Age and Comorbidity Burden of Patients Critically Ill with COVID-19 Affect Both Access to and Outcome of Ventilation Therapy in Intensive Care Units
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Study Design
2.3. Data Quality
2.4. Statistical Analysis
3. Results
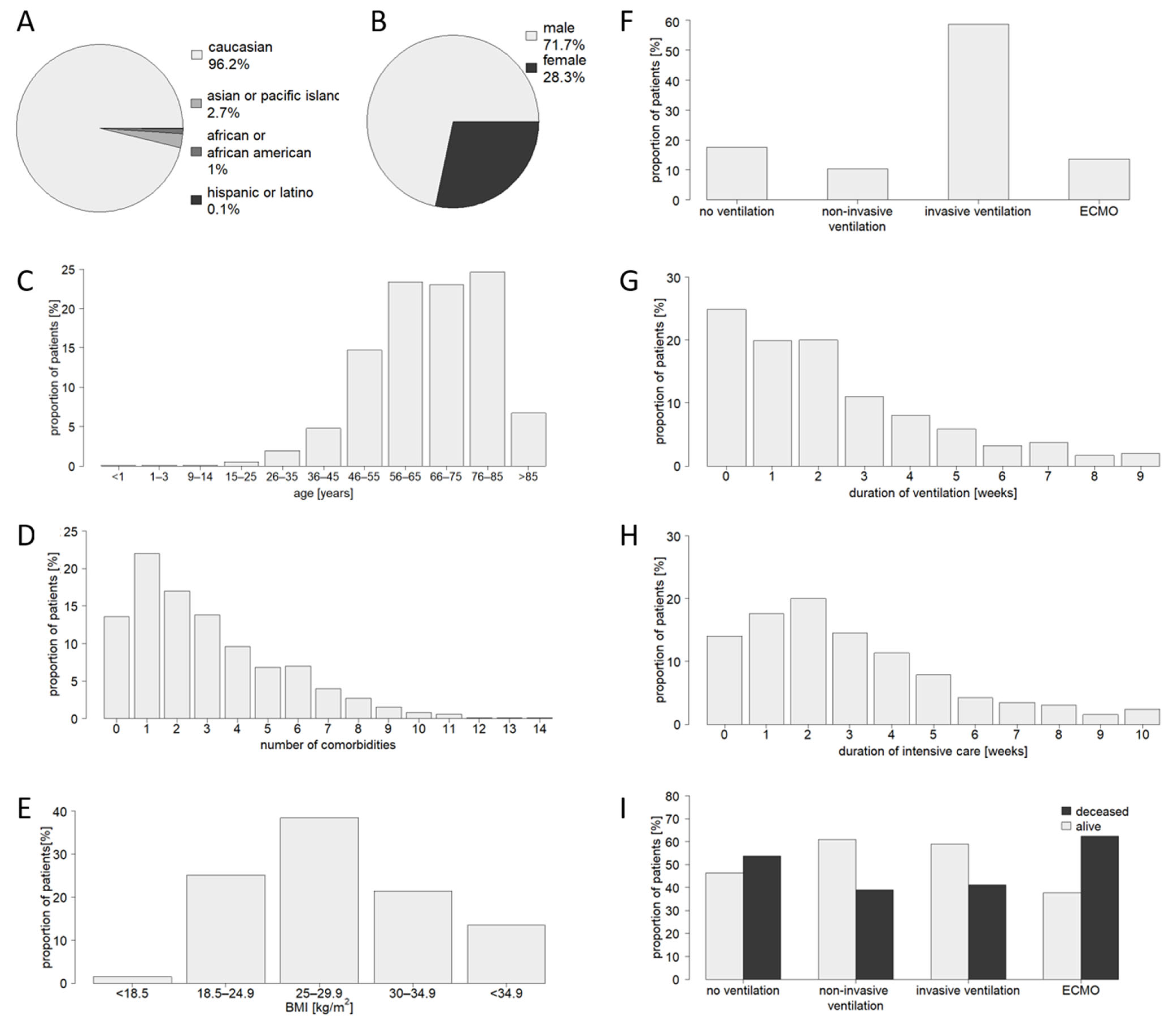
3.1. Characteristics of the Study Population
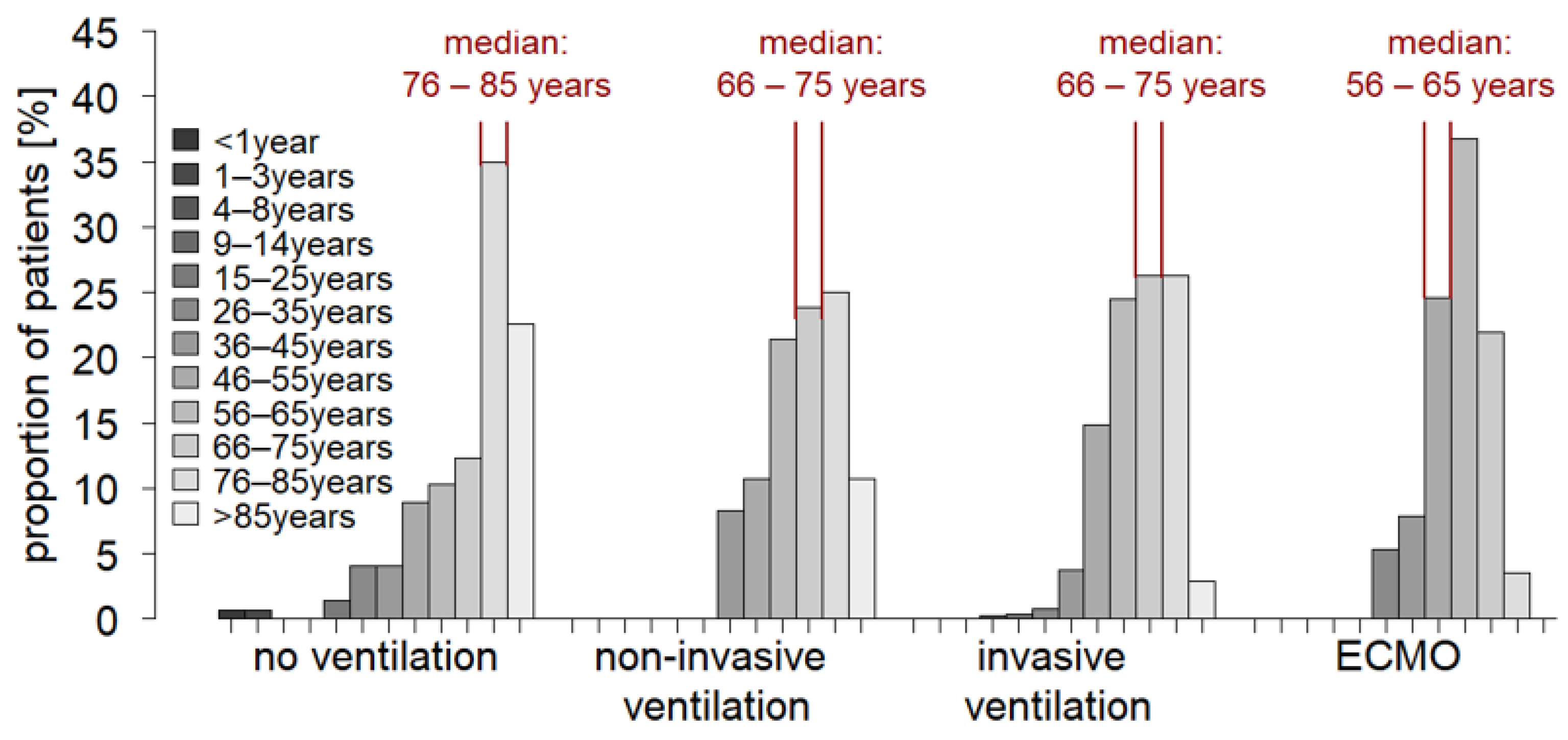
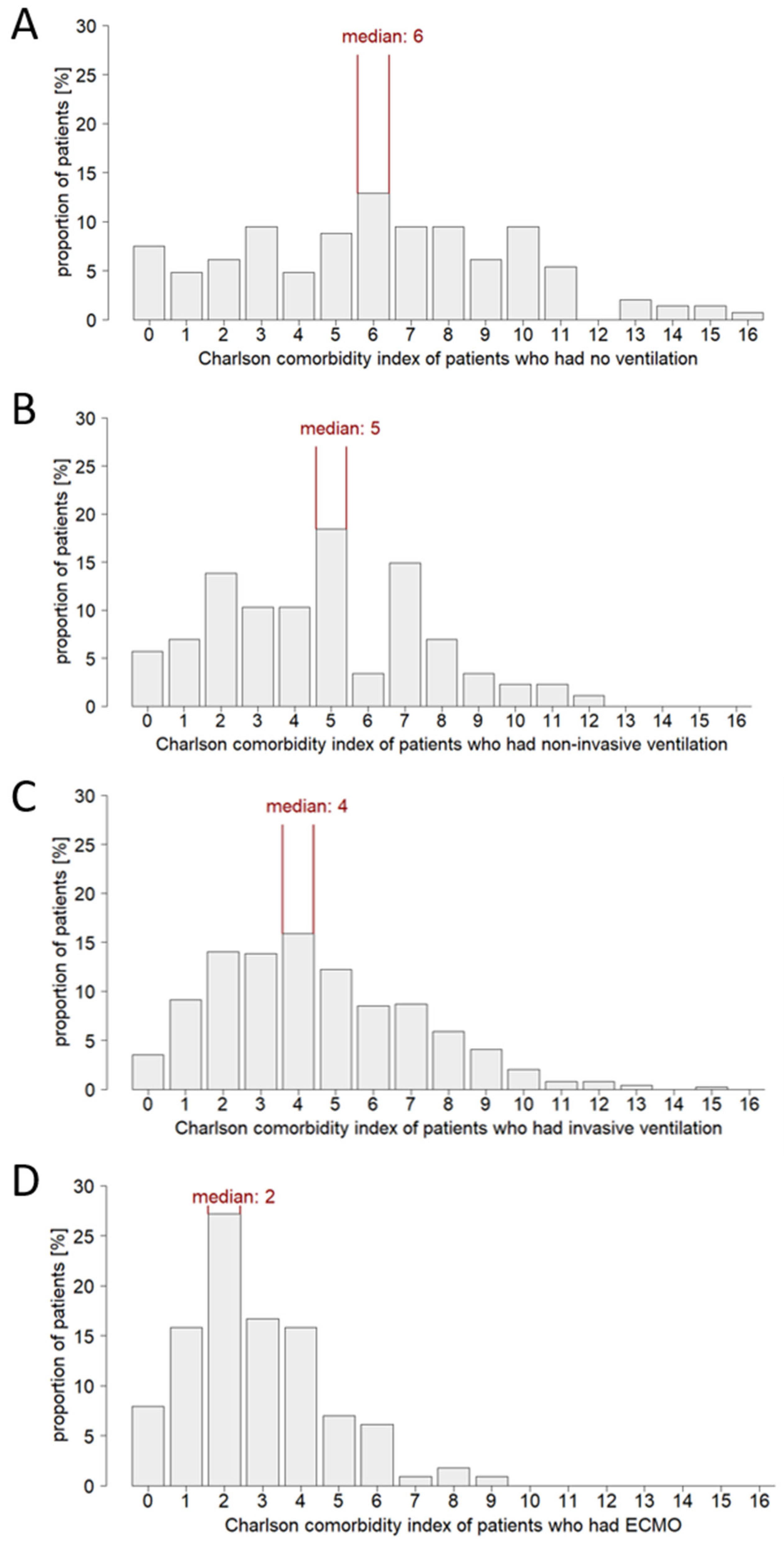
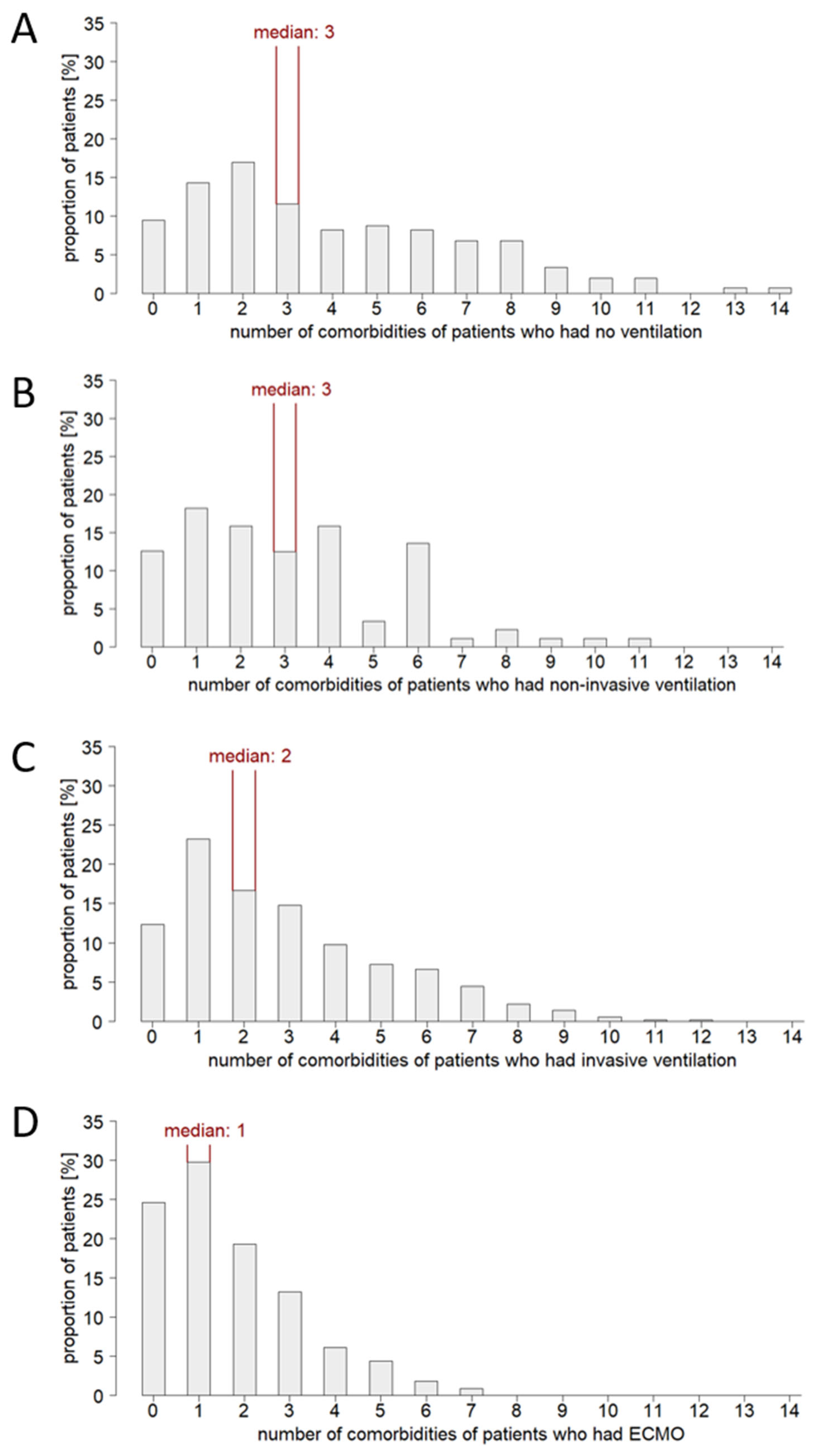
3.2. Patient Age and Comorbidity Influence the Ventilation Strategy in Critical Care
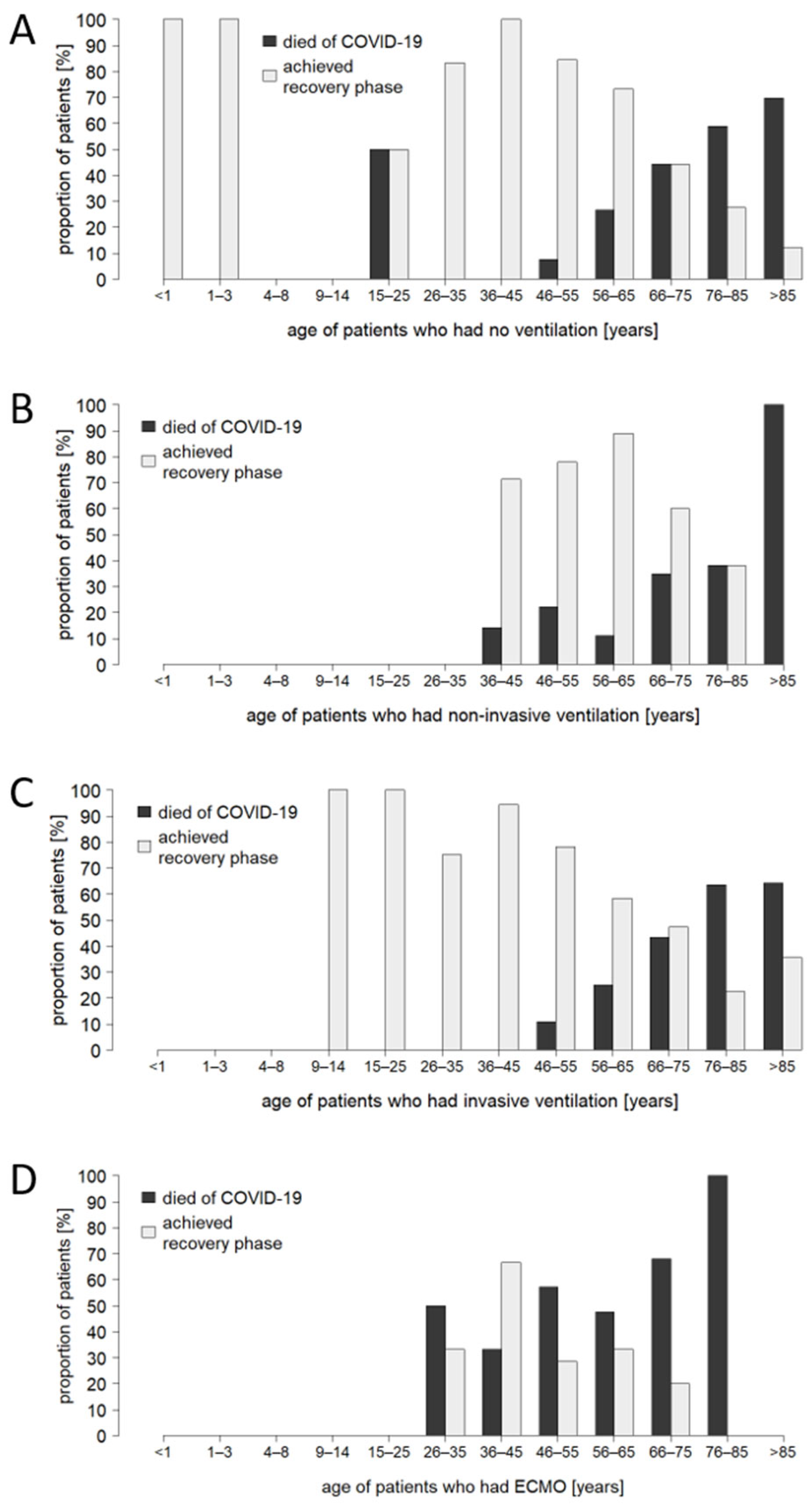
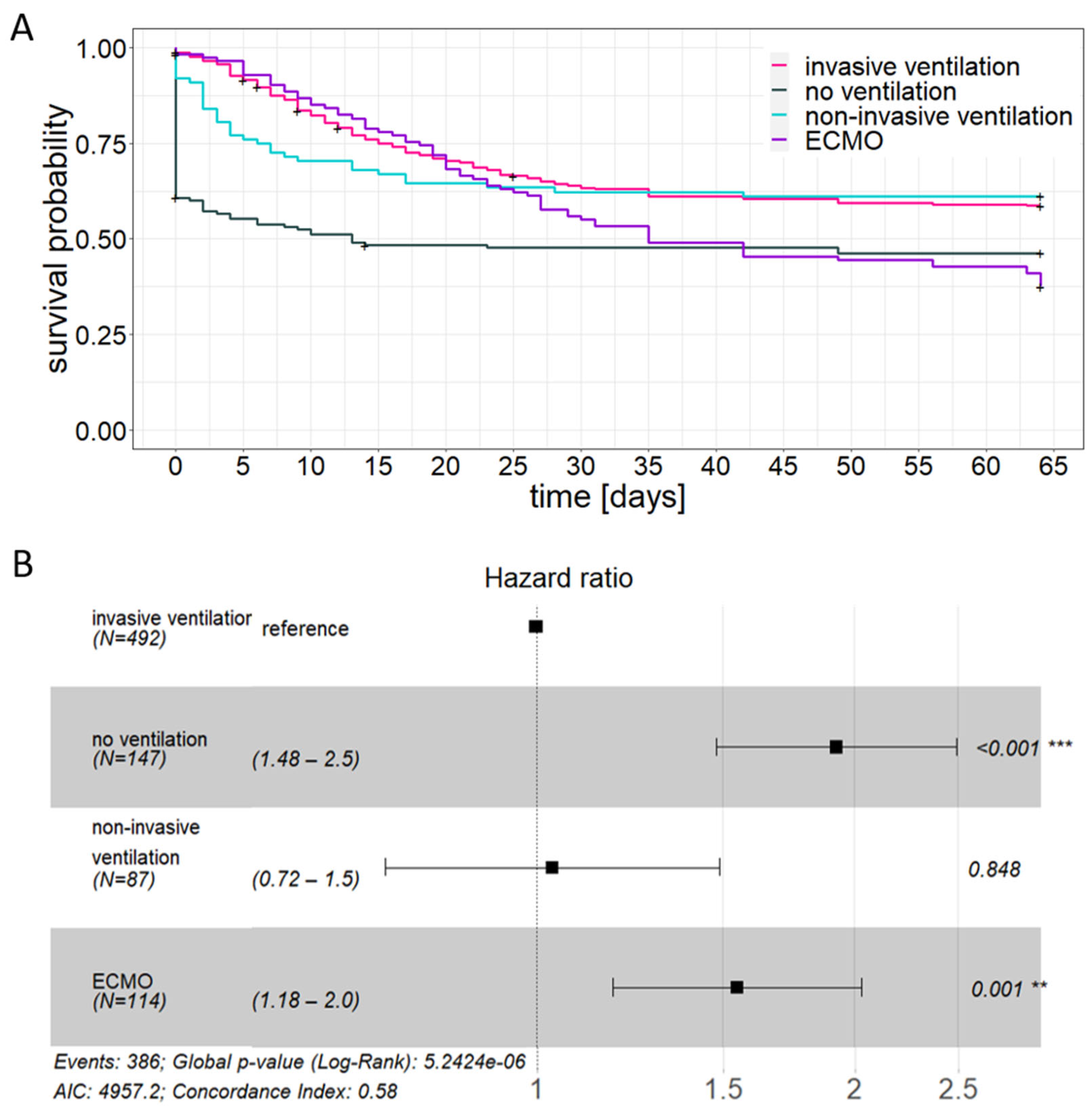
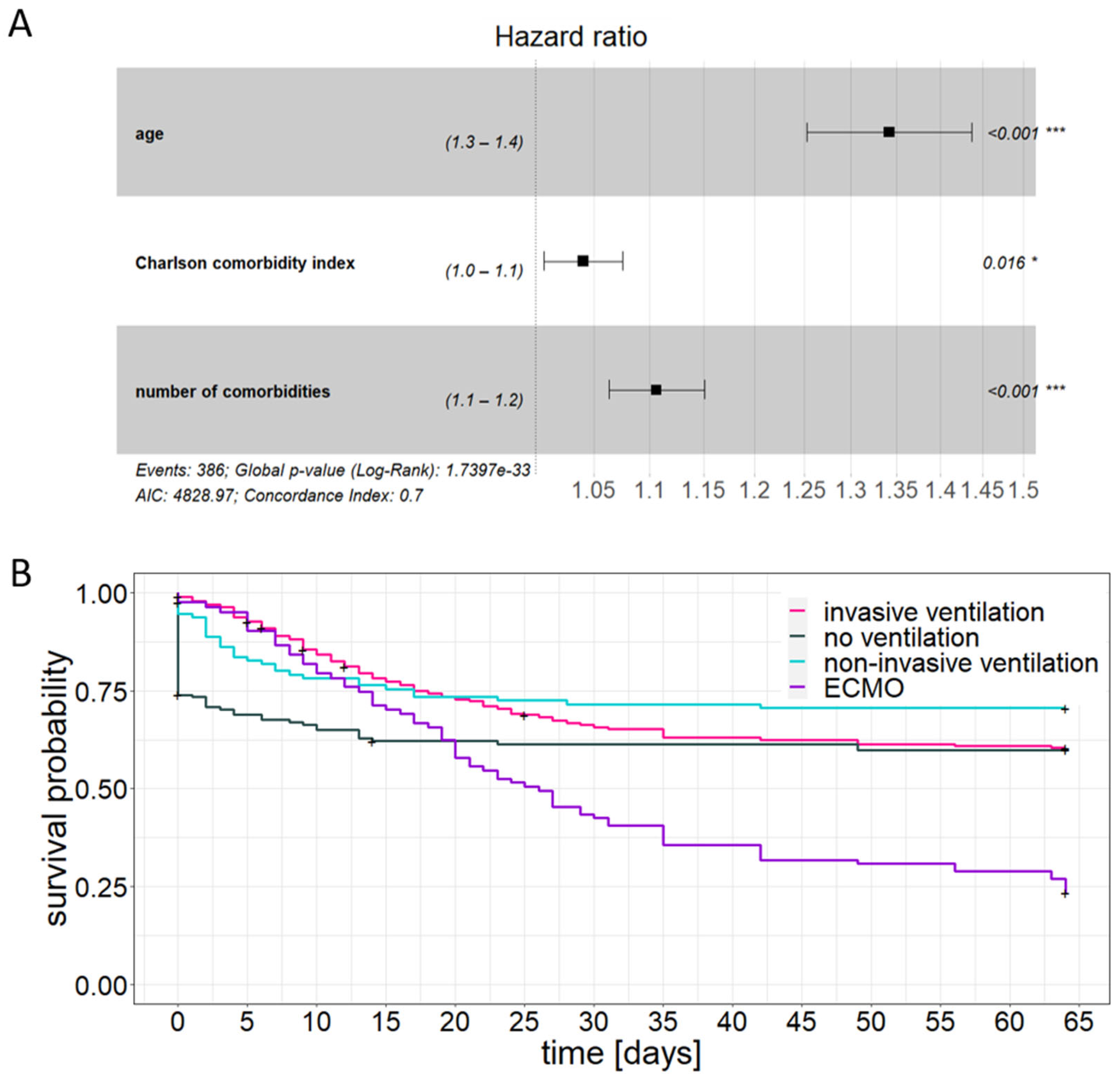
3.3. Patient Age and Comorbidity Have an Impact on the Outcome of Critical Care Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef]
- Jung, C.; Flaatten, H.; Fjolner, J.; Bruno, R.R.; Wernly, B.; Artigas, A.; Pinto, B.B.; Schefold, J.C.; Wolff, G.; Kelm, M.; et al. The impact of frailty on survival in elderly intensive care patients with COVID-19: The COVIP study. Crit. Care 2021, 25, 149. [Google Scholar] [CrossRef] [PubMed]
- Oba, S.; Altinay, M.; Salkaya, A.; Turk, H.S. Evaluation of the effect of clinical characteristics and intensive care treatment methods on the mortality of covid-19 patients aged 80 years and older. BMC Anesthesiol. 