Prognostic Role of Visual Evoked Potentials in Non-Neuritic Eyes at Multiple Sclerosis Diagnosis
Abstract
1. Introduction
2. Patients and Methods
3. Results
4. Discussion
- (1)
- We employed traditional full-field VEPs as an easily repeatable and largely used marker. Some authors evaluated multifocal (mf) VEPs in smaller groups and found some correlations with disability. Blanco et al. showed delayed and reduced visual responses in more than 70% of non-ON cases among a group of 28 patients. They also described lower wave amplitudes with higher disability scores [17].
- (2)
- Since our study included a large cohort of 181 MS patients at diagnosis, our results could not reflect long-term treatment-related prognosis. The choice of starting highly effective DMTs could be considered as a marker of disease severity at diagnosis in our cohort.
- (3)
- Pivotal data on optical coherence tomography we performed in a limited subgroup with no definite results. Nonetheless, VEPs are currently diffusely employed in MS neurological assessments at first MS diagnostic work-up.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Lapiscina, E.H.; Sanchez-Dalmau, B.F.; Fraga-Pumar, E.; Ortiz-Perez, S.; Tercero-Uribe, A.I.; Torres-Torres, R.; Villoslada, P. The visual pathway as a model to understand brain damage in multiple sclerosis. Mult. Scler. 2014, 20, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Klistorner, A.; Garrick, R.; Barnett, M.H.; Graham, S.L.; Arvind, H.; Sriram, P.; Yiannikas, C. Axonal loss in non-optic neuritis eyes of patients with multiple sclerosis linked to delayed visual evoked potential. Neurology 2013, 80, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Janáky, M.; Jánossy; Horváth, G.; Benedek, G.; Braunitzer, G. VEP and PERG in patients with multiple sclerosis, with and without a history of optic neuritis. Doc. Ophthalmol. 2017, 134, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Odom, J.V.; International Society for Clinical Electrophysiology of Vision; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Mizota, A.; Tormene, A.P. International Society for Clinical Electrophysiology of Vision. