Objective Classification of Glistening in Implanted Intraocular Lenses Using Optical Coherence Tomography: Proposal for a New Classification and Grading System
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Study Protocol
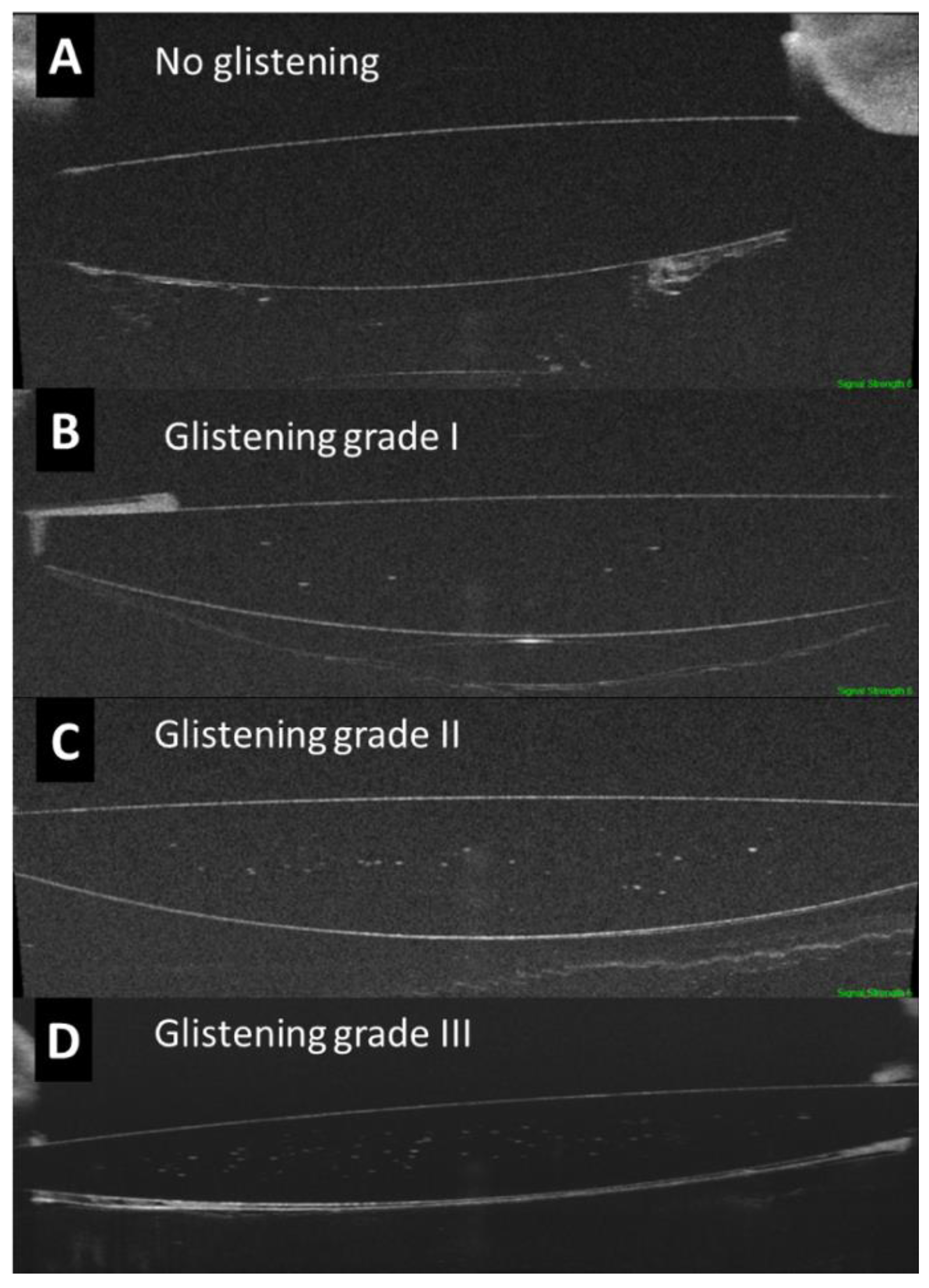
2.3. Assessment of the IOL Glistening by OCT
2.4. Statistical Analysis
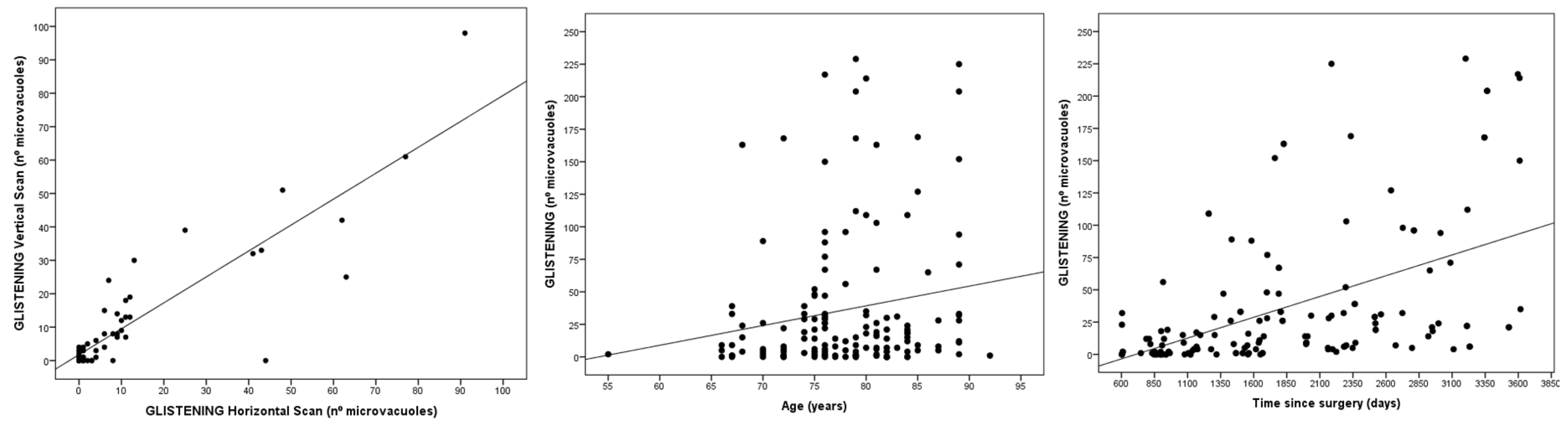
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanclerz, P.; Yildirim, T.M.; Khoramnia, R. A review of late intraocular lens opacifications. Curr. Opin. Ophthalmol. 2021, 32, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Durr, G.M.; Ahmed, I.I.K. Intraocular Lens Complications: Decentration, Uveitis–Glaucoma–Hyphema Syndrome, Opacification, and Refractive Surprises. Ophthalmology 2021, 128, e186–e194. [Google Scholar] [CrossRef]
- Colin, J.; Orignac, I. Glistenings on intraocular lenses in healthy eyes: Effects and associations. J. Refract. Surg. 2011, 27, 869–875. [Google Scholar] [CrossRef]
- Yildirim, T.M.; Schickhardt, S.K.; Wang, Q.; Friedmann, E.; Khoramnia, R.; Auffarth, G.U. Quantitative evaluation of microvacuole formation in five intraocular lens models made of different hydrophobic materials. PLoS ONE 2021, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Markeviciute, A.; Zemaitiene, R. A narrative review of intraocular lens opacifications: Update 2020. Ann. Transl. Med. 2020, 8, 1547. [Google Scholar] [CrossRef] [PubMed]
- Werner, L. Intraocular Lenses: Overview of Designs, Materials, and Pathophysiologic Features. Ophthalmology 2021, 128, e74–e93. [Google Scholar] [CrossRef] [PubMed]
- Werner, L. Glistenings and surface light scattering in intraocular lenses. J. Cataract Refract. Surg. 2010, 36, 1398–1420. [Google Scholar] [CrossRef] [PubMed]
- Biwer, H.; Schuber, E.; Honig, M.; Spratte, B.; Baumeister, M.; Kohnen, T. Objective classification of glistenings in implanted intraocular lenses using Scheimpflug tomography. J. Cataract Refract. Surg. 2015, 41, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Montañés, J.; Alvarez, A.; Rodríguez-Conde, R.; Fernández-Hortelano, A. Clinical factors related to the frequency and intensity of glistenings in AcrySof intraocular lenses. J. Cataract Refract. Surg. 2003, 29, 1980–1984. [Google Scholar] [CrossRef]