2021, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Welch, C. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: Results of an international multi-centre study. Age Ageing 2021, 50, 617–630. [Google Scholar] [CrossRef]
- Hussien, H.; Nastasa, A.; Apetrii, M.; Nistor, I.; Petrovic, M.; Covic, A. Different aspects of frailty and COVID-19: Points to consider in the current pandemic and future ones. BMC Geriatr. 2021, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.G.; Breckons, M.; Lee, R.P.; Dotchin, C.; Walker, R. Rationing care by frailty during the COVID-19 pandemic. Age Ageing 2021, 50, 7–10. [Google Scholar] [CrossRef]
- Polidori, M.C.; Sies, H.; Ferrucci, L.; Benzing, T. COVID-19 mortality as a fingerprint of biological age. Ageing Res. Rev. 2021, 67, 101308. [Google Scholar] [CrossRef]
- Pijls, B.G.; Jolani, S.; Atherley, A.; Derckx, R.T.; Dijkstra, J.I.R.; Franssen, G.H.L.; Hendriks, S.; Richters, A.; Venemans-Jellema, A.; Zalpuri, S.; et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: A meta-analysis of 59 studies. BMJ Open 2021, 11, e044640. [Google Scholar] [CrossRef]
- Hewitt, J.; Carter, B.; Vilches-Moraga, A.; Quinn, T.J.; Braude, P.; Verduri, A.; Pearce, L.; Stechman, M.; Short, R.; Price, A.; et al. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. Lancet Public Health 2020, 5, E444–E451. [Google Scholar] [CrossRef]
