects on mean arterial pressure (MAP) and CI, but epinephrine was associated with higher rates of refractory shock, tachycardia, lactic acidosis [101] and mortality [102,103]. Moreover, compared to dopamine, norepinephrine had a safer profile in patients with CS, due to a lower trend for arrhythmic events and mortality [104]. Vasopressin lacks inotropic properties and may be used as a second-line vasoactive agent, concomitantly to norepinephrine, if hemodynamic status does not improve with single use of norepinephrine [1,99,105]. Its administration may be appealing in special circumstances, like in patients with right ventricular failure, as it does not affect pulmonary arterial pressure [93,97,106], or in combination with milrinone in order to counteract its vasodilatory effect [1], but evidence is lacking [93].
Inotropes may be considered in addition to vasopressors in order to augment cardiac output and end-organ perfusion (Class IIb/C recommendation) [4]. Dobutamine is recommended over other inotropic agents if signs of hypoperfusion persist despite first-line vasopressor therapy [1]. However, a systematic review failed to show any benefit of dobutamine over levosimendan for short- and long-term survival [107]. Levosimendan and milrinone may be preferable over dobutamine in special cases such as long b-blockade, right ventricular dysfunction, pulmonary hypertension, or Takotsubo cardiomyopathy [1,4,108].
In CS patients with refractory hypotension, vasopressin may aid in preserving arterial blood pressure, as a third vasoactive agent, in conjunction with norepinephrine and dobutamine [99].
5.5. Short-Term Mechanical Circulatory Support
Patients who present with deteriorating or extremis CS, or those who fail to stabilize hemodynamically with two vasoactive agents may benefit from devices for temporary MCS in an individualized manner (Class IIa/C recommendation) [4,22]. Early initiation of MCS may provide univentricular or biventricular support by improving cardiac contractility, reducing left/right ventricular end-diastolic pressures, enhancing coronary perfusion and decreasing myocardial oxygen demand [109,110], resulting in effective weaning from cardiotoxic vasoactive agents [111]. However, controversial results with respect to mortality [112,113,114,115] incite the consideration of challenging issues regarding their application in practice, such as patient selection, type of device, appropriate timing, and prognostic impact. A characteristic recent example is the ECMO-CS randomized controlled trial where patients with deteriorating CS were randomized to immediate ECMO or early conservative therapy (with the ECMO kept as a bailout option at a later stage). The study did not show a benefit for the early ECMO approach. Despite having several limitations (i.e., relatively small sample size, mixed cohort of AMI- and non-AMI-related CS, and significant crossover rate (39% crossover to VA-ECMO in early conservative arm)), the study provides important data for the CS population.
Available choices for left ventricular assistance include the intra-aortic balloon pump (IABP), microaxial flow pumps (Impella CP, Impella 5), or the left-atrium-to-femoral-artery system device (Tandem Heart), while right ventricular assistance may be supported by the Impella RP, Tandem Heart RA-PA and Protek Duo devices. Venous–arterial extracorporeal membrane oxygenation (VA-ECMO) may reinforce biventricular performance and improve oxygenation [1,55,110]. Regarding the down-side of advanced percutaneous left ventricular devices, IABP use has subsided, taking into account no proven survival benefit for patients with AMI-CS [112], and currently its implementation may be considered for patients with refractory CS of non-AMI etiology (class IIb/C recommendation) [4] or for AMI-CS patients with mechanical complications as a bridge to more advanced MCS devices (class IIa/C recommendation) [42]. Even if Impella devices seem like promising approaches, data regarding their beneficial effect on mortality are scarce [113,114,116]. Although ECMO may ensure hemodynamic stabilization in cardiopulmonary resuscitation [117], it may also increase LV afterload, making its use reasonable with concomitant LV unloading (IABP, Impella, septostomy and hybrid circuit configuration) [1,110,118]. It must be emphasized that devices like Impella and ECMO necessitate the insertion of large-bore cannulas into major vessels and carry a high risk of complications, including vascular and bleeding complications. Characteristically, for Impella used in AMI-related CS, the rate of severe bleeding reported in the literature ranged from 8.5% to 31% [119,120]. Thus, to improve the efficacy of advanced MCS devices and increase the chances of positive studies in the CS field, specific measures should be taken to reduce complications. Such measures include comprehensive training in device insertion and maintenance, formation of dedicated teams (e.g., ECMO team including perfusionists), ultrasound guided vascular access, use of vascular closure systems, etc.
The uniqueness of each device, indicated by distinct hemodynamic effects, favorable profiles, contraindications and complications, limits their use to selected patients and under the supervision of expert teams [121]. The comprehensive approach by multidisciplinary teams in the context of a shock network emerges as an ultimate but not impossible goal to improve survival. Interestingly, recent data support that early initiation of MCS in the initial stages of CS, even before the administration of inotropes or the PCI strategy, is significantly associated with increased survival rates in patients presenting with AMI-CS [122], supporting the need to achieve shorter “door to support” intervals, so as to anticipate the deleterious effects of the fatal spiral of cardiac compromise [123].
6. Shock Teams and Networks
The holistic management of CS, which includes early identification, comprehensive diagnostic work-up and treatment interventions, requires a multidisciplinary approach. The variety of CS presentations, severity and causes, the lack of strong evidence for proposed treatments (e.g., t-MCS), and the need for an individualized therapeutic approach make collective decision making and pooling of specialized expertise even more important. There is increasing evidence that the introduction of dedicated shock teams and networks could improve clinical outcomes in CS patients.
The introduction of the hub-and-spoke model has a positive impact on patient outcomes [124,125,126]. The “hub” hospital has the central role, warranting the presence of a multidisciplinary CS team consisting of the interventional cardiologist, critical care specialist, cardiothoracic surgeon and advanced heart failure specialist (Level of care I). The “spoke” hospitals are either PCI-capable centers without advanced MCS (Level of care II) or non-PCI-capable hospitals, both referring to the hub hospital [121,127]. The role of the shock team is the timely recognition of CS and the provision of appropriate consultation to spoke hospitals regarding escalation of therapy and the need for advanced MCS, right heart catheterization (RHC) for the employment of the appropriate MCS modality, initiation of MCS, advanced postintervention care and monitoring, and eventually weaning from the MCS device and patient recovery [11,127]. Moreover, Rab comments on the fundamental role of a “shock doc”, a distinct member of the shock team who coordinates patient pathways and is responsible for the synchronization of intervention strategies [128]. Implementation of regionalized shock protocols in both AMI-CS and non-AMI-CS populations has proven to be feasible and was associated with improved survival [34,69,129,130]. A multicenter observational study, which compared CICUs with or without shock teams, concluded that the presence of shock teams was linked to more extensive use of pulmonary catheter catheterization and decreased administration of vasoactive agents. Furthermore, centers with a shock team used less MCS overall, but when MCS was used, it more often involved an advanced device (e.g., IMPELLA, ECMO or Tandem Heart). Mortality was substantially lower (23% versus 29%; adjusted OR: 0,72; 95% CI: 0.55–0.94; p = 0.016), as was the proportion of patients necessitating renal replacement therapy or mechanical ventilation, depicting a moderate degree of end-organ damage [131].
