Microwave Ablation of Recurrent Hepatocellular Carcinoma after Curative Surgical Resection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Committee Approval
2.2. Patients
2.3. Protocol of MWA
2.4. Follow-Up
2.5. Parameters’ Analyzed and Definitions
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dimitroulis, D.; Damaskos, C.; Valsami, S.; Davakis, S.; Garmpis, N.; Spartalis, E.; Athanasiou, A.; Moris, D.; Sakellariou, S.; Kykalos, S.; et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J. Gastroenterol. 2017, 23, 5282–5294. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Lim, K.-C.; Chow, P.K.-H.; Allen, J.C.; Siddiqui, F.J.; Chan, E.S.-Y.; Tan, S.-B. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br. J. Surg. 2012, 99, 1622–1629. [Google Scholar] [CrossRef]
- Belghiti, J.; Kianmanesh, R. Surgical treatment of hepatocellular carcinoma. HPB 2005, 7, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Lo, C. Downstaging of hepatocellular carcinoma before transplantation: An advance in therapy or just another selection criterion. Am. J. Transplant. 2008, 8, 2485–2486. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.M.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Deng, K.; Zhang, C.; Li, H.; Luo, Y.; Yang, Y.; Li, C.; Li, X.; Geng, Z.; Xie, C. Nomograms for Predicting Hepatocellular Carcinoma Recurrence and Overall Postoperative Patient Survival. Front. Oncol. 2022, 12, 843589. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-F.; Xing, H.; Han, J.; Li, Z.-L.; Lau, W.-Y.; Zhou, Y.-H.; Gu, W.-M.; Wang, H.; Chen, T.-H.; Zeng, Y.-Y.; et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma. JAMA Surg. 2019, 154, 209–217. