Radiogenomics Reveals Correlation between Quantitative Texture Radiomic Features of Biparametric MRI and Hypoxia-Related Gene Expression in Men with Localised Prostate Cancer
Abstract
1. Introduction
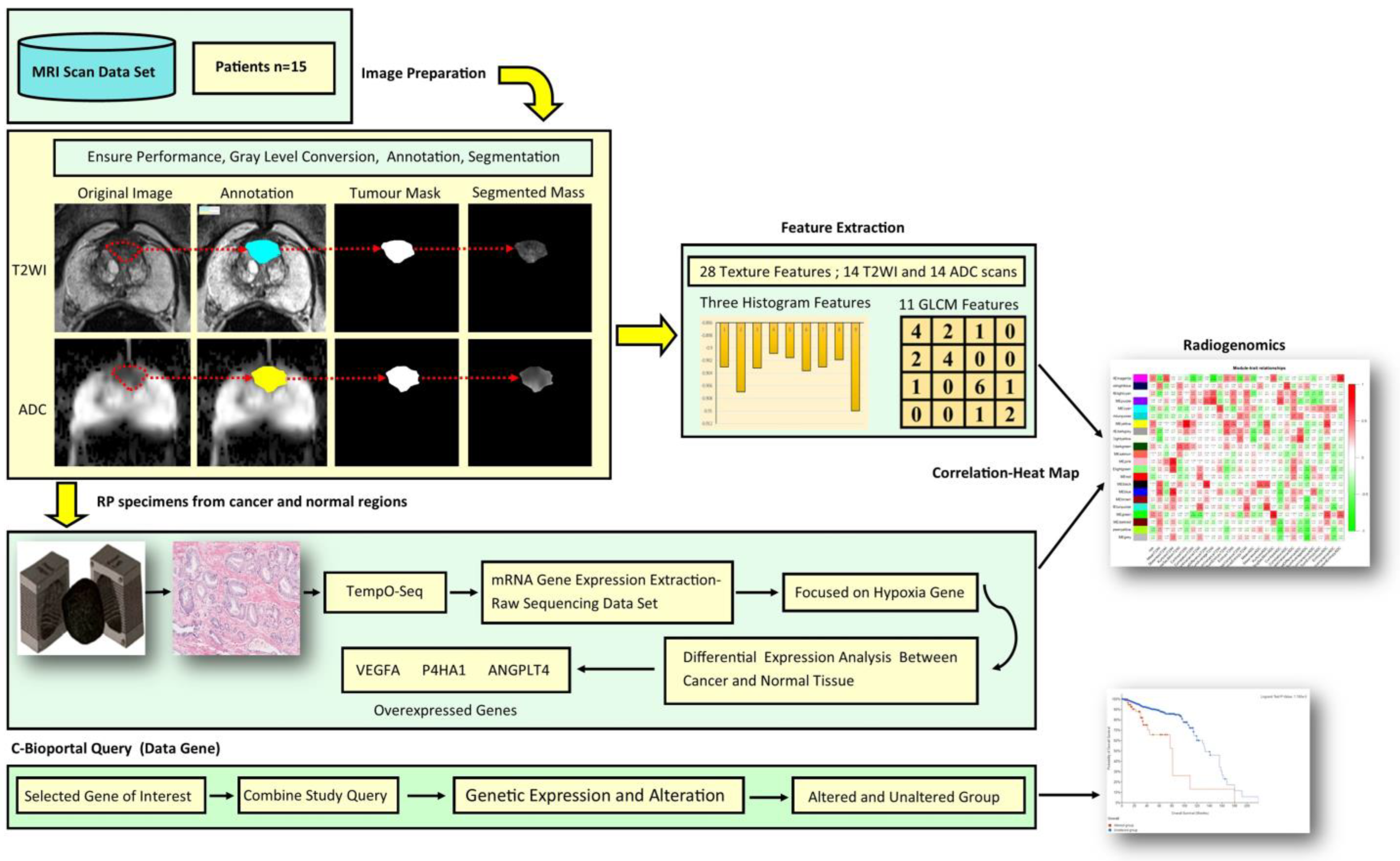
2. Materials and Methods
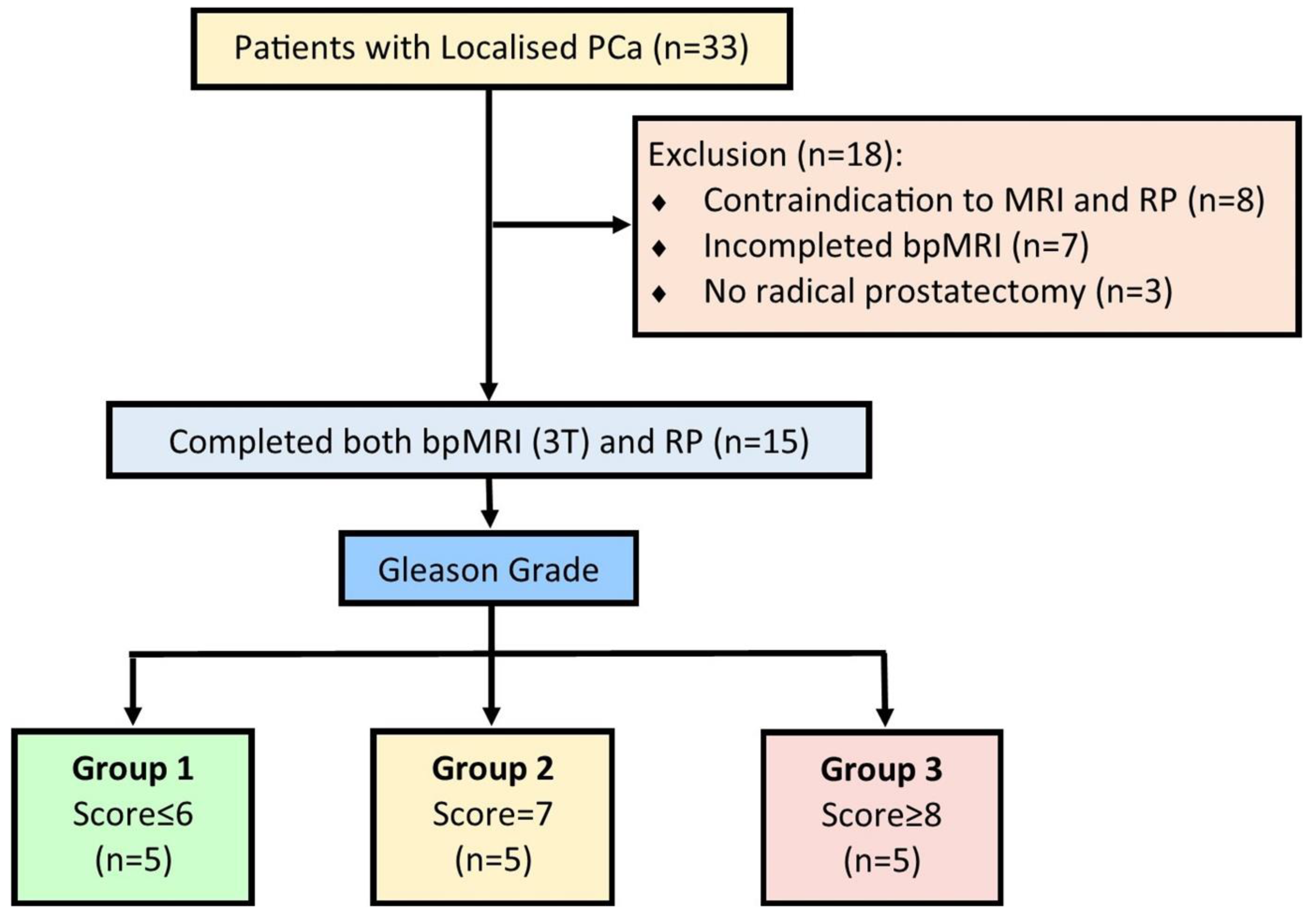
2.1. Proof-of-Concept Cohort
2.2. Biparametric MRI (BpMRI) Image Acquisition
2.3. Image Processing and Analysis of Textural Feature Extraction
2.4. Histopathology Protocol
2.5. Profiling of Gene Expression
2.6. TempO-Seq Assay Protocol Summary
2.7. Statistical Analysis
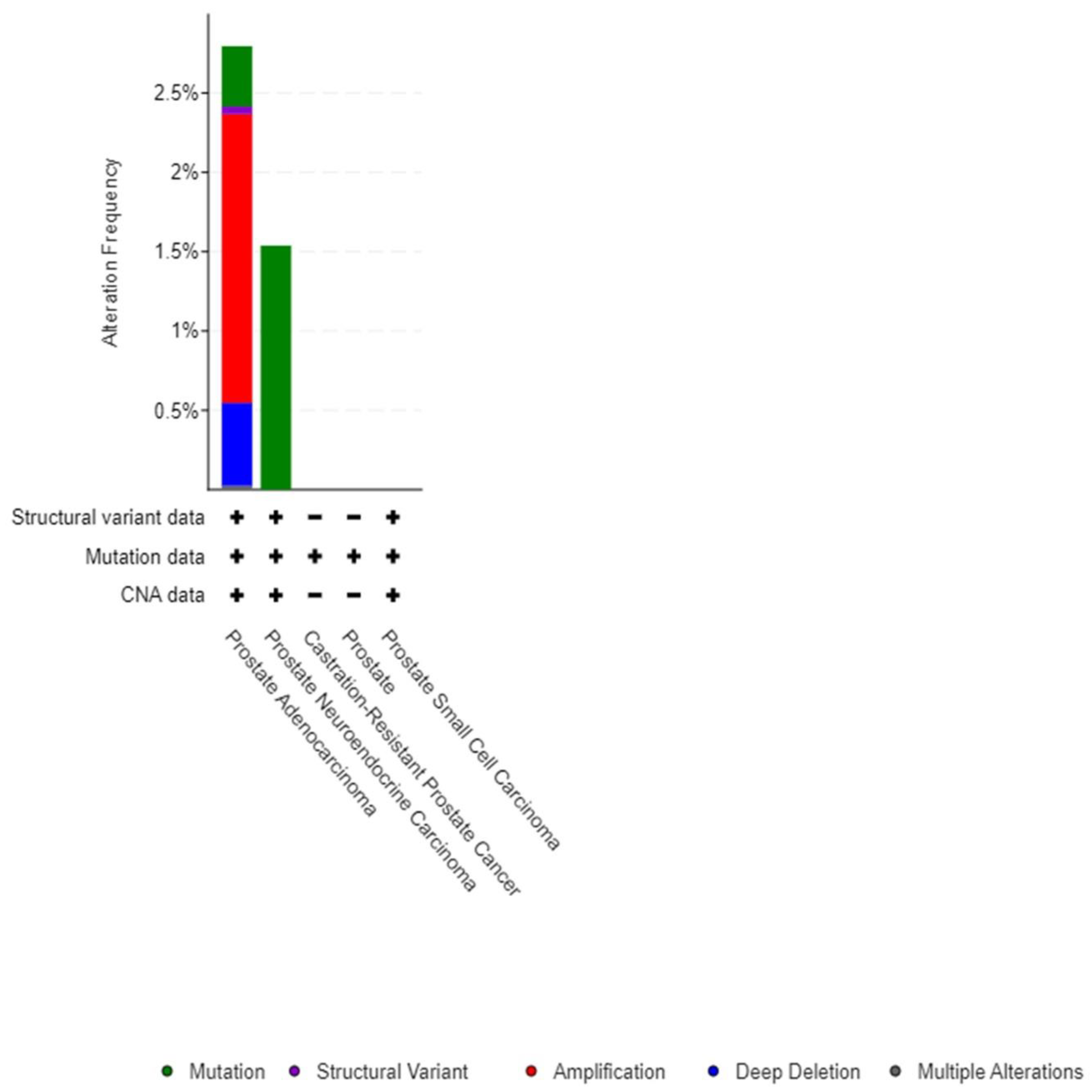
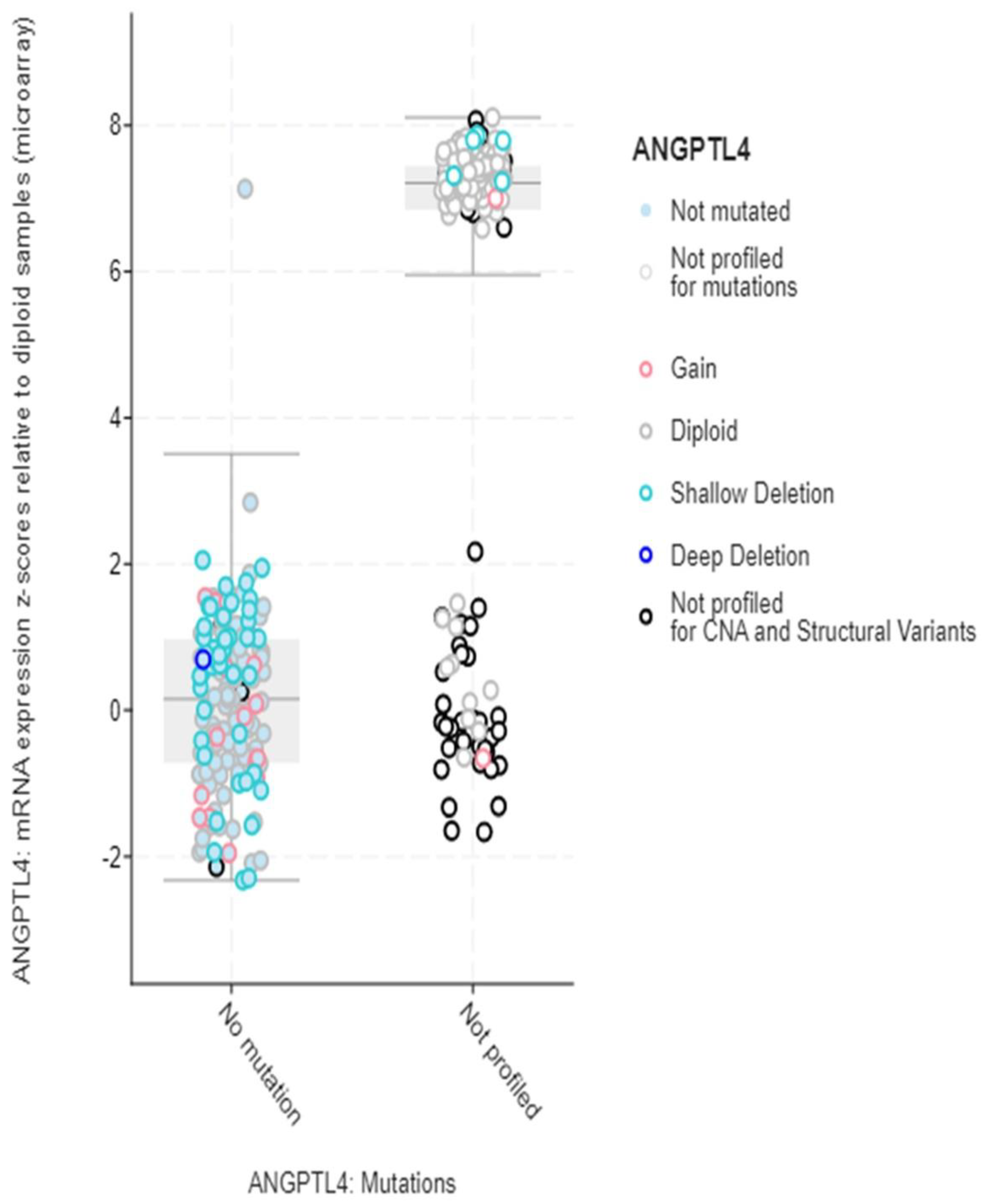
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Lucero García Rojas, E.Y.; Villanueva, C.; Bond, R.A. Hypoxia Inducible Factors as Central Players in the Pathogenesis and Pathophysiology of Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 853, 709509. [Google Scholar] [CrossRef] [PubMed]
- Nojima, Y.; Takeda, Y.; Maeda, Y.; Bamba, T.; Fukusaki, E.; Itoh, M.N.; Mizuguchi, K.; Kumanogoh, A. Metabolomic analysis of fibrotic mice combined with public RNA-Seq