In this model, the ED has a strategic role outlined by the early identification of CS patients, initial stabilization, and prompt triage to the appropriate level of care [127]. Emergency physicians should endorse unified shock protocols in alliance with the hub hospital and activate the shock team through a one-call line for multidisciplinary consultation. The importance of expedient triage is underscored by an observational retrospective study that reviewed CS patient admissions from the ED directly to a CICU or an ICU. Patient admission to a non-CICU was associated with significant treatment delays for disease-modifying and evidenced-based therapies like RHC, PCI or MCS, and eventually higher mortality [132]. Therefore, direct transfer of CS patients to the spoke hospital is a priority in order to guarantee optimal management without delays.
Criteria for shock team activation vary among different shock team initiatives that implement regional multidisciplinary strategies. Prespecified inclusion criteria in the Detroit Cardiogenic Shock Initiative comprise AMI (indicated by ischemic symptoms/ECG and/or biomarker investigations) or CS (indicated by hypotension < 90/60 mmHg or the need for vasopressors/inotropes to maintain SBP > 90 mmHg) and evidence of hypoperfusion (cool extremities, oliguria, lactic acidosis) that settle the activation of the catheterization lab, in the absence of any exclusion criteria, where hemodynamic criteria will tailor further management [34]. The INOVA pathway and the Utah Cardiac Recovery Shock Team propose the activation of the shock team upon even the suspicion of CS with clinical criteria (SBP, evidence of end-organ perfusion, lactate) [69,130]. Finally, the University of Ottawa Heart Institute recommends that upon clinical identification of CS, the coronary cardiac unit senior resident has the key role in assessing the patient for possible exclusion criteria and successively activating “CODE SHOCK” [129]. Thus, it seems that clinical evaluation is the mainstay for setting the alarm code.
The roadmap of the patient presenting to the ED with CS is summarized in Figure 1.
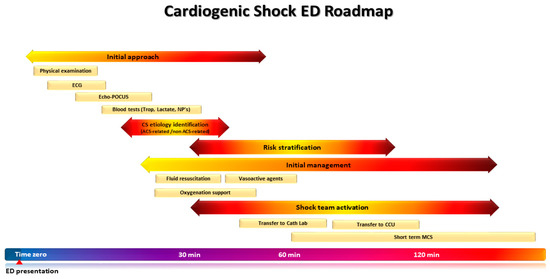
Figure 1.
The roadmap of the patients presenting in the emergency department (ED) with cardiogenic shock (CS). A timely and structured approach upon presentation to the ED ensures prompt identification of CS, its underlying etiology, and risk stratification, in order to guide decision making and patient disposition without delay.
7. Conclusions
The battle against CS necessitates a deep insight into the complexity of the syndrome and a prompt multidisciplinary approach. Current trends in epidemiology and a more nuanced stage classification may help clarify the broad spectrum of clinical phenotypes and optimize individualized management according the the culprit cardiac insult. Prompt identification and continuous assessment of CS patients are the foundations of the appropriate treatment plan. Critical evaluation in the acute setting should be directly correlated with the activation of a medical “defense cascade”, which in turn should guarantee high quality interventions. The ED, which occupies a crucial position, in alliance with the fundamental performance of the shock team, is a promising bundle for the improvement of CS mortality. Attention should not only be driven towards the development of proficient MCS devices but also to the adoption of readily available diagnostic tools and decision-making algorithms that will ensure expedient patient direction through the CS network. The establishment of validated risk stratification scores, the determination of uniform and readily available clinical criteria for shock team activation, and the endorsement of precise exclusion criteria are special issues to be addressed. Thus, further clinical studies should be organized on this basis, so as to facilitate optimal CS patient management.
Author Contributions
Conceptualization and supervision, E.P. and J.P.; Writing—original draft preparation, S.B.; writing—review and editing, G.K. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
S.B., G.K., A.B. and E.P. have no conflicts of interest or financial ties to disclose. J.P. received honoraria for lectures from Orion Pharma, Pfizer, Servier, Astra, AO Orphan and Roche Diagnostics. For the present study, the authors have no conflicts of interest to declare.
References
- Chioncel, O.; Parissis, J.; Mebazaa, A.; Thiele, H.; Desch, S.; Bauersachs, J.; Harjola, V.; Antohi, E.; Arrigo, M.; Ben Gal, T.; et al. Epidemiology, Pathophysiology and Contemporary Management of Cardiogenic Shock—A Position Statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1315–1341. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, L.B.; Cox, B.G. The Fundamental Mechanisms of Shock: Proceedings of a Symposium Held in Oklahoma City, Oklahoma, 1–2 October 1971; Springer: Boston, MA, USA, 1972. [Google Scholar]
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.N.; Kaier, K.; Zotzmann, V.; Stachon, P.; Pottgiesser, T.; von zur Muehlen, C.; Zehender, M.; Duerschmied, D.; Schmid, B.; Bode, C.; et al. Cardiogenic Shock: Incidence, Survival and Mechanical Circulatory Support Usage 2007–2017-Insights from a National Registry. Clin. Res. Cardiol. 2021, 110, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Syed, M.; Patibandla, S.; Sulaiman, S.; Kheiri, B.; Shah, M.K.; Bianco, C.; Balla, S.; Patel, B. Fifteen-Year Trends in Incidence of Cardiogenic Shock Hospitalization and In-Hospital Mortality in the United States. JAHA 2021, 10, e021061. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.D.; Bohula, E.A.; van Diepen, S.; Katz, J.N.; Alviar, C.L.; Baird-Zars, V.M.; Barnett, C.F.; Barsness, G.W.; Burke, J.A.; Cremer, P.C.; et al. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units: Data from the Critical Care Cardiology Trials Network Registry. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005618. [Google Scholar] [CrossRef]