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, P.; Qu, S.; Zhou, J.; Yang, J.; Yang, X.; Xia, Y.; Li, J.; Wang, K.; Yan, Z.; et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB 2015, 17, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; MDT of West China Hospital; Jin, C.; Facciorusso, A.; Donadon, M.; Han, H.-S.; Mao, Y.; Dai, C.; Cheng, S.; Zhang, B.; et al. Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: An international expert consensus. HepatoBiliary Surg. Nutr. 2018, 7, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Goh, B.K.P. Management of recurrent hepatocellular carcinoma after resection. HepatoBiliary Surg. Nutr. 2020, 9, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system: The 2022 update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Wang, Y. Microwave Ablation of Hepatocellular Carcinoma. Oncology 2007, 72, 124–131. [Google Scholar] [CrossRef]
- Vogl, T.J.; Nour-Eldin, N.A.; Hammerstingl, R.M.; Panahi, B.; Naguib, N.N.N. Microwave Ablation (MWA): Basics, Technique and Results in Primary and Metastatic Liver Neoplasms—Review Article. Mikrowellenablation (MWA): Grundlagen, Technik und Ergebnisse in primären und sekundären Lebertumoren—Übersichtsarbeit. RoFo 2017, 189, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, K.; Luo, H.; Zhang, W.; Zhang, L.; Gao, M. Long-term outcomes of percutaneous microwave ablation versus repeat hepatectomy for treatment of late recurrent small hepatocellular carcinoma: A retrospective study. Zhonghua Yi Xue Za Zhi 2014, 94, 2570–2572. [Google Scholar]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc. Interv. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.-H.; Choi, B.I.; de Baère, T.; Dodd, G.D.; et al. Image-Guided Tumor Ablation: Standardization of Terminology and Reporting Criteria—A 10-Year Update. J. Vasc. Interv. Radiol. 2014, 25, 1691–1705.e4. [Google Scholar] [CrossRef]