- Smithard, D.G.; Abdelhameed, N.; Han, T.; Pieris, A. Age, Frailty, Resuscitation and Intensive Care: With Reference to COVID-19. Geriatrics 2021, 6, 36. [Google Scholar] [CrossRef]
- Pott Junior, H.; Cominetti, M.R. Comorbidities predict 30-day hospital mortality of older adults with COVID-19. Geriatr. Nurs. 2021, 42, 1024–1028. [Google Scholar] [CrossRef]
- Eiras Poco, P.C.; Romero Aliberti, M.J.; Dias, M.B.; Takahashi, S.d.F.; Leonel, F.C.; Altona, M.; Venys, A.L.; Shin-Ike, I.A.; Garcia, B.A.; Sumita, L.H.; et al. Divergent: Age, Frailty, and Atypical Presentations of COVID-19 in Hospitalized Patients. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2021, 76, E46–E51. [Google Scholar] [CrossRef]
- Meintrup, D.; Borgmann, S.; Seidl, K.; Stecher, M.; Jakob, C.E.M.; Pilgram, L.; Spinner, C.D.; Rieg, S.; Isberner, N.; Hower, M.; et al. Specific Risk Factors for Fatal Outcome in Critically Ill COVID-19 Patients: Results from a European Multicenter Study. J. Clin. Med. 2021, 10, 3855. [Google Scholar] [CrossRef] [PubMed]
- Jakob, C.E.M.; Kohlmayer, F.; Meurers, T.; Vehreschild, J.J.; Prasser, F. Design and evaluation of a data anonymization pipeline to promote Open Science on COVID-19. Sci. Data 2020, 7, 435. [Google Scholar] [CrossRef]