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. 2016, 133, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bsteh, G.; Ehling, R.; Lutterotti, A.; Hegen, H.; Di Pauli, F.; Auer, M.; Deisenhammer, F.; Reindl, M.; Berger, T. Long Term Clinical Prognostic Factors in Relapsing-Remitting Multiple Sclerosis: Insights from a 10-Year Observational Study. PLoS ONE 2016, 11, e0158978. [Google Scholar] [CrossRef] [PubMed]
- Caverzasi, E.; Cordano, C.; Zhu, A.H.; Zhao, C.; Bischof, A.; Kirkish, G.; Bennett, D.J.; Devereux, M.; Baker, N.; Inman, J.; et al. Imaging correlates of visual function in multiple sclerosis. PLoS ONE 2020, 15, e0235615. [Google Scholar] [CrossRef] [PubMed]
- Fuhr, P.; Borggrefe-Chappuis, A.; Schindler, C.; Kappos, L. Visual and motor evoked potentials in the course of multiple sclerosis. Brain 2001, 124 Pt 11, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Kallmann, B.A.; Fackelmann, S.; Toyka, K.V.; Rieckmann, P.; Reiners, K. Early abnormalities of evoked potentials and future disability in patients with multiple sclerosis. Mult. Scler. 2006, 12, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Giffroy, X.; Maes, N.; Albert, A.; Maquet, P.; Crielaard, J.-M.; Dive, D. Multimodal evoked potentials for functional quantification and prognosis in multiple sclerosis. BMC Neurol. 2016, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Schlaeger, R.; D’Souza, M.; Schindler, C.; Grize, L.; Kappos, L.; Fuhr, P. Electrophysiological markers and predictors of the disease course in primary progressive multiple sclerosis. Mult. Scler. 2014, 20, 51–56. [Google Scholar] [CrossRef] [PubMed]
- London, F.; El Sankari, S.; van Pesch, V. Early disturbances in multimodal evoked potentials as a prognostic factor for long-term disability in relapsing-remitting multiple sclerosis patients. Clin. Neurophysiol. 2017, 128, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Leocani, L.; Guerrieri, S.; Comi, G. Visual Evoked Potentials as a Biomarker in Multiple Sclerosis and Associated Optic Neuritis. J. Neuroophthalmol. 