- Harjola, V.-P.; Lassus, J.; Sionis, A.; Køber, L.; Tarvasmäki, T.; Spinar, J.; Parissis, J.; Banaszewski, M.; Silva-Cardoso, J.; Carubelli, V.; et al. Clinical Picture and Risk Prediction of Short-Term Mortality in Cardiogenic Shock: Clinical Picture and Outcome of Cardiogenic Shock. Eur. J. Heart Fail. 2015, 17, 501–509. [Google Scholar] [CrossRef]
- Sinha, S.S.; Rosner, C.M.; Tehrani, B.N.; Maini, A.; Truesdell, A.G.; Lee, S.B.; Bagchi, P.; Cameron, J.; Damluji, A.A.; Desai, M.; et al. Cardiogenic Shock from Heart Failure Versus Acute Myocardial Infarction: Clinical Characteristics, Hospital Course, and 1-Year Outcomes. Circ. Heart Fail. 2022, 15, e009279. [Google Scholar] [CrossRef]
- Shah, M.; Patnaik, S.; Patel, B.; Ram, P.; Garg, L.; Agarwal, M.; Agrawal, S.; Arora, S.; Patel, N.; Wald, J.; et al. Trends in Mechanical Circulatory Support Use and Hospital Mortality among Patients with Acute Myocardial Infarction and Non-Infarction Related Cardiogenic Shock in the United States. Clin. Res. Cardiol. 2018, 107, 287–303. [Google Scholar] [CrossRef]
- Truesdell, A.G.; Tehrani, B.; Singh, R.; Desai, S.; Saulino, P.; Barnett, S.; Lavanier, S.; Murphy, C. ‘Combat’ Approach to Cardiogenic Shock. Interv. Cardiol. Rev. 2018, 1, 81. [Google Scholar] [CrossRef]
- Zannad, F.; Mebazaa, A.; Juillière, Y.; Cohen-Solal, A.; Guize, L.; Alla, F.; Rougé, P.; Blin, P.; Barlet, M.-H.; Paolozzi, L.; et al. Clinical Profile, Contemporary Management and One-Year Mortality in Patients with Severe Acute Heart Failure Syndromes: The EFICA Study☆. Eur. J. Heart Fail. 2006, 8, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.J.; Gore, J.M.; Alpert, J.S.; Osganian, V.; de Groot, J.; Bade, J.; Chen, Z.; Frid, D.; Dalen, J.E. Cardiogenic Shock after Acute Myocardial Infarction: Incidence and Mortality from a Community-Wide Perspective, 1975 to 1988. N. Engl. J. Med. 1991, 325, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Buller, C.E.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.D.; Bohula, E.A.; Morrow, D.A. Epidemiology and Causes of Cardiogenic Shock. Curr. Opin. Crit. Care 2021, 27, 401–408. [Google Scholar] [CrossRef]
- Shah, R.U.; Henry, T.D.; Rutten-Ramos, S.; Garberich, R.F.; Tighiouart, M.; Bairey Merz, C.N. Increasing Percutaneous Coronary Interventions for ST-Segment Elevation Myocardial Infarction in the United States. JACC Cardiovasc. Interv. 2015, 8, 139–146. [Google Scholar] [CrossRef]
- Holcomb, J.B. Major Scientific Lessons Learned in the Trauma Field over the Last Two Decades. PLoS Med. 2017, 14, e1002339. [Google Scholar] [CrossRef]
- Gitz Holler, J.; Jensen, H.K.; Henriksen, D.P.; Rasmussen, L.M.; Mikkelsen, S.; Pedersen, C.; Lassen, A.T. Etiology of Shock in the Emergency Department: A 12-Year Population-Based Cohort Study. Shock 2019, 51, 60–67. [Google Scholar] [CrossRef]
- Thiele, H.; Ohman, E.M.; de Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of Cardiogenic Shock Complicating Myocardial Infarction: An Update 2019. Eur. Heart J. 2019, 40, 2671–2683. [Google Scholar] [CrossRef]
- Menon, V.; Slater, J.N.; White, H.D.; Sleeper, L.A.; Cocke, T.; Hochman, J.S. Acute Myocardial Infarction Complicated by Systemic Hypoperfusion without Hypotension: Report of the SHOCK Trial Registry. Am. J. Med. 2000, 108, 374–380. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Burstein, B.; Van Diepen, S.; Murphy, J.; Holmes, D.R.; Bell, M.R.; Barsness, G.W.; Henry, T.D.; Menon, V.; Rihal, C.S.; et al. Defining Shock and Preshock for Mortality Risk Stratification in Cardiac Intensive Care Unit Patients. Circ. Heart Fail. 2021, 14, e007678. [Google Scholar] [CrossRef]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI Clinical Expert Consensus Statement on the Classification of Cardiogenic Shock: This Document Was Endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100008. [Google Scholar] [CrossRef]
- Hanson, I.D.; Tagami, T.; Mando, R.; Kara Balla, A.; Dixon, S.R.; Timmis, S.; Almany, S.; Naidu, S.S.; Baran, D.; Lemor, A.; et al. SCAI Shock Classification in Acute Myocardial Infarction: Insights from the National Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2020, 96, 1137–1142. [Google Scholar] [CrossRef]
- Jentzer, J.C.; van Diepen, S.; Barsness, G.W.; Henry, T.D.; Menon, V.; Rihal, C.S.; Naidu, S.S.; Baran, D.A. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2019, 74, 2117–2128. [Google Scholar] [CrossRef]
- Lawler, P.R.; Berg, D.D.; Park, J.-G.; Katz, J.N.; Baird-Zars, V.M.; Barsness, G.W.; Bohula, E.A.; Carnicelli, A.P.; Chaudhry, S.-P.; Jentzer, J.C.; et al. The Range of Cardiogenic Shock Survival by Clinical Stage: Data from the Critical Care Cardiology Trials Network Registry. Crit. Care Med. 2021, 49, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Thayer, K.L.; Zweck, E.; Ayouty, M.; Garan, A.R.; Hernandez-Montfort, J.; Mahr, C.; Morine, K.J.; Newman, S.; Jorde, L.; Haywood, J.L.; et al. Invasive Hemodynamic Assessment and Classification of In-Hospital Mortality Risk among Patients with Cardiogenic Shock. Circ. Heart Fail. 2020, 13, e007099. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.; Dabboura, S.; Yan, I.; Hilal, R.; Neumann, J.T.; Sörensen, N.A.; Goßling, A.; Becher, P.M.; Grahn, H.; Wagner, T.; et al. Application of the SCAI Classification in a Cohort of Patients with Cardiogenic Shock. Catheter. Cardiovasc. Interv. 2020, 96, E213–E219. [Google Scholar] [CrossRef]