- Kow, A.W.C.; Elkhouly, A.G.; Cristino, B.; Alhasan, A.; Dauri, M.; Pompeo, E. Transplantation versus liver resection in patients with hepatocellular carcinoma. Transl. Gastroenterol. Hepatol. 2019, 4, 33. [Google Scholar] [CrossRef]
- Decaens, T. Liver transplantation for hepatocellular carcinoma: Time for an international consensus. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Samuel, D.; Coilly, A. Management of patients with liver diseases on the waiting list for transplantation: A major impact to the success of liver transplantation. BMC Med. 2018, 16, 113. [Google Scholar] [CrossRef]
- De’Angelis, N.; Landi, F.; Carra, M.C.; Azoulay, D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J. Gastroenterol. 2015, 21, 11185–11198. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Ivanics, T.; Claasen, M.P.; Muaddi, H.; Sapisochin, G. The management of post-transplantation recurrence of hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Santopaolo, F.; Lenci, I.; Milana, M.; Manzia, T.M.; Baiocchi, L. Liver transplantation for hepatocellular carcinoma: Where do we stand? World J. Gastroenterol. 2019, 25, 2591–2602. [Google Scholar] [CrossRef]
- Azzam, A.Z. Liver transplantation as a management of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1347–1354. [Google Scholar] [CrossRef]
- Crissien, A.M.; Frenette, C. Current management of hepatocellular carcinoma. Gastroenterol. Hepatol. 2014, 10, 153–161. [Google Scholar]
- Lacaze, L.; Scotté, M. Surgical treatment of intra hepatic recurrence of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1755–1760. [Google Scholar] [CrossRef]
- Liang, X.; Cai, J.; Zheng, J.; Xie, Y.; Kirih, M.; Tao, L. Laparoscopic repeat hepatectomy for treating recurrent liver cancer. J. Minimal Access Surg. 2021, 17, 1–6. [Google Scholar] [CrossRef]