- Jakob, C.E.M.; Borgmann, S.; Duygu, F.; Behrends, U.; Hower, M.; Merle, U.; Friedrichs, A.; Tometten, L.; Hanses, F.; Jung, N.; et al. First results of the “Lean European Open Survey on SARS-CoV-2-Infected Patients (LEOSS)”. Infection 2020, 49, 63–73. [Google Scholar] [CrossRef]
- Kluge, S.; Malin, J.J.; Fichtner, F.J.; Müller, O.; Skoetz, N.; Karagiannidis, C. Recommendations on the In-Hospital Treatment of Patients with COVID-19. Dtsch. Arztebl. Int. 2021, 118, 865–871. [Google Scholar] [CrossRef] [PubMed]
- IfD Allensbach. Patientenverfügungen–Umfrage zur Verbreitung in Deutschland nach Altersgruppe 2014. Available online: https://de.statista.com/statistik/daten/studie/375267/umfrage/patientenverfuegungen-verbreitung-in-deutschland-nach-altersgruppen/ (accessed on 8 March 2023).
- Guidet, B.; Jung, C.; Flaatten, H.; Fjolner, J.; Artigas, A.; Pinto, B.B.; Schefold, J.C.; Beil, M.; Sigal, S.; van Heerden, P.V.; et al. Increased 30-day mortality in very old ICU patients with COVID-19 compared to patients with respiratory failure without COVID-19. Intensive Care Med. 2022, 48, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Guidet, B.; Flaatten, H.; Boumendil, A.; Morandi, A.; Andersen, F.H.; Artigas, A.; Bertolini, G.; Cecconi, M.; Christensen, S.; Faraldi, L.; et al. Withholding or withdrawing of life-sustaining therapy in older adults (=80 years) admitted to the intensive care unit. Intensive Care Med. 2018, 44, 1598–1601. [Google Scholar] [CrossRef]