- Tetz, M.; Jorgensen, M.R. New Hydrophobic IOL Materials and Understanding the Science of Glistenings. Curr. Eye Res. 2015, 40, 969–981. [Google Scholar] [CrossRef]
- Rønbeck, M.; Behndig, A.; Taube, M.; Koivula, A.; Kugelberg, M. Comparison of glistenings in intraocular lenses with three different materials: 12-year follow-up. Acta Ophthalmol. 2013, 91, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Bao, X.; Qin, Y.; Hou, M.; Wu, M. Subjective Visual Performance and Objective Optical Quality With Intraocular Lens Glistening and Surface Light Scattering. J. Refract. Surg. 2018, 34, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Neuhann, T.; Yildirim, T.M.; Son, H.S.; Merz, P.R.; Khoramnia, R.; Auffarth, G.U. Reasons for explantation, demographics, and material analysis of 200 intraocular lens explants. J. Cataract Refract. Surg. 2020, 46, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Dehoog, E.; Doraiswamy, A. Evaluation of loss in optical quality of multifocal intraocular lenses with glistenings. J. Cataract Refract. Surg. 2016, 42, 606–612. [Google Scholar] [CrossRef]
- Kawahara, S.; Nagai, Y.; Kawakami, E.; Yamanaka, R.; Ida, N.; Takeuchi, M.; Uyama, M.; Miyata, A.; Uchida, N.; Nakajima, K.; et al. Clinical and experimental observation of glistening in acrylic intraocular lenses. Expression of the Varicella Zoster Virus Thymidine Kinase and Cytokines in Patients with Acute Retinal Necrosis Syndrome 2000. Analysis of Proteins During Recovery fr. J. Jpn. Ophthalmol. Soc. 2000, 104, 349–353. [Google Scholar]
- Tognetto, D.; Toto, L.; Sanguinetti, G.; Ravalico, G. Glistenings in foldable intraocular lenses. J. Cataract Refract. Surg. 2002, 28, 1211–1216. [Google Scholar] [CrossRef]
- Thomes, B.E.; Callaghan, T.A. Evaluation of in vitro glistening formation in hydrophobic acrylic intraocular lenses. Clin. Ophthalmol. 2013, 7, 1529–1534. [Google Scholar] [CrossRef]
- Łabuz, G.; Knebel, D.; Auffarth, G.U.; Fang, H.; van den Berg, T.J.; Yildirim, T.M.; Son, H.S.; Khoramnia, R. Glistening Formation and Light Scattering in Six Hydrophobic-Acrylic Intraocular Lenses. Am. J. Ophthalmol. 2018, 196, 112–120. [Google Scholar] [CrossRef]