- Vogl, T.J.; Naguib, N.N.; Nour-Eldin, N.-E.A.; Rao, P.; Emami, A.H.; Zangos, S.; Nabil, M.; Abdelkader, A. Review on transarterial chemoembolization in hepatocellular carcinoma: Palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur. J. Radiol. 2009, 72, 505–516. [Google Scholar] [CrossRef]
- Zu, Q.-Q.; Liu, S.; Zhou, C.-G.; Yang, Z.-Q.; Xia, J.-G.; Zhao, L.-B.; Shi, H.-B. Chemoembolization of Recurrent Hepatoma After Curative Resection: Prognostic Factors. Am. J. Roentgenol. 2015, 204, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Glassberg, M.B.; Ghosh, S.; Clymer, J.W.; Qadeer, R.A.; Ferko, N.C.; Sadeghirad, B.; Wright, G.; Amaral, J.F. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: A systematic review and meta-analysis. OncoTargets Ther. 2019, 12, 6407–6438. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jin, Y.-J.; Shin, S.K.; Kwon, J.H.; Kim, S.G.; Suh, Y.J.; Jeong, Y.; Yu, J.H.; Lee, J.-W.; Kwon, O.S.; et al. Surgery versus radiofrequency ablation in patients with Child- Pugh class-A/single small (≤3 cm) hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Eisele, R.; Chopra, S.; Lock, J.; Glanemann, M. Treatment of recurrent hepatocellular carcinoma confined to the liver with repeated resection and radiofrequency ablation: A single center experience. Technol. Health Care 2013, 21, 9–18. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, H.; Huang, D.Q.; Xu, C.; Zheng, H.; Maeda, M.; Zhao, X.; Wang, L.; Xiao, F.; Lv, H.; et al. Radiofrequency ablation versus repeat resection for recurrent hepatocellular carcinoma (≤ 5 cm) after initial curative resection. Eur. Radiol. 2020, 30, 6357–6368. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Gan, Y.; Ge, N.; Chen, Y.; Wang, Y.; Zhang, B.; Wang, Y.; Ye, S.; Ren, Z. Transarterial Chemoembolization versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma after Resection within Barcelona Clinic Liver Cancer Stage 0/A: A Retrospective Comparative Study. J. Vasc. Interv. Radiol. 