- Baran, D.A.; Long, A.; Badiye, A.P.; Stelling, K. Prospective Validation of the SCAI Shock Classification: Single Center Analysis. Catheter. Cardiovasc. Interv. 2020, 96, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Pareek, N.; Dworakowski, R.; Webb, I.; Barash, J.; Emezu, G.; Melikian, N.; Hill, J.; Shah, A.; MacCarthy, P.; Byrne, J. SCAI Cardiogenic Shock Classification after out of Hospital Cardiac Arrest and Association with Outcome. Catheter. Cardiovasc. Interv. 2021, 97, E288–E297. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; Henry, T.D.; Barsness, G.W.; Menon, V.; Baran, D.A.; Diepen, S. Influence of Cardiac Arrest and SCAI Shock Stage on Cardiac Intensive Care Unit Mortality. Catheter. Cardiovasc. Interv. 2020, 96, 1350–1359. [Google Scholar] [CrossRef]
- Kapur, N.K.; Kanwar, M.; Sinha, S.S.; Thayer, K.L.; Garan, A.R.; Hernandez-Montfort, J.; Zhang, Y.; Li, B.; Baca, P.; Dieng, F.; et al. Criteria for Defining Stages of Cardiogenic Shock Severity. J. Am. Coll. Cardiol. 2022, 80, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Tolppanen, H.; Mueller, C.; Lassus, J.; DiSomma, S.; Baksyte, G.; Cecconi, M.; Choi, D.J.; Cohen Solal, A.; Christ, M.; et al. Acute Heart Failure and Cardiogenic Shock: A Multidisciplinary Practical Guidance. Intensive Care Med. 2016, 42, 147–163. [Google Scholar] [CrossRef]
- Basir, M.B.; Schreiber, T.; Dixon, S.; Alaswad, K.; Patel, K.; Almany, S.; Khandelwal, A.; Hanson, I.; George, A.; Ashbrook, M.; et al. Feasibility of Early Mechanical Circulatory Support in Acute Myocardial Infarction Complicated by Cardiogenic Shock: The Detroit Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2018, 91, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Thim, T.; Krarup, N.H.V.; Grove, E.L.; Rohde, C.V.; Løfgren, B. Initial Assessment and Treatment with the Airway, Breathing, Circulation, Disability, Exposure (ABCDE) Approach. IJGM 2012, 117. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Mebazaa, A.; Harjola, V.-P.; Coats, A.J.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R.; et al. Clinical Phenotypes and Outcome of Patients Hospitalized for Acute Heart Failure: The ESC Heart Failure Long-Term Registry: Outcome of Patients Hospitalized for Acute Heart Failure. Eur. J. Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.S.; Diamond, G.; Chatterjee, K.; Swan, H.J.C. Medical Therapy of Acute Myocardial Infarction by Application of Hemodynamic Subsets. N. Engl. J. Med. 1976, 295, 1356–1362. [Google Scholar] [CrossRef]
- Binanay, C.; Califf, R.M.; Hasselblad, V.; O’Connor, C.M.; Shah, M.R.; Sopko, G.; Stevenson, L.W.; Francis, G.S.; Leier, C.V.; Miller, L.W.; et al. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness: The ESCAPE Trial. JAMA 2005, 294, 1625. [Google Scholar] [CrossRef]
- Richard, C. Early Use of the Pulmonary Artery Catheter and Outcomes in Patients with Shock and Acute Respiratory Distress Syndrome: A Randomized Controlled Trial. JAMA 2003, 290, 2713. [Google Scholar] [CrossRef]
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.L.; et al. Consensus on Circulatory Shock and Hemodynamic Monitoring. Task Force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef]
- Harjola, V.-P.; Parissis, J.; Brunner-La Rocca, H.-P.; Čelutkienė, J.; Chioncel, O.; Collins, S.P.; De Backer, D.; Filippatos, G.S.; Gayat, E.; Hill, L.; et al. Comprehensive In-Hospital Monitoring in Acute Heart Failure: Applications for Clinical Practice and Future Directions for Research. A Statement from the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardio: In-Hospital Monitoring of AHF. Eur. J. Heart Fail. 2018, 20, 1081–1099. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- For the Acute Heart Failure Study Group of the European Society of Cardiology Acute Cardiovascular Care Association; Price, S.; Platz, E.; Cullen, L.; Tavazzi, G.; Christ, M.; Cowie, M.R.; Maisel, A.S.; Masip, J.; Miro, O.; et al. Echocardiography and Lung Ultrasonography for the Assessment and Management of Acute Heart Failure. Nat. Rev. Cardiol. 2017, 14, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Berg, I.; Walpot, K.; Lamprecht, H.; Valois, M.; Lanctôt, J.-F.; Srour, N.; van den Brand, C. A Systemic Review on the Diagnostic Accuracy of Point-of-Care Ultrasound in Patients with Undifferentiated Shock in the Emergency Department. Cureus 2022, 14, e23188. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.; Mailhot, T.; Riley, D.; Mandavia, D. The RUSH Exam: Rapid Ultrasound in SHock in the Evaluation of the Critically Lll. Emerg. Med. Clin. N. Am. 2010, 28, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Adedipe, A.; Morse, S.; McCabe, J.; Mahr, C.; Nichol, G. Feasibility of Very Early Identification of Cardiogenic Shock by Semi-Automated Ultrasound Exam in the Emergency Department. Cureus 2022, 14, e30927. [Google Scholar] [CrossRef] [PubMed]
- Ruben, M.; Molinas, M.S.; Paladini, H.; Khalife, W.; Barbagelata, A.; Perrone, S.; Kaplinsky, E. Emerging Concepts in Heart Failure Management and Treatment: Focus on Point-of-Care Ultrasound in Cardiogenic Shock. DIC 2023, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Soliman-Aboumarie, H.; Denault, A.Y. How to Assess Systemic Venous Congestion with Point of Care Ultrasound. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 177–180. [Google Scholar] [CrossRef]