- Jung, C.; Flaatten, H.; Lange, D.; de Beil, M.; Guidet, B. The relationship between treatment limitations and pressure on intensive care units in elderly patients. Intensive Care Med. 2022, 48, 124–125. [Google Scholar] [CrossRef]
- Sjogren, L.; Stenberg, E.; Thuccani, M.; Martikainen, J.; Rylander, C.; Wallenius, V.; Olbers, T.; Kindblom, J.M. Impact of obesity on intensive care outcomes in patients with COVID-19 in Sweden-A cohort study. PLoS ONE 2021, 16, e0257891. [Google Scholar] [CrossRef]
- Kwok, S.; Adam, S.; Ho, J.H.; Iqbal, Z.; Turkington, P.; Razvi, S.; Le Roux, C.W.; Soran, H.; Syed, A.A. Obesity: A critical risk factor in the COVID-19 pandemic. Clin. Obes. 2020, 10, 12403. [Google Scholar] [CrossRef]
- Mason, K.E.; Maudsley, G.; McHale, P.; Pennington, A.; Day, J.; Barr, B. Age-Adjusted Associations between Comorbidity and Outcomes of COVID-19: A Review of the Evidence from the Early Stages of the Pandemic. Front. Public Health 2021, 9, 584182. [Google Scholar] [CrossRef]