2016, 27, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-W.; Zhang, Y.-J.; Liang, H.-H.; Lin, X.-J.; Guo, R.-P.; Chen, M.-S. Recurrent Hepatocellular Carcinoma Treated with Sequential Transcatheter Arterial Chemoembolization and RF Ablation versus RF Ablation Alone: A Prospective Randomized Trial. Radiology 2012, 262, 689–700. [Google Scholar] [CrossRef]
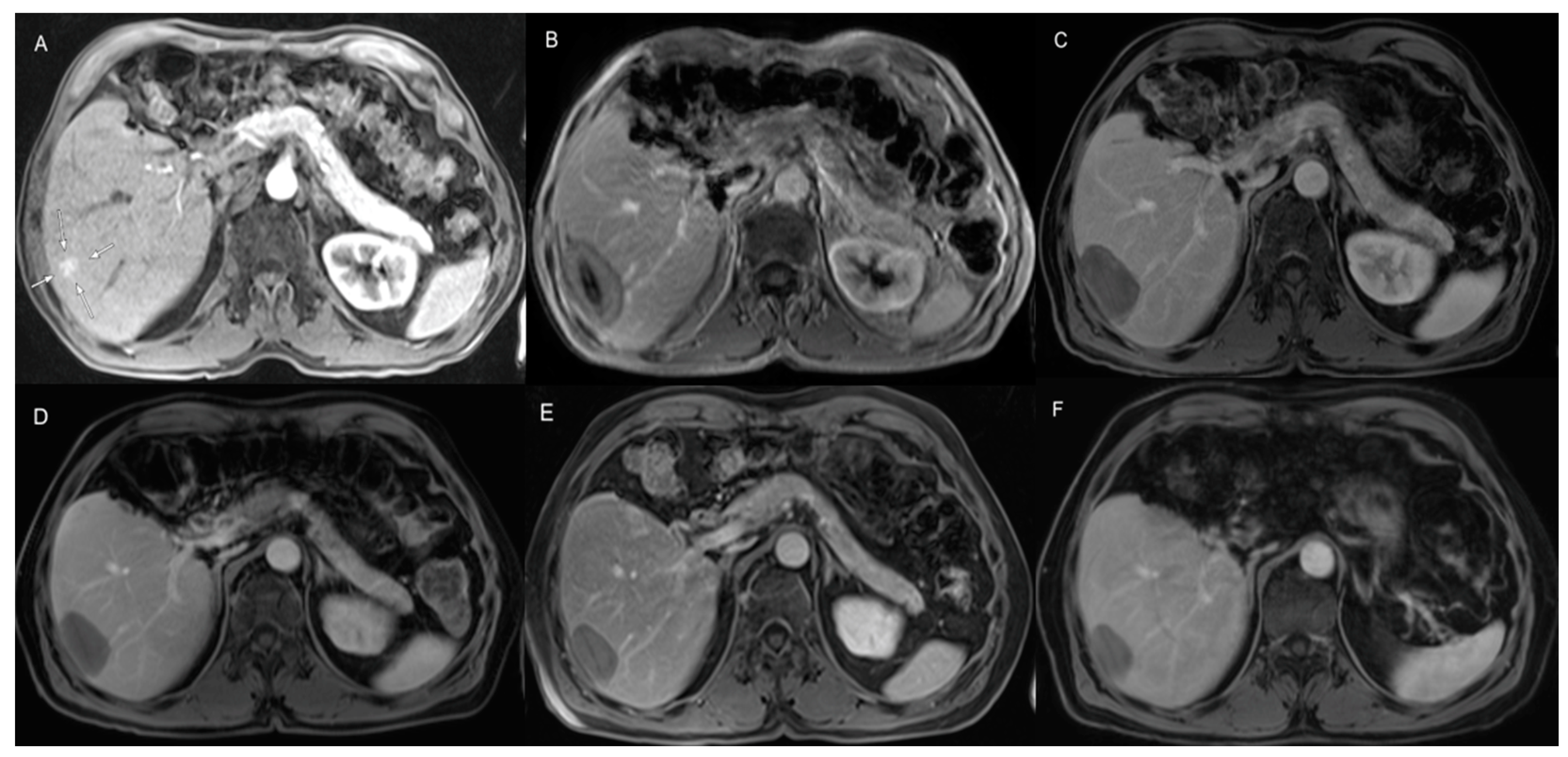
| Parameter | Result |
|---|---|
| Number of patients | 40 |
| Mean age | 62.3 ± 11.7 years |
| Gender n (%) | |
| Women | 11 (22.5) |
| Men | 29 (77.5) |
| Etiology of liver disease n (%) | |
| Chronic viral hepatitis | 16 (40) |
| Alcoholic liver disease | 9 (22.5) |
| Non-alcoholic fatty liver disease | 4 (10) |
| Chronic viral hepatitis and alcoholic liver disease | 3 (7.5) |
| Other/Cryptogenic | 8 (20) |
| Cirrhosis n (%) | 27 (67.5) |
| Child-Pugh class A | 26 (96.3) |
| Child-Pugh class B | 1 (3.7) |
| Surgical approach n (%) | |
| Laparotomy | 31 (77.5) |
| Laparoscopic | 9 (22.5) |
| Time to recurrence after surgical resection | 1.5 years |
| Recurrence within ≤6 months n (%) | 7 (17.5) |
| Recurrence within 6 to ≤12 months n (%) | 13 (32.5) |
| Recurrence within 12 to ≤24 months n (%) | 10 (25) |
| Recurrence after more than 24 months n (%) | 10 (25) |
| No. of tumors | 48 |
| Tumor size | 1.8 ± 0.8 cm |
| Distribution of recurrent tumors n (%) | |
| One tumor | 33 (82.5) |
| Two tumors | 6 (15) |
| Three tumors | 1 (2.5) |
| <2 cm | 36 (75) |
| =2 cm | 2 (4.2) |
| >2 cm | 10 (20.8) |
| Location n (%) | |
| Right lobe | 27 (56.25) |
| Left lobe | 21 (43.75) |
| Parameter | Result |
|---|---|
| Diameter of ablation area | 4.5 ± 0.9 cm |