human lung data reveal potential diagnostic biomarker candidates for lung fibrosis. FEBS Open Bio 2020, 10, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Agahozo, M.C.; van Bockstal, M.; Westenend, P.J.; Galant, C.; Lambein, K.; Reisenbichler, E.; Sinke, R.; Wong, S.; van Deurzen, C.H.M. Stromal Changes are Associated with High P4HA2 Expression in Ductal Carcinoma in Situ of the Breast. J. Mammary Gland. Biol. Neoplasia 2022, 26, 367–375. [Google Scholar] [CrossRef]
- Ahmadi, M.; Eftekhari Kenzerki, M.; Akrami, S.M.; Pashangzadeh, S.; Hajiesmaeili, F.; Rahnavard, S.; Habibipour, L.; Saffarzadeh, N.; Mousavi, P. Overexpression of HPRT1 is associated with poor prognosis in head and neck squamous cell carcinoma. FEBS Open Bio 2021, 11, 2525–2540. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Higgins, L.; Withers, H.; Garbens, A.; Love, H.; Magnoni, L.; Hayward, S.; Moyes, C. Hypoxia and the metabolic phenotype of prostate cancer cells. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2009, 1787, 1433–1443. [Google Scholar] [CrossRef]
- Natua, S.; Dhamdhere, S.G.; Mutnuru, S.A.; Shukla, S. Interplay within tumor microenvironment orchestrates neoplastic RNA metabolism and transcriptome diversity. Wiley Interdiscip. Rev. RNA 2022, 13, e1676. [Google Scholar] [CrossRef]
- Raghunand, N.; Gatenby, R.A.; Gillies, R.J. Microenvironmental and cellular consequences of altered blood flow in tumours. Br. J. Radiol. 2003, 76 (Suppl. S1), S11–S22. [Google Scholar] [CrossRef]
- Dai, Y.; Bae, K.; Siemann, D.W. Impact of hypoxia on the metastatic potential of human prostate cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 521–528. [Google Scholar] [CrossRef]
- Ghafar, M.A.; Anastasiadis, A.G.; Chen, M.; Burchardt, M.; Olsson, L.E.; Xie, H.; Benson, M.C.; Buttyan, R. Acute hypoxia increases the aggressive characteristics and survival properties of prostate cancer cells. Prostate 2003, 54, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.D.; Ross, J.A.; McLaren, D.B.; Parker, C.C.; Habib, F.K.; Riddick, A.C.P. The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int. 2010, 105, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Fraga, A.; Ribeiro, R.; Príncipe, P.; Lopes, C.; Medeiros, R. Hypoxia and prostate cancer aggressiveness: A tale with many endings. Clin. Genitourin Cancer 2015, 13, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; McMorran, R.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. The comparative toxicogenomics database: Update 2019. Nucleic. Acids Res. 2019, 47, D948–D954. [Google Scholar] [CrossRef]
- Chitalia, R.D.; Kontos, D. Role of texture analysis in breast MRI as a cancer biomarker: A review. J. Magn. Reson. Imaging 2019, 49, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Löfstedt, T.; Brynolfsson, P.; Asklund, T.; Nyholm, T.; Garpebring, A. Gray-level invariant Haralick texture features. PLoS ONE 2019, 14, e0212110. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; de Cobelli, O.; Musi, G.; del Giudice, F.; Carrieri, G.; Busetto, G.M.; Falagario, U.G.; Sciarra, A.; Maggi, M.; Crocetto, F.; et al. Radiomics in prostate cancer: An up-to-date review. Ther. Adv. Urol. 2022, 14, 17562872221109020. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, D. Image Quality Analysis Using GLCM. 2004. Available online: http://purl.fcla.edu/fcla/etd/CFE0000273 (accessed on 27 March 2023).