- Treskova-Schwarzbach, M.; Haas, L.; Reda, S.; Pilic, A.; Borodova, A.; Karimi, K.; Koch, J.; Nygren, T.; Scholz, S.; Schoenfeld, V.; et al. Pre-existing health conditions and severe COVID-19 outcomes: An umbrella review approach and meta-analysis of global evidence. BMC Med. 2021, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Tonna, J.E.; Abrams, D.; Brodie, D.; Greenwood, J.C.; Mateo-Sidron, J.A.R.; Usman, A.; Fan, E. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). Asaio J. 2021, 67, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Badulak, J.; Antonini, M.V.; Stead, C.M.; Shekerdemian, L.; Raman, L.; Paden, M.L.; Agerstrand, C.; Bartlett, R.H.; Barrett, N.; Combes, A.; et al. Extracorporeal Membrane Oxygenation for COVID-19: Updated 2021 Guidelines from the Extracorporeal Life Support Organization. Asaio J. 2021, 67, 485–495. [Google Scholar] [CrossRef]
- Chinnadurai, R.; Ogedengbe, O.; Agarwal, P.; Money-Coomes, S.; Abdurrahman, A.Z.; Mohammed, S.; Kalra, P.A.; Rothwell, N.; Pradhan, S. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting—A cohort study. BMC Geriatr. 2020, 20, 409. [Google Scholar] [CrossRef] [PubMed]
| Comorbidity | No. (%) |
|---|---|
| Hypertension | 512 (61.0) |
| Diabetes without end-organ damage | 155 (18.5) |
| Chronic kidney disease | 145 (17.3) |
| Coronary artery disease | 140 (16.7) |
| Atrial fibrillation | 134 (16.0) |
| Chronic heart failure | 94 (11.2) |
| Chronic obstructive pulmonary disease (COPD) | 83 (9.9) |
| Diabetes with end-organ damage | 81 (9.6) |