| Number of microwave ablation treatments | 48 |
| Number of microwave ablation antennas | 48 |
| Technical success n (%) | 48 (100) |
| Ablation time | 9 ± 2.5 min |
| Power | 86 ± 20.7 watts |
| Complete hepatic response n (%) | 15 (37.5) |
| Local tumor progression n (%) | 2 (5) |
| Intrahepatic distant recurrence n (%) | 23 (57.5) |
| Treatment-related complications/deaths n (%) | 0.0 (0.0) |
| Parameter | Result |
|---|---|
| Follow-up time | 3.2 ± 2.5 years |
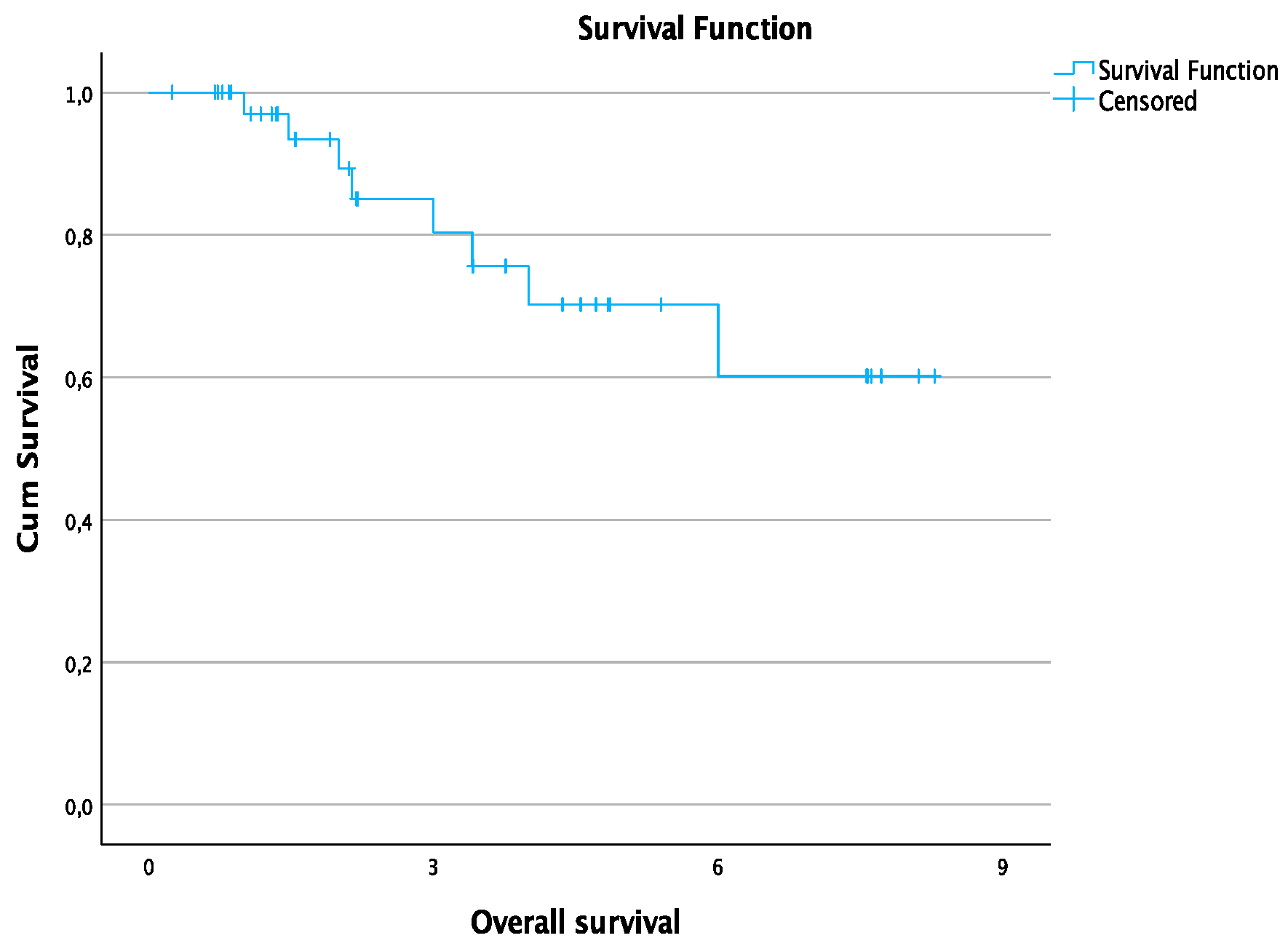
| 1-year overall survival rate | 97% |
| 2-year overall survival rate | 89.2% |
| 3-year overall survival rate | 80.3% |
| 4-year overall survival rate | 70.2% |
| 6-year overall survival rate | 60.2% |
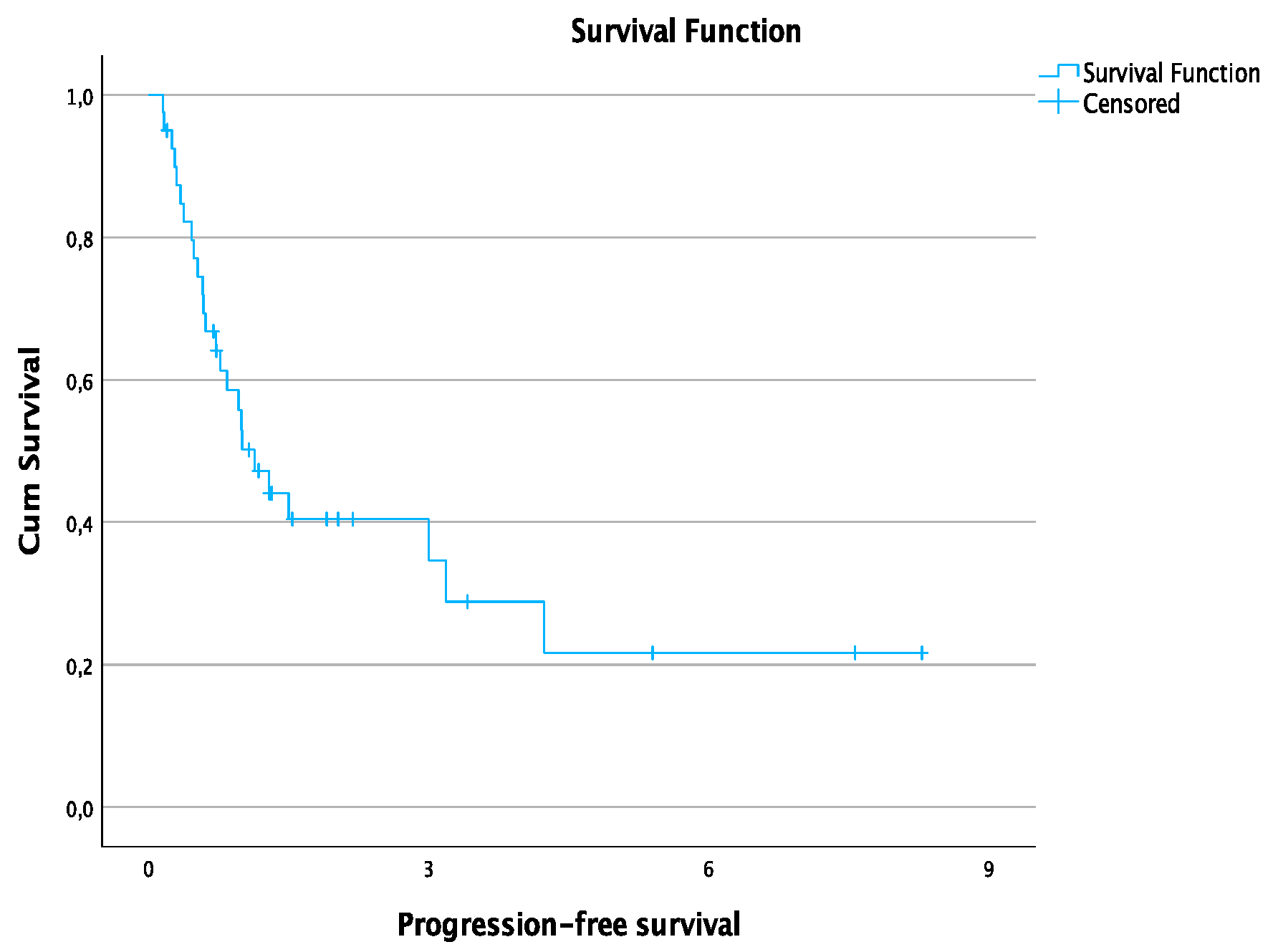
| Median progression-free survival time | 13.6 months |
| 1-year progression-free survival rate | 50.2% |
| 3-year progression-free survival rate | 34.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adwan, H.; Hammann, L.; Vogl, T.J. Microwave Ablation of Recurrent Hepatocellular Carcinoma after Curative Surgical Resection. J. Clin. Med. 2023, 12, 2560. https://doi.org/10.3390/jcm12072560
Adwan H, Hammann L, Vogl TJ. Microwave Ablation of Recurrent Hepatocellular Carcinoma after Curative Surgical Resection. Journal of Clinical Medicine. 2023; 12(7):2560. https://doi.org/10.3390/jcm12072560
Chicago/Turabian StyleAdwan, Hamzah, Lars Hammann, and Thomas J. Vogl. 2023. "Microwave Ablation of Recurrent Hepatocellular Carcinoma after Curative Surgical Resection" Journal of Clinical Medicine 12, no. 7: 2560. https://doi.org/10.3390/jcm12072560
APA StyleAdwan, H., Hammann, L., & Vogl, T. J. (2023). Microwave Ablation of Recurrent Hepatocellular Carcinoma after Curative Surgical Resection. Journal of Clinical Medicine, 12(7), 2560. https://doi.org/10.3390/jcm12072560