2018, 38, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Weinstock-Guttman, B.; Baier, M.; Stockton, R.; Weinstock, A.; Justinger, T.; Munschauer, F.; Brownscheidle, C.; Williams, J.; Fisher, E.; Miller, D.; et al. Pattern reversal visual evoked potentials as a measure of visual pathway pathology in multiple sclerosis. Mult. Scler. 2003, 9, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, S.; Comi, G.; Leocani, L. Optical Coherence Tomography and Visual Evoked Potentials as Prognostic and Monitoring Tools in Progressive Multiple Sclerosis. Front. Neurosci. 2021, 15, 692599. [Google Scholar] [CrossRef] [PubMed]
- Sater, R.A.; Rostami, A.; Galetta, S.; Farber, R.E.; Bird, S.J. Serial evoked potential studies and MRI imaging in chronic progressive multiple sclerosis. J. Neurol. Sci. 1999, 171, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Piedrabuena, R.; Bittar, M. Optical coherence tomography and visual evoked potential and its relationship with neurological disability in patients with relapsing-remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 57, 103420. [Google Scholar] [CrossRef] [PubMed]
- Blanco, R.; Pérez-Rico, C.; Puertas-Muñoz, I.; Ayuso-Peralta, L.; Boquete, L.; Arévalo-Serrano, J. Functional assessment of the visual pathway with multifocal visual evoked potentials, and their relationship with disability in patients with multiple sclerosis. Mult. Scler. 2014, 20, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Pihl-Jensen, G.; Schmidt, M.F.; Frederiksen, J.L. Multifocal visual evoked potentials in optic neuritis and multiple sclerosis: A review. Clin. Neurophysiol. 2017, 128, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
| Female (N, %) | 113, 62% |
|---|---|
| Age at onset (years: mean, SD) Age at diagnosis (years: mean, SD) | 35.4, 11.3 36.9, 11.7 |
| MS course at diagnosis (N, %): relapsing-remitting progressive (primary or secondary) | 166, 92% 15, 8% |
| Previous history of ON or at onset (N, %) | 54, 30% |
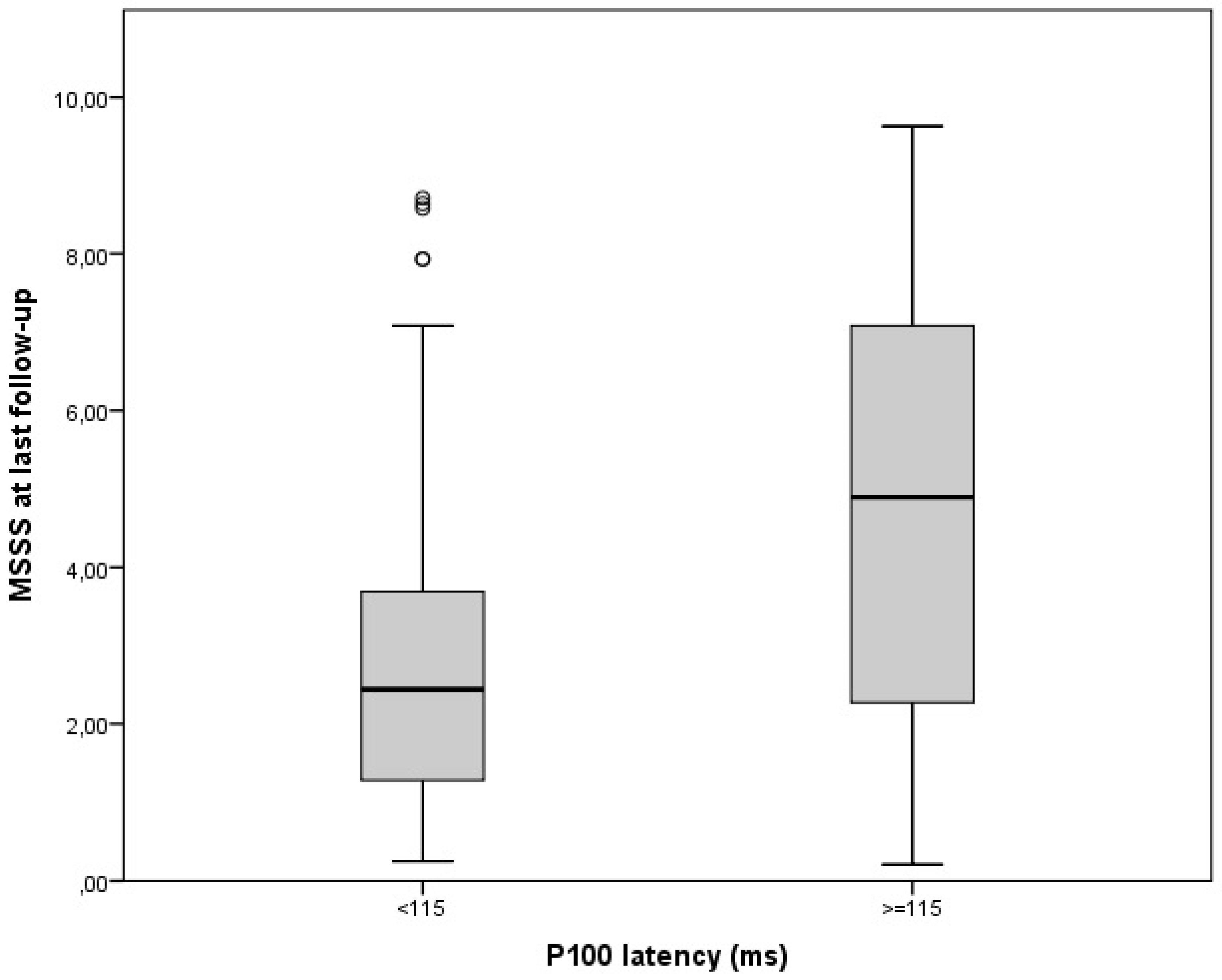
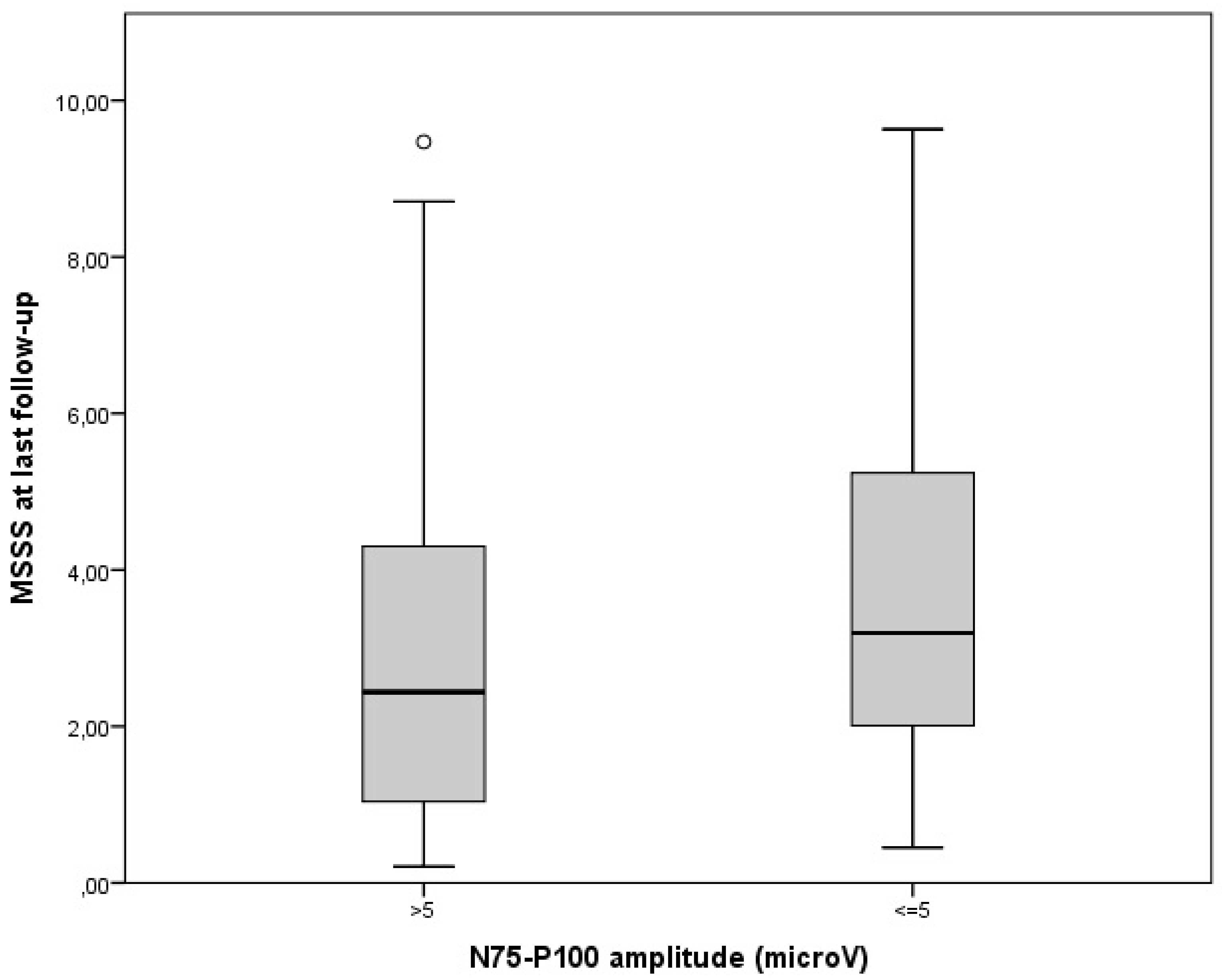