| Acute kidney injury | 80 (9.5) |
| Cerebrovascular disease | 78 (9.3) |
| Solid tumor | 73 (8.7) |
| Myocardial infarction | 64 (7.6) |
| Dementia | 63 (7.5) |
| Chronic pulmonary disease | 51 (6.1) |
| Peripheral vascular disease | 42 (5.0) |
| On dialysis | 34 (4.0) |
| Asthma | 31 (3.7) |
| Carotid artery disease | 31 (3.7) |
| Rheumatic disease | 30 (3.6) |
| Hemiplegia | 27 (3.2) |
| Lymphoma | 27 (3.2) |
| Atrioventricular block | 25 (3.0) |
| Chronic liver disease | 25 (3.0) |
| Organ transplantation | 20 (2.4) |
| Peptic ulcer | 20 (2.4) |
| Aortic stenosis | 18 (2.1) |
| Leukemia | 15 (1.8) |
| Solid tumor, metastasized | 12 (1.4) |
| Liver cirrhosis | 7 (0.8) |
| Median Survival Time [Days] | 30-Day Mortality [%] | |||
|---|---|---|---|---|
| unadjusted | adjusted | unadjusted | adjusted | |
| no ventilation | 13 | - | 52.4 | 38.6 |
| non-invasive ventilation | - | - | 37.7 | 28.4 |
| invasive ventilation | - | - | 36.6 | 34.5 |
| ECMO | 35 | 26 | 44.9 | 57.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Hesselle, M.L.; Borgmann, S.; Rieg, S.; Vehreschild, J.J.; Rasch, S.; Koll, C.E.M.; Hower, M.; Stecher, M.; Ebert, D.; Hanses, F.; et al. Age and Comorbidity Burden of Patients Critically Ill with COVID-19 Affect Both Access to and Outcome of Ventilation Therapy in Intensive Care Units. J. Clin. Med. 2023, 12, 2469. https://doi.org/10.3390/jcm12072469