- Kim, H.S.; Kim, Y.J.; Kim, K.G.; Park, J.S. Preoperative CT texture features predict prognosis after curative resection in pancreatic cancer. Sci. Rep. 2019, 9, 17389. [Google Scholar] [CrossRef]
- Thomas, R.; Qin, L.; Alessandrino, F.; Sahu, S.P.; Guerra, P.J.; Krajewski, K.M.; Shinagare, A. A review of the principles of texture analysis and its role in imaging of genitourinary neoplasms. Abdom. Radiol. 2019, 44, 2501–2510. [Google Scholar] [CrossRef]
- El Adoui, M.; Drisis, S.; Larhmam, M.A.; Lemort, M.; Benjelloun, M. Breast cancer heterogeneity analysis as index of response to treatment using MRI images: A review. Imaging Med. 2017, 9, 109–119. [Google Scholar]
- Molina, D.; Pérez-Beteta, J.; Luque, B.; Arregui, E.; Calvo, M.; Borrás, J.M.; López, C.; Martino, J.; Velasquez, C.; Asenjo, B.; et al. Tumour heterogeneity in glioblastoma assessed by MRI texture analysis: A potential marker of survival. Br. J. Radiol. 2016, 89, 20160242. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.; Engelsen, A.S.T.; Buart, S.; Elsayed, W.S.; Venkatesh, G.H.; Chouaib, S. Hypoxia-driven intratumor heterogeneity and immune evasion. Cancer Lett. 2020, 492, 1–10. [Google Scholar] [CrossRef]
- Qian, J.; Rankin, E.B. Hypoxia-induced phenotypes that mediate tumor heterogeneity. In Hypoxia Cancer Metastasis; Springer: Berlin/Heidelberg, Germany, 2019; pp. 43–55. [Google Scholar]
- Massanova, M.; Robertson, S.; Barone, B.; Dutto, L.; Caputo, V.F.; Bhatt, J.R.; Ahmad, I.; Bada, M.; Obeidallah, A.; Crocetto, F. The comparison of imaging and clinical methods to estimate prostate volume: A single-centre retrospective study. Urol. Int. 2021, 105, 804–810. [Google Scholar] [CrossRef] [PubMed]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Imaging 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Mehralivand, S.; Shih, J.H.; Rais-Bahrami, S.; Oto, A.; Bednarova, S.; Nix, J.W.; Thomas, J.V.; Gordetsky, J.B.; Gaur, S.; Harmon, S.A.; et al. A magnetic resonance imaging–based prediction model for prostate biopsy risk stratification. JAMA Oncol. 2018, 4, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, S.; Bussetti, M.; Bassani, N.; Rossi, R.S.; Incarbone, G.P.; Bianchi, F.; Maggioni, M.; Runza, L.; Ceriotti, F.; Panteghini, M. Definition of outcome-based prostate-specific antigen (PSA) thresholds for advanced prostate cancer risk prediction. Cancers 2021, 13, 3381. [Google Scholar] [CrossRef]
- Callender, T.; Emberton, M.; Morris, S.; Pharoah, P.D.P.; Pashayan, N. Benefit, harm, and cost-effectiveness associated with magnetic resonance imaging before biopsy in age-based and risk-stratified screening for prostate cancer. JAMA Netw Open 2021, 4, e2037657. [Google Scholar] [CrossRef]
- Yang, L.; Roberts, D.; Takhar, M.; Erho, N.; Bibby, B.A.S.; Thiruthaneeswaran, N.; Bhandari, V.; Cheng, W.-C.; Haider, S.; McCorry, A.M.; et al. Development and validation of a 28-gene hypoxia-related prognostic signature for localized prostate cancer. EBioMedicine 2018, 31, 182–189. [Google Scholar] [CrossRef]
- Sun, Y.; Williams, S.; Byrne, D.; Keam, S.; Reynolds, H.M.; Mitchell, C.; Wraith, D.; Murphy, D.; Haworth, A. Association analysis between quantitative MRI features and hypoxia-related genetic profiles in prostate cancer: A pilot study. Br. J. Radiol. 2019, 92, 20190373. [Google Scholar] [CrossRef]
- Sørensen, B.S.; Knudsen, A.; Wittrup, C.F.; Nielsen, S.; Aggerholm-Pedersen, N.; Busk, M.; Horsman, M.; Høyer, M.; Bouchelouche, P.N.; Overgaard, J.; et al. The usability of a 15-gene hypoxia classifier as a universal hypoxia profile in various cancer cell types. Radiother. Oncol. 2015, 116, 346–351. [Google Scholar] [CrossRef]
- Chi, J.T.; Wang, Z.; Nuyten, D.S.A.; Rodriguez, E.H.; Schaner, M.E.; Salim, A.; Wang, Y.; Kristensen, G.B.; Helland, Å.; Børresen-Dale, A.-L.; et al. Gene expression programs in response to hypoxia: Cell type specificity and prognostic significance in human cancers. PLoS Med. 2006, 3, e47. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, D. CRIA: An Interactive Gene Selection Algorithm for Cancers Prediction Based on Copy Number Variations. Front. Plant Sci. 2022, 13, 839044. [Google Scholar] [CrossRef]