- Lazzeri, C.; Valente, S.; Chiostri, M.; Gensini, G.F. Clinical Significance of Lactate in Acute Cardiac Patients. WJC 2015, 7, 483. [Google Scholar] [CrossRef]
- Fuernau, G. Lactate and Other Biomarkers as Treatment Target in Cardiogenic Shock. Curr. Opin. Crit. Care 2019, 25, 403–409. [Google Scholar] [CrossRef]
- Jansen, T.C.; van Bommel, J.; Schoonderbeek, F.J.; Sleeswijk Visser, S.J.; van der Klooster, J.M.; Lima, A.P.; Willemsen, S.P.; Bakker, J. Early Lactate-Guided Therapy in Intensive Care Unit Patients: A Multicenter, Open-Label, Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2010, 182, 752–761. [Google Scholar] [CrossRef]
- Park, I.H.; Yang, J.H.; Jang, W.J.; Chun, W.J.; Oh, J.H.; Park, Y.H.; Ko, Y.-G.; Yu, C.W.; Kim, B.S.; Kim, H.-J.; et al. Clinical Significance of Lactate Clearance in Patients with Cardiogenic Shock: Results from the RESCUE Registry. J. Intensive Care 2021, 9, 63. [Google Scholar] [CrossRef]
- Fuernau, G.; Desch, S.; de Waha-Thiele, S.; Eitel, I.; Neumann, F.-J.; Hennersdorf, M.; Felix, S.B.; Fach, A.; Böhm, M.; Pöss, J.; et al. Arterial Lactate in Cardiogenic Shock. JACC Cardiovasc. Interv. 2020, 13, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Fernando, S.M.; Hu, K.; Parlow, S.; Di Santo, P.; Brodie, D.; Hibbert, B. Optimal Perfusion Targets in Cardiogenic Shock. JACC Adv. 2022, 1, 100034. [Google Scholar] [CrossRef]
- Vahdatpour, C.; Collins, D.; Goldberg, S. Cardiogenic Shock. JAHA 2019, 8, e011991. [Google Scholar] [CrossRef] [PubMed]
- Boeddinghaus, J.; Nestelberger, T.; Koechlin, L.; Wussler, D.; Lopez-Ayala, P.; Walter, J.E.; Troester, V.; Ratmann, P.D.; Seidel, F.; Zimmermann, T.; et al. Early Diagnosis of Myocardial Infarction with Point-of-Care High-Sensitivity Cardiac Troponin I. J. Am. Coll. Cardiol. 2020, 75, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Kremer, D.; Geven, C.; ter Maaten, J.M.; Struck, J.; Bergmann, A.; Pickkers, P.; Metra, M.; Mebazaa, A.; Düngen, H.-D.; et al. Adrenomedullin in Heart Failure: Pathophysiology and Therapeutic Application. Eur. J. Heart Fail. 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Emmens, J.E.; ter Maaten, J.M.; Damman, K.; van Veldhuisen, D.J.; de Boer, R.A.; Struck, J.; Bergmann, A.; Sama, I.E.; Streng, K.W.; Anker, S.D.; et al. Proenkephalin, an Opioid System Surrogate, as a Novel Comprehensive Renal Marker in Heart Failure. Circ. Heart Fail. 2019, 12, e005544. [Google Scholar] [CrossRef]
- Molvin, J.; Jujic, A.; Navarin, S.; Melander, O.; Zoccoli, G.; Hartmann, O.; Bergmann, A.; Struck, J.; Bachus, E.; Di Somma, S.; et al. Bioactive Adrenomedullin, Proenkephalin A and Clinical Outcomes in an Acute Heart Failure Setting. Open Heart 2019, 6, e001048. [Google Scholar] [CrossRef]
- Siranart, N.; Laohasurayotin, K.; Chokesuwattanaskul, R.; Ariyachaipanich, A. Proenkephalin as a Novel Prognostic Marker in Heart Failure Patients: A Meta-Analysis. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544.908. [Google Scholar] [CrossRef]
- Fuernau, G.; Poenisch, C.; Eitel, I.; de Waha, S.; Desch, S.; Schuler, G.; Adams, V.; Werdan, K.; Zeymer, U.; Thiele, H. Growth-Differentiation Factor 15 and Osteoprotegerin in Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Biomarker Substudy of the IABP-SHOCK II-Trial. Eur. J. Heart Fail. 2014, 16, 880–887. [Google Scholar] [CrossRef]
- Pöss, J.; Fuernau, G.; Denks, D.; Desch, S.; Eitel, I.; de Waha, S.; Link, A.; Schuler, G.; Adams, V.; Böhm, M.; et al. Angiopoietin-2 in acute myocardial infarction complicated by cardiogenic shock—A biomarker substudy of the IABP-SHOCK II-Trial. Eur. J. Heart Fail. 2015, 17, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Koreny, M.; Karth, G.D.; Geppert, A.; Neunteufl, T.; Priglinger, U.; Heinz, G.; Siostrzonek, P. Prognosis of Patients Who Develop Acute Renal Failure during the First 24 Hours of Cardiogenic Shock after Myocardial Infarction. Am. J. Med. 2002, 112, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, O.; Nguyen, T.; Bansal, S.; Prasad, A. Acute Kidney Injury in Cardiogenic Shock: A Comprehensive Review. Catheter. Cardiovasc. Interv. 2021, 98, E91–E105. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, M.; Parissis, J.; Yilmaz, M.B.; Seronde, M.-F.; Kivikko, M.; Laribi, S.; Paugam-Burtz, C.; Cai, D.; Pohjanjousi, P.; Laterre, P.-F.; et al. Liver Function Abnormalities, Clinical Profile, and Outcome in Acute Decompensated Heart Failure. Eur. Heart J. 2013, 34, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Cottin, Y.; Leurent, G.; Gilard, M.; Beer, J.-C.; Zabalawi, A.; Chagué, F.; Filippi, E.; Brunet, D.; Hacot, J.-P.; et al. Predicting the Development of In-Hospital Cardiogenic Shock in Patients with ST-Segment Elevation Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention: The ORBI Risk Score. Eur. Heart J. 2018, 39, 2090–2102. [Google Scholar] [CrossRef]
- Sleeper, L.A.; Reynolds, H.R.; White, H.D.; Webb, J.G.; Džavík, V.; Hochman, J.S. A Severity Scoring System for Risk Assessment of Patients with Cardiogenic Shock: A Report from the SHOCK Trial and Registry. Am. Heart J. 2010, 160, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Pöss, J.; Köster, J.; Fuernau, G.; Eitel, I.; de Waha, S.; Ouarrak, T.; Lassus, J.; Harjola, V.-P.; Zeymer, U.; Thiele, H.; et al. Risk Stratification for Patients in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 69, 1913–1920. [Google Scholar] [CrossRef]
- Tehrani, B.N.; Truesdell, A.G.; Sherwood, M.W.; Desai, S.; Tran, H.A.; Epps, K.C.; Singh, R.; Psotka, M.; Shah, P.; Cooper, L.B.; et al. Standardized Team-Based Care for Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 1659–1669. [Google Scholar] [CrossRef]