| VEP measures in non-ON eyes at diagnosis: abnormal P100 latency (patients: N, %) P100 latency (ms: mean, ES) abnormal N75-P100 amplitude (patients: N, %) N75-P100 amplitude (microV: mean, ES) | 38, 21% 106.4, 1.1 63, 35% 7.1, 0.3 |
| Disease duration at diagnosis (years: mean, ES) | 1.4, 0.3 |
| EDSS at diagnosis (median, range) | 1, 0–8 |
| Prognostic Factors | OR | IC 95% | p-Value | Overall p-Value |
|---|---|---|---|---|
| A: N = 181 Patients (All Cases); R = 0.47, R-Square = 0.22, F-Value = 8 | ||||
| Gender Age at onset Previous history of ON or at onset MS course P100 latency N75-P100 amplitude | 0.29 0.04 0.10 2.07 0.04 −0.04 | −0.42–0.10 0.01–0.07 −0.62–0.82 0.80–3.33 0.01–0.06 −0.13–0.06 | NS 0.005 NS 0.002 0.002 NS | <0.001 |
| B: N = 166 patients (RRMS); R = 0.35, R-square = 0.13, F-value = 4 | ||||
| Gender Age at onset Previous history of ON or at onset P100 latency N75-P100 amplitude DMTs | 0.44 0.04 0.11 0.04 −0.05 0.11 | −0.29–1.16 0.01–0.07 −0.61–0.82 0.01–0.06 −0.14–0.04 0.17–1.28 | NS 0.025 NS 0.005 NS 0.010 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecchio, D.; Barbero, P.; Galli, G.; Virgilio, E.; Naldi, P.; Comi, C.; Cantello, R. Prognostic Role of Visual Evoked Potentials in Non-Neuritic Eyes at Multiple Sclerosis Diagnosis. J. Clin. Med. 2023, 12, 2382. https://doi.org/10.3390/jcm12062382
Vecchio D, Barbero P, Galli G, Virgilio E, Naldi P, Comi C, Cantello R. Prognostic Role of Visual Evoked Potentials in Non-Neuritic Eyes at Multiple Sclerosis Diagnosis. Journal of Clinical Medicine. 2023; 12(6):2382. https://doi.org/10.3390/jcm12062382
Chicago/Turabian StyleVecchio, Domizia, Paolo Barbero, Giulia Galli, Eleonora Virgilio, Paola Naldi, Cristoforo Comi, and Roberto Cantello. 2023. "Prognostic Role of Visual Evoked Potentials in Non-Neuritic Eyes at Multiple Sclerosis Diagnosis" Journal of Clinical Medicine 12, no. 6: 2382. https://doi.org/10.3390/jcm12062382
APA StyleVecchio, D., Barbero, P., Galli, G., Virgilio, E., Naldi, P., Comi, C., & Cantello, R. (2023). Prognostic Role of Visual Evoked Potentials in Non-Neuritic Eyes at Multiple Sclerosis Diagnosis. Journal of Clinical Medicine, 12(6), 2382. https://doi.org/10.3390/jcm12062382