- Wu, P.; Heins, Z.J.; Muller, J.T.; Katsnelson, L.; de Bruijn, I.; Abeshouse, A.A.; Schultz, N.; Fenyö, D.; Gao, J. Integration and Analysis of CPTAC Proteomics Data in the Context of Cancer Genomics in the cBioPortal*[S]. Mol. Cell. Proteom. 2019, 18, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, N.; Wei, C.; Szewczyk-Bieda, M.; Campbell, A.; Memon, S.; Lang, S.; Nabi, G. Combined T2 and diffusion-weighted MR imaging with template prostate biopsies in men suspected with prostate cancer but negative transrectal ultrasound-guided biopsies. World J. Urol. 2017, 35, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Brynolfsson, P.; Nilsson, D.; Torheim, T.; Asklund, T.; Karlsson, C.T.; Trygg, J.; Nyholm, T.; Garpebring, A. Haralick texture features from apparent diffusion coefficient (ADC) MRI images depend on imaging and pre-processing parameters. Sci. Rep. 2017, 7, 4041. [Google Scholar] [CrossRef]
- Gnep, K.; Fargeas, A.; Gutiérrez-Carvajal, R.E.; Commandeur, F.; Mathieu, R.; Ospina, J.D.; Rolland, Y.; Rohou, T.; Vincendeau, S.; Hatt, M.; et al. Haralick textural features on T2-weighted MRI are associated with biochemical recurrence following radiotherapy for peripheral zone prostate cancer. J. Magn. Reson. Imaging 2017, 45, 103–117. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Haralick, R.M.; Sternberg, S.R.; Zhuang, X. Image analysis using mathematical morphology. IEEE Trans. Pattern Anal. Mach Intell. 1987, 4, 532–550. [Google Scholar] [CrossRef]
- Guha, A.; Anjari, M.; Cook, G.; Goh, V.; Connor, S. Radiomic analysis of tumour heterogeneity using MRI in head and neck cancer following chemoradiotherapy: A feasibility study. Front Oncol. 2022, 12, 200. [Google Scholar] [CrossRef]
- Epstein, J.I.; Allsbrook, W.C., Jr.; Amin, M.B.; Egevad, L.L.; Committee, I.G. The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am. J. Surg. Pathol. 2005, 29, 1228–1242. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Egevad, L.; Delahunt, B.; Srigley, J.R.; Samaratunga, H. International Society of Urological Pathology (ISUP) Grading of Prostate Cancer–An ISUP Consensus on Contemporary Grading; Apmis. Wiley Online Library: Pittsburgh, PA, USA, 2016; Volume 124, pp. 433–435. [Google Scholar]
- Yeakley, J.M.; Shepard, P.J.; Goyena, D.E.; VanSteenhouse, H.C.; McComb, J.D.; Seligmann, B.E. A trichostatin A expression signature identified by TempO-Seq targeted whole transcriptome profiling. PLoS ONE 2017, 12, e0178302. [Google Scholar] [CrossRef] [PubMed]
- Trejo, C.L.; Babić, M.; Imler, E.; Gonzalez, M.; Bibikov, S.I.; Shepard, P.J.; VanSteenhouse, H.C.; Yeakley, J.M.; Seligmann, B.E. Extraction-free whole transcriptome gene expression analysis of FFPE sections and histology-directed subareas of tissue. PLoS ONE 2019, 14, e0212031. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Bushel, P.R.; Ferguson, S.S.; Ramaiahgari, S.C.; Paules, R.S.; Auerbach, S.S. Comparison of normalization methods for analysis of TempO-Seq Targeted RNA sequencing data. Front. Genet. 2020, 11, 594. [Google Scholar] [CrossRef]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.Y.; Dillies, M.A. SARTools: A DESeq2-and EdgeR-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, X.; Song, X.; Xie, L. Identification of Diagnostic Exosomal LncRNA-miRNA-mRNA Biomarkers in Colorectal Cancer Based on the ceRNA Network. Pathol. Oncol. Res. 2022, 116, 1610493. [Google Scholar] [CrossRef]
- Salciccia, S.; Capriotti, A.L.; Laganà, A.; Fais, S.; Logozzi, M.; de Berardinis, E.; Busetto, G.; Di Pierro, G.; Ricciuti, G.; Del Giudice, F.; et al. Biomarkers in prostate cancer diagnosis: From current knowledge to the role of metabolomics and exosomes. Int. J. Mol. Sci. 2021, 22, 4367. [Google Scholar] [CrossRef]