- Rivas-Lasarte, M.; Sans-Roselló, J.; Collado-Lledó, E.; González-Fernández, V.; Noriega, F.J.; Hernández-Pérez, F.J.; Fernández-Martínez, J.; Ariza, A.; Lidón, R.-M.; Viana-Tejedor, A.; et al. External Validation and Comparison of the CardShock and IABP-SHOCK II Risk Scores in Real-World Cardiogenic Shock Patients. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 16–24. [Google Scholar] [CrossRef]
- Kalra, S.; Ranard, L.S.; Memon, S.; Rao, P.; Garan, A.R.; Masoumi, A.; O’Neill, W.; Kapur, N.K.; Karmpaliotis, D.; Fried, J.A.; et al. Risk Prediction in Cardiogenic Shock: Current State of Knowledge, Challenges and Opportunities. J. Card. Fail. 2021, 27, 1099–1110. [Google Scholar] [CrossRef]
- Tien, Y.-T.; Chen, W.-J.; Huang, C.-H.; Wang, C.-H.; Chen, W.-T.; Hung, C.-S.; Lin, J.-J.; Huang, C.-C.; Chang, W.-T.; Tsai, M.-S. The CSP (Cardiogenic Shock Prognosis) Score: A Tool for Risk Stratification of Cardiogenic Shock. Front. Cardiovasc. Med. 2022, 9, 842056. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Antonescu, C.; Ravindranath, S.; Dong, J.; Lu, M.; Vicario, F.; Wondrely, L.; Thompson, P.; Swearingen, D.; Acharya, D. Early Prediction of Cardiogenic Shock Using Machine Learning. Front. Cardiovasc. Med. 2022, 9, 862424. [Google Scholar] [CrossRef] [PubMed]
- Henning, D.J.; Kearney, K.E.; Hall, M.K.; Mahr, C.; Shapiro, N.I.; Nichol, G. Identification of Hypotensive Emergency Department Patients with Cardiogenic Etiologies. Shock 2018, 49, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ceglarek, U.; Schellong, P.; Rosolowski, M.; Scholz, M.; Willenberg, A.; Kratzsch, J.; Zeymer, U.; Fuernau, G.; de Waha-Thiele, S.; Büttner, P.; et al. The Novel Cystatin C, Lactate, Interleukin-6, and N-Terminal pro-B-Type Natriuretic Peptide (CLIP)-Based Mortality Risk Score in Cardiogenic Shock after Acute Myocardial Infarction. Eur. Heart J. 2021, 42, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Gil, V.; Domínguez-Rodríguez, A.; Masip, J.; Peacock, W.F.; Miró, Ò. Morphine Use in the Treatment of Acute Cardiogenic Pulmonary Edema and Its Effects on Patient Outcome: A Systematic Review. Curr. Heart Fail. Rep. 2019, 16, 81–88. [Google Scholar] [CrossRef]
- Cherpanath, T.G.V.; Hirsch, A.; Geerts, B.F.; Lagrand, W.K.; Leeflang, M.M.; Schultz, M.J.; Groeneveld, A.B.J. Predicting Fluid Responsiveness by Passive Leg Raising: A Systematic Review and Meta-Analysis of 23 Clinical Trials*. Crit. Care Med. 2016, 44, 981–991. [Google Scholar] [CrossRef]
- Monnet, X.; Marik, P.; Teboul, J.-L. Passive Leg Raising for Predicting Fluid Responsiveness: A Systematic Review and Meta-Analysis. Intensive Care Med. 2016, 42, 1935–1947. [Google Scholar] [CrossRef]
- Monnet, X.; Teboul, J.-L. Assessment of Fluid Responsiveness: Recent Advances. Curr. Opin. Crit. Care 2018, 24, 190–195. [Google Scholar] [CrossRef]
- Blanco, P. Rationale for Using the Velocity–Time Integral and the Minute Distance for Assessing the Stroke Volume and Cardiac Output in Point-of-Care Settings. Ultrasound J. 2020, 12, 21. [Google Scholar] [CrossRef]
- Ashley, M.; Justin, M. Predicting and Measuring Fluid Responsiveness with Echocardiography. Echo Res. Pract. 2016, 3, G1–G12. [Google Scholar] [CrossRef]
- Kellum, J.A. Abnormal Saline and the History of Intravenous Fluids. Nat. Rev. Nephrol. 2018, 14, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, M.D.; Gammelager, H.; Schmidt, M.; Rasmussen, T.B.; Shaw, R.E.; Bøtker, H.E.; Sørensen, H.T.; Christiansen, C.F. Acute Kidney Injury Treated with Renal Replacement Therapy and 5-Year Mortality after Myocardial Infarction-Related Cardiogenic Shock: A Nationwide Population-Based Cohort Study. Crit. Care 2015, 19, 452. [Google Scholar] [CrossRef] [PubMed]
- Semler, M.W.; Self, W.H.; Wanderer, J.P.; Ehrenfeld, J.M.; Wang, L.; Byrne, D.W.; Stollings, J.L.; Kumar, A.B.; Hughes, C.G.; Hernandez, A.; et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N. Engl. J. Med. 2018, 378, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Ranzani, O.T.; Azevedo, L.C.P.; Martins, I.D.S.; Kellum, J.A.; Libório, A.B. Lactated Ringer Is Associated with Reduced Mortality and Less Acute Kidney Injury in Critically Ill Patients: A Retrospective Cohort Analysis*. Crit. Care Med. 2016, 44, 2163–2170. [Google Scholar] [CrossRef]
- Hammond, N.E.; Zampieri, F.G.; Di Tanna, G.L.; Garside, T.; Adigbli, D.; Cavalcanti, A.B.; Machado, F.R.; Micallef, S.; Myburgh, J.; Ramanan, M.; et al. Balanced Crystalloids versus Saline in Critically Ill Adults—A Systematic Review with Meta-Analysis. NEJM Evid. 2022, 1, EVIDoa2100010. [Google Scholar] [CrossRef]
- Dong, W.-H.; Yan, W.-Q.; Song, X.; Zhou, W.-Q.; Chen, Z. Fluid Resuscitation with Balanced Crystalloids versus Normal Saline in Critically Ill Patients: A Systematic Review and Meta-Analysis. Scand. J. Trauma Resusc. Emerg. Med. 2022, 30, 28. [Google Scholar] [CrossRef]
- Zayed, Y.Z.M.; Aburahma, A.M.Y.; Barbarawi, M.O.; Hamid, K.; Banifadel, M.R.N.; Rashdan, L.; Bachuwa, G.I. Balanced Crystalloids versus Isotonic Saline in Critically Ill Patients: Systematic Review and Meta-Analysis. J. Intensive Care 2018, 6, 51. [Google Scholar] [CrossRef]
- Alviar, C.L.; Miller, P.E.; McAreavey, D.; Katz, J.N.; Lee, B.; Moriyama, B.; Soble, J.; van Diepen, S.; Solomon, M.A.; Morrow, D.A. Positive Pressure Ventilation in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2018, 72, 1532–1553. [Google Scholar] [CrossRef]