- Miyata, A.; Uchida, N.; Nakajima, K.; Yaguchi, S. Clinical and experimental observation of glistening in acrylic intraocular lenses. Jpn. J. Ophthalmol. 2001, 45, 564–569. [Google Scholar] [CrossRef]
- Tripathy, K.; Sridhar, U. Optical coherence tomography of intraocular lens glistening. Indian J. Ophthalmol. 2019, 67, 138–139. [Google Scholar] [CrossRef]
- Werner, L.; Michelson, J.; Ollerton, A.; Leishman, L.; Bodnar, Z. Anterior segment optical coherence tomography in the assessment of postoperative intraocular lens optic changes. J. Cataract Refract. Surg. 2012, 38, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Vigo, J.I.; de-Pablo Gómez de Liaño, L.; Sánchez-Guillen, I.; Macarro-Merino, A.; Fernández-Vigo, C.; García-Feijóo, J.; Fernández-Vigo, J.A. Pseudoexfoliation signs in the anterior segment assessed by optical coherence tomography and Scheimpflug device. Arch. Soc. Esp. Oftalmol. 2018, 93, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.M.; Fang, H.; Schickhardt, S.K.; Wang, Q.; Merz, P.R.; Auffarth, G.U. Glistening formation in a new hydrophobic acrylic intraocular lens. BMC Ophthalmol. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Stanojcic, N.; O’Brart, D.P.S.; Maycock, N.; Hull, C.C. Effects of intraocular lens glistenings on visual function: A prospective study and presentation of a new glistenings grading methodology. BMJ Open Ophthalmol. 2019, 4, e000266. [Google Scholar] [CrossRef] [PubMed]
- Stanojcic, N.; OʼBrart, D.; Hull, C.; Wagh, V.; Azan, E.; Bhogal, M.; Robbie, S.; Li, J.-P.O. Visual and refractive outcomes and glistenings occurrence after implantation of 2 hydrophobic acrylic aspheric monofocal IOLs. J. Cataract Refract. Surg. 2020, 46, 986–994. [Google Scholar] [CrossRef]
- Henriksen, B.S.; Kinard, K.; Olson, R.J. Effect of intraocular lens glistening size on visual quality. J. Cataract Refract. Surg. 2015, 41, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Waite, A.; Faulkner, N.; Olson, R.J. Glistenings in the single-piece, hydrophobic, acrylic intraocular lenses. Am. J. Ophthalmol. 2007, 144, 143–144. [Google Scholar] [CrossRef]
- Hayashi, K.; Hirata, A.; Yoshida, M.; Yoshimura, K.; Hayashi, H. Long-term effect of surface light scattering and glistenings of intraocular lenses on visual function. Am. J. Ophthalmol. 2012, 154, 240–251.e2. [Google Scholar] [CrossRef]
- Geniusz, M.; Zając, M