- Krishnan, S.; Kanthaje, S.; Punchappady, D.R.; Mujeeburahiman, M.; Ratnacaram, C.K. Circulating metabolite biomarkers: A game changer in the human prostate cancer diagnosis. J. Cancer Res. Clin. Oncol. 2022, 149, 951–967. [Google Scholar] [CrossRef]
- Lima, A.R.; Pinto, J.; Carvalho-Maia, C.; Jerónimo, C.; Henrique, R.; de Bastos, M.L.; Carvalho, M.; Guedes de Pinho, P. A panel of urinary volatile biomarkers for differential diagnosis of prostate cancer from other urological cancers. Cancers 2020, 12, 2017. [Google Scholar] [CrossRef]
- Spohn, S.K.B.; Bettermann, A.S.; Bamberg, F.; Benndorf, M.; Mix, M.; Nicolay, N.H.; Fechter, T.; Hölscher, T.; Grosu, R.; Chiti, A.; et al. Radiomics in prostate cancer imaging for a personalized treatment approach-current aspects of methodology and a systematic review on validated studies. Theranostics 2021, 11, 8027. [Google Scholar] [CrossRef] [PubMed]
- Dalman, M.R.; Deeter, A.; Nimishakavi, G.; Duan, Z.H. Fold change and p-value cutoffs significantly alter microarray interpretations. In BMC Bioinformatics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–4. [Google Scholar]
- Her, E.J.; Haworth, A.; Sun, Y.; Williams, S.; Reynolds, H.M.; Kennedy, A.; Ebert, M.A. Biologically Targeted Radiation Therapy: Incorporating Patient-Specific Hypoxia Data Derived from Quantitative Magnetic Resonance Imaging. Cancers 2021, 13, 4897. [Google Scholar] [CrossRef] [PubMed]
- Dercle, L.; Ammari, S.; Bateson, M.; Durand, P.B.; Haspinger, E.; Massard, C.; Jaudet, C.; Varga, A.; Deutsch, E.; Soria, J.-C.; et al. Limits of radiomic-based entropy as a surrogate of tumor heterogeneity: ROI-area, acquisition protocol and tissue site exert substantial influence. Sci. Rep. 2017, 7, 7952. [Google Scholar] [CrossRef] [PubMed]
- Chaddad, A.; Kucharczyk, M.J.; Desrosiers, C.; Okuwobi, I.P.; Katib, Y.; Zhang, M.; Rathore, S.; Sargos, P.; Niazi, T. Deep radiomic analysis to predict gleason score in prostate cancer. IEEE Access 2020, 8, 167767–167778. [Google Scholar] [CrossRef]
- Nketiah, G.; Elschot, M.; Kim, E.; Bathen, T.F.; Selnæs, K.M. Evaluation of T2W MRI-Derived Textural Entropy for Assessment of Prostate Cancer Aggressiveness; ISMRM: Concord, CA, USA, 2016. [Google Scholar]
- Fiz, F.; Viganò, L.; Gennaro, N.; Costa, G.; La Bella, L.; Boichuk, A.; Cavinato, L.; Sollini, M.; Politi, L.S.; Chiti, A.; et al. Radiomics of liver metastases: A systematic review. Cancers 2020, 12, 2881. [Google Scholar] [CrossRef]
- Coppola, F.; Mottola, M.; Monaco, S.L.; Cattabriga, A.; Cocozza, M.; Yuan, J.; De Benedittis, C.; Cuicchi, D.; Guido, A.; Llimpe, F.R.; et al. The Heterogeneity of Skewness in T2W-Based Radiomics Predicts the Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Diagnostics 2021, 11, 795. [Google Scholar] [CrossRef]
- Just, N. Improving tumour heterogeneity MRI assessment with histograms. Br. J. Cancer 2014, 111, 2205–2213. [Google Scholar] [CrossRef]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Rui, W.; Wu, Y.; Ma, Z.; Wang, Y.; Wang, Y.; Xu, X.; Zhang, J.; Yao, Z. MR textural analysis on contrast enhanced 3D-SPACE images in assessment of consistency of pituitary macroadenoma. Eur. J. Radiol. 2019, 110, 219–224. [Google Scholar] [CrossRef]
- Litjens, G.J.S.; Hambrock, T.; Hulsbergen–van de Kaa, C.; Barentsz, J.O.; Huisman, H.J. Interpatient variation in normal peripheral zone apparent diffusion coefficient: Effect on the prediction of prostate cancer aggressiveness. Radiology 2012, 265, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Kobus, T.; Vos, P.C.; Hambrock, T.; De Rooij, M.; Hulsbergen—Van de Kaa, C.A.; Barentsz, J.O.; Heerschap, A.; Scheenen, T.W.J. Prostate cancer aggressiveness: In vivo assessment of MR spectroscopy and diffusion-weighted imaging at 3 T. Radiology 2012, 265, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Vos, E.K.; Litjens, G.J.S.; Kobus, T.; Hambrock, T.; Hulsbergen-van de Kaa, C.A.; Barentsz, J.O.; Huisman, H.; Scheenen, T.W. Assessment of prostate cancer aggressiveness using dynamic contrast-enhanced magnetic resonance imaging at 3 T. Eur. Urol. 2013, 64, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Kim, S.; Lim, R.P.; Hindman, N.; Deng, F.M.; Babb, J.S.; Taneja, S.S. Prostate cancer localization using multiparametric MR imaging: Comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales. Radiology 2013, 269, 482–492. [Google Scholar] [CrossRef]