- Alviar, C.L.; Rico-Mesa, J.S.; Morrow, D.A.; Thiele, H.; Miller, P.E.; Maselli, D.J.; van Diepen, S. Positive Pressure Ventilation in Cardiogenic Shock: Review of the Evidence and Practical Advice for Patients with Mechanical Circulatory Support. Can. J. Cardiol. 2020, 36, 300–312. [Google Scholar] [CrossRef]
- Masip, J.; Peacock, W.F.; Price, S.; Cullen, L.; Martin-Sanchez, F.J.; Seferovic, P.; Maisel, A.S.; Miro, O.; Filippatos, G.; Vrints, C.; et al. Indications and Practical Approach to Non-Invasive Ventilation in Acute Heart Failure. Eur. Heart J. 2018, 39, 17–25. [Google Scholar] [CrossRef]
- Berbenetz, N.; Wang, Y.; Brown, J.; Godfrey, C.; Ahmad, M.; Vital, F.M.; Lambiase, P.; Banerjee, A.; Bakhai, A.; Chong, M. Non-Invasive Positive Pressure Ventilation (CPAP or Bilevel NPPV) for Cardiogenic Pulmonary Oedema. Cochrane Database Syst. Rev. 2019, 4, CD005351. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.-P.; Mebazaa, A.; Čelutkienė, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary Management of Acute Right Ventricular Failure: A Statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology: Contemporary Management of Acute RV Failure. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Combes, A.; van Diepen, S.; Hollinger, A.; Katz, J.N.; Landoni, G.; Hajjar, L.A.; Lassus, J.; Lebreton, G.; Montalescot, G.; et al. Management of Cardiogenic Shock Complicating Myocardial Infarction. Intensive Care Med. 2018, 44, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Ramani, G.V. Assessment and Management of Cardiogenic Shock in the Emergency Department. Cardiol. Clin. 2012, 30, 651–664. [Google Scholar] [CrossRef]
- Thiele, H.; Ohman, E.M.; Desch, S.; Eitel, I.; de Waha, S. Management of Cardiogenic Shock. Eur. Heart J. 2015, 36, 1223–1230. [Google Scholar] [CrossRef]
- Levy, B.; Buzon, J.; Kimmoun, A. Inotropes and Vasopressors Use in Cardiogenic Shock: When, Which and How Much? Curr. Opin. Crit. Care 2019, 25, 384–390. [Google Scholar] [CrossRef]
- Delmas, C.; Roubille, F.; Lamblin, N.; Bonello, L.; Leurent, G.; Levy, B.; Elbaz, M.; Danchin, N.; Champion, S.; Lim, P.; et al. Baseline Characteristics, Management, and Predictors of Early Mortality in Cardiogenic Shock: Insights from the FRENSHOCK Registry. ESC Heart Fail. 2022, 9, 408–419. [Google Scholar] [CrossRef]
- Polyzogopoulou, E.; Arfaras-Melainis, A.; Bistola, V.; Parissis, J. Inotropic Agents in Cardiogenic Shock. Curr. Opin. Crit. Care 2020, 26, 403–410. [Google Scholar] [CrossRef]
- Levy, B.; Klein, T.; Kimmoun, A. Vasopressor Use in Cardiogenic Shock. Curr. Opin. Crit. Care 2020, 26, 411–416. [Google Scholar] [CrossRef]
- Levy, B.; Clere-Jehl, R.; Legras, A.; Morichau-Beauchant, T.; Leone, M.; Frederique, G.; Quenot, J.-P.; Kimmoun, A.; Cariou, A.; Lassus, J.; et al. Epinephrine Versus Norepinephrine for Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 173–182. [Google Scholar] [CrossRef]
- Léopold, V.; Gayat, E.; Pirracchio, R.; Spinar, J.; Parenica, J.; Tarvasmäki, T.; Lassus, J.; Harjola, V.-P.; Champion, S.; Zannad, F.; et al. Epinephrine and Short-Term Survival in Cardiogenic Shock: An Individual Data Meta-Analysis of 2583 Patients. Intensive Care Med. 2018, 44, 847–856. [Google Scholar] [CrossRef] [PubMed]
- For the CardShock Study Investigators; Tarvasmäki, T.; Lassus, J.; Varpula, M.; Sionis, A.; Sund, R.; Køber, L.; Spinar, J.; Parissis, J.; Banaszewski, M.; et al. Current Real-Life Use of Vasopressors and Inotropes in Cardiogenic Shock—Adrenaline Use Is Associated with Excess Organ Injury and Mortality. Crit. Care 2016, 20, 208. [Google Scholar] [CrossRef]
- De Backer, D.; Biston, P.; Devriendt, J.; Madl, C.; Chochrad, D.; Aldecoa, C.; Brasseur, A.; Defrance, P.; Gottignies, P.; Vincent, J.-L. Comparison of Dopamine and Norepinephrine in the Treatment of Shock. N. Engl. J. Med. 2010, 362, 779–789. [Google Scholar] [CrossRef]
- Jolly, S.; Newton, G.; Horlick, E.; Seidelin, P.H.; Ross, H.J.; Husain, M.; Dzavik, V. Effect of Vasopressin on Hemodynamics in Patients with Refractory Cardiogenic Shock Complicating Acute Myocardial Infarction. Am. J. Cardiol. 2005, 96, 1617–1620. [Google Scholar] [CrossRef]
- Wallace, A.W.; Tunin, C.M.; Shoukas, A.A. Effects of Vasopressin on Pulmonary and Systemic Vascular Mechanics. Am. J. Physiol.-Heart Circ. Physiol. 1989, 257, H1228–H1234. [Google Scholar] [CrossRef]
- Uhlig, K.; Efremov, L.; Tongers, J.; Frantz, S.; Mikolajczyk, R.; Sedding, D.; Schumann, J. Inotropic Agents and Vasodilator Strategies for the Treatment of Cardiogenic Shock or Low Cardiac Output Syndrome. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar] [CrossRef]
- Mebazaa, A.; Nieminen, M.S.; Filippatos, G.S.; Cleland, J.G.; Salon, J.E.; Thakkar, R.; Padley, R.J.; Huang, B.; Cohen-Solal, A. Levosimendan vs. Dobutamine: Outcomes for Acute Heart Failure Patients on β-Blockers in SURVIVE†. Eur. J. Heart Fail. 2009, 11, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Burkhoff, D.; Sayer, G.; Doshi, D.; Uriel, N. Hemodynamics of Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2015, 66, 2663–2674. [Google Scholar] [CrossRef]
- Salter, B.S.; Gross, C.R.; Weiner, M.M.; Dukkipati, S.R.; Serrao, G.W.; Moss, N.; Anyanwu, A.C.; Burkhoff, D.; Lala, A. Temporary Mechanical Circulatory Support Devices: Practical Considerations for All Stakeholders. Nat. Rev. Cardiol. 2022, 20, 263–277. [Google Scholar] [CrossRef]