de Hesselle ML, Borgmann S, Rieg S, Vehreschild JJ, Rasch S, Koll CEM, Hower M, Stecher M, Ebert D, Hanses F, et al. Age and Comorbidity Burden of Patients Critically Ill with COVID-19 Affect Both Access to and Outcome of Ventilation Therapy in Intensive Care Units. Journal of Clinical Medicine. 2023; 12(7):2469. https://doi.org/10.3390/jcm12072469
Chicago/Turabian Stylede Hesselle, Marie Louise, Stefan Borgmann, Siegbert Rieg, Jörg Janne Vehreschild, Sebastian Rasch, Carolin E. M. Koll, Martin Hower, Melanie Stecher, Daniel Ebert, Frank Hanses, and et al. 2023. "Age and Comorbidity Burden of Patients Critically Ill with COVID-19 Affect Both Access to and Outcome of Ventilation Therapy in Intensive Care Units" Journal of Clinical Medicine 12, no. 7: 2469. https://doi.org/10.3390/jcm12072469
APA Stylede Hesselle, M. L., Borgmann, S., Rieg, S., Vehreschild, J. J., Rasch, S., Koll, C. E. M., Hower, M., Stecher, M., Ebert, D., Hanses, F., Schumann, J., & on behalf of the LEOSS Study Group. (2023). Age and Comorbidity Burden of Patients Critically Ill with COVID-19 Affect Both Access to and Outcome of Ventilation Therapy in Intensive Care Units. Journal of Clinical Medicine, 12(7), 2469. https://doi.org/10.3390/jcm12072469