- Sun, X.; Yu, Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol. Sin. 2015, 36, 1219–1227. [Google Scholar] [CrossRef]
- Marusyk, A.; Almendro, V.; Polyak, K. Intra-tumour heterogeneity: A looking glass for cancer? Nat. Rev. Cancer 2012, 12, 323–334. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Fei, S.; Wang, R.; Wang, H.; Zhu, W.; Zhao, Y. Small Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Enhanced Proangiogenic Potential of Cardiac Fibroblasts via Angiopoietin-Like 4. Stem. Cells Int. 2022, 2022, 3229289. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, H.; Sun, J.; Mao, W.; Chen, S.; Chen, M. Multi-Omics analysis identifies a lncRNA-related prognostic signature to predict bladder cancer recurrence. Bioengineered 2021, 12, 11108–11125. [Google Scholar] [CrossRef]



| Characteristics Median | PCa (n) | (Median-IQR) |
|---|---|---|
| Age (years) | 15 | 66–13 |
| Size (mL) | 15 | 51.5–22 |
| Prostate location | ||
| Transitional zone | 4 | |
| Peripheral zone | 11 | |
| PSA (ng/mL) | ||
| PSA 4–20 | 15 | 10.8–4.2 |
| Genes | Gleason Risk Category | BaseMean | log2Fold-Change | p-Value |
|---|---|---|---|---|
| P4HA1 | low | 84.1 | 2.16 | 0.047 |
| VEGFA | Intermediate | 902.9 | −2.17 | 0.006 |
| ANGPLT4 | high | 6.07 | 6.07 | 0.050 |
| Radiomic Features | ANGPLT4 | P4HA1 | VEGFA | |||
|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | |
| Sum Variance T2WI | 0.600 | 0.020 | 0.045 | 0.048 | −0.151 | 0.042 |
| Skewness T2WI | −0.312 | 0.034 | 0.524 | 0.024 | −0.061 | 0.047 |
| Entropy T2WI | 0.600 | 0.020 | 0.043 | 0.048 | −0.079 | 0.046 |
| Skewness ADC | 0.247 | 0.038 | 0.493 | 0.025 | 0.060 | 0.047 |
| Panel A | Panel B | Neither | A Not B | B Not A | Both | Log2 Odds Ratio | p-Value | q-Value | Tendency |
|---|---|---|---|---|---|---|---|---|---|
| ANGPLT4 | VEGFA | 4373 | 15 | 55 | 8 | >3 | <0.001 | <0.001 | Co-occurrence |
| P4HA1 | VEGFA | 4246 | 142 | 55 | 8 | 2.121 | 0.001 | 0.001 | Co-occurrence |
| ANGPLT4 | P4HA1 | 4282 | 19 | 146 | 4 | 2.826 | 0.007 | 0.001 | Co-occurrence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogbonnaya, C.N.; Alsaedi, B.S.O.; Alhussaini, A.J.; Hislop, R.; Pratt, N.; Nabi, G. Radiogenomics Reveals Correlation between Quantitative Texture Radiomic Features of Biparametric MRI and Hypoxia-Related Gene Expression in Men with Localised Prostate Cancer. J. Clin. Med. 2023, 12, 2605. https://doi.org/10.3390/jcm12072605
Ogbonnaya CN, Alsaedi BSO, Alhussaini AJ, Hislop R, Pratt N, Nabi G. Radiogenomics Reveals Correlation between Quantitative Texture Radiomic Features of Biparametric MRI and Hypoxia-Related Gene Expression in Men with Localised Prostate Cancer. Journal of Clinical Medicine. 2023; 12(7):2605. https://doi.org/10.3390/jcm12072605
Chicago/Turabian StyleOgbonnaya, Chidozie N., Basim S. O. Alsaedi, Abeer J. Alhussaini, Robert Hislop, Norman Pratt, and Ghulam Nabi. 2023. "Radiogenomics Reveals Correlation between Quantitative Texture Radiomic Features of Biparametric MRI and Hypoxia-Related Gene Expression in Men with Localised Prostate Cancer" Journal of Clinical Medicine 12, no. 7: 2605. https://doi.org/10.3390/jcm12072605
APA StyleOgbonnaya, C. N., Alsaedi, B. S. O., Alhussaini, A. J., Hislop, R., Pratt, N., & Nabi, G. (2023). Radiogenomics Reveals Correlation between Quantitative Texture Radiomic Features of Biparametric MRI and Hypoxia-Related Gene Expression in Men with Localised Prostate Cancer. Journal of Clinical Medicine, 12(7), 2605. https://doi.org/10.3390/jcm12072605