- Tongers, J.; Sieweke, J.-T.; Kühn, C.; Napp, L.C.; Flierl, U.; Röntgen, P.; Schmitto, J.D.; Sedding, D.G.; Haverich, A.; Bauersachs, J.; et al. Early Escalation of Mechanical Circulatory Support Stabilizes and Potentially Rescues Patients in Refractory Cardiogenic Shock. Circ. Heart Fail. 2020, 13, e005853. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, W.W.; Kleiman, N.S.; Moses, J.; Henriques, J.P.S.; Dixon, S.; Massaro, J.; Palacios, I.; Maini, B.; Mulukutla, S.; Džavík, V.; et al. A Prospective, Randomized Clinical Trial of Hemodynamic Support with Impella 2.5 Versus Intra-Aortic Balloon Pump in Patients Undergoing High-Risk Percutaneous Coronary Intervention: The PROTECT II Study. Circulation 2012, 126, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Ouweneel, D.M.; Eriksen, E.; Sjauw, K.D.; van Dongen, I.M.; Hirsch, A.; Packer, E.J.S.; Vis, M.M.; Wykrzykowska, J.J.; Koch, K.T.; Baan, J.; et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 69, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Basir, M.B.; Kapur, N.K.; Patel, K.; Salam, M.A.; Schreiber, T.; Kaki, A.; Hanson, I.; Almany, S.; Timmis, S.; Dixon, S.; et al. Improved Outcomes Associated with the Use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2019, 93, 1173–1183. [Google Scholar] [CrossRef]
- Burkhoff, D.; Cohen, H.; Brunckhorst, C.; O’Neill, W.W. A Randomized Multicenter Clinical Study to Evaluate the Safety and Efficacy of the TandemHeart Percutaneous Ventricular Assist Device versus Conventional Therapy with Intraaortic Balloon Pumping for Treatment of Cardiogenic Shock. Am. Heart J. 2006, 152, 469.e1–469.e8. [Google Scholar] [CrossRef]
- Tsangaris, A.; Alexy, T.; Kalra, R.; Kosmopoulos, M.; Elliott, A.; Bartos, J.A.; Yannopoulos, D. Overview of Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) Support for the Management of Cardiogenic Shock. Front. Cardiovasc. Med. 2021, 8, 686558. [Google Scholar] [CrossRef]
- Schrage, B.; Becher, P.M.; Bernhardt, A.; Bezerra, H.; Blankenberg, S.; Brunner, S.; Colson, P.; Cudemus Deseda, G.; Dabboura, S.; Eckner, D.; et al. Left Ventricular Unloading Is Associated with Lower Mortality in Patients with Cardiogenic Shock Treated with Venoarterial Extracorporeal Membrane Oxygenation: Results from an International, Multicenter Cohort Study. Circulation 2020, 142, 2095–2106. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.S.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs. Intra-Aortic Balloon Pump with In-Hospital Mortality and Major Bleeding among Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2020, 323, 734. [Google Scholar] [CrossRef]
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.-M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock: Matched-Pair IABP-SHOCK II Trial 30-Day Mortality Analysis. Circulation 2019, 139, 1249–1258. [Google Scholar] [CrossRef]
- Tehrani, B.N.; Truesdell, A.G.; Psotka, M.A.; Rosner, C.; Singh, R.; Sinha, S.S.; Damluji, A.A.; Batchelor, W.B. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail. 2020, 8, 879–891. [Google Scholar] [CrossRef]
- Basir, M.B.; Schreiber, T.L.; Grines, C.L.; Dixon, S.R.; Moses, J.W.; Maini, B.S.; Khandelwal, A.K.; Ohman, E.M.; O’Neill, W.W. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am. J. Cardiol. 2017, 119, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.L.; Kapur, N.K. Acute Mechanical Circulatory Support for Cardiogenic Shock: The “Door to Support” Time. F1000Research 2017, 6, 737. [Google Scholar] [CrossRef] [PubMed]
- McBride, L.R.; Lowdermilk, G.A.; Fiore, A.C.; Moroney, D.A.; Brannan, J.A.; Swartz, M.T. Transfer of Patients Receiving Advanced Mechanical Circulatory Support. J. Thorac. Cardiovasc. Surg. 2000, 119, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Jaroszewski, D.E.; Kleisli, T.; Staley, L.; Pierce, C.; Scott, R.; Steidley, D.E.; DeValeria, P.; Arabia, F.A. A Traveling Team Concept to Expedite the Transfer and Management of Unstable Patients in Cardiopulmonary Shock. J. Heart Lung Transplant. 2011, 30, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Aponte, M.M.P.; Manrique, C.; Kar, B. Systems of Care in Cardiogenic Shock. Methodist DeBakey Cardiovasc. J. 2020, 16, 50. [Google Scholar] [CrossRef] [PubMed]
- Rab, T.; Ratanapo, S.; Kern, K.B.; Basir, M.B.; McDaniel, M.; Meraj, P.; King, S.B.; O’Neill, W. Cardiac Shock Care Centers. J. Am. Coll. Cardiol. 2018, 72, 1972–1980. [Google Scholar] [CrossRef]
- Rab, T. “Shock Teams” and “Shock Docs”. J. Am. Coll. Cardiol. 2019, 73, 1670–1672. [Google Scholar] [CrossRef]
- Lee, F.; Hutson, J.H.; Boodhwani, M.; McDonald, B.; So, D.; De Roock, S.; Rubens, F.; Stadnick, E.; Ruel, M.; Le May, M.; et al. Multidisciplinary Code Shock Team in Cardiogenic Shock: A Canadian Centre Experience. CJC Open 2020, 2, 249–257. [Google Scholar] [CrossRef]
- Taleb, I.; Koliopoulou, A.G.; Tandar, A.; McKellar, S.H.; Tonna, J.E.; Nativi-Nicolau, J.; Alvarez Villela, M.; Welt, F.; Stehlik, J.; Gilbert, E.M.; et al. Shock Team Approach in Refractory Cardiogenic Shock Requiring Short-Term Mechanical Circulatory Support: A Proof of Concept. Circulation 2019, 140, 98–100. [Google Scholar] [CrossRef]
- Papolos, A.I.; Kenigsberg, B.B.; Berg, D.D.; Alviar, C.L.; Bohula, E.; Burke, J.A.; Carnicelli, A.P.; Chaudhry, S.-P.; Drakos, S.; Gerber, D.A.; et al. Management and Outcomes of Cardiogenic Shock in Cardiac ICUs with versus without Shock Teams. J. Am. Coll. Cardiol. 2021, 78, 1309–1317. [Google Scholar] [CrossRef]
- de la Paz, A.; Orgel, R.; Hartsell, S.E.; Pauley, E.; Katz, J.N. Getting Cardiogenic Shock Patients to the Right Place—How Initial Intensive Care Unit Triage Decisions Impact Processes of Care and Outcomes. Am. Heart J. 2020, 230, 66–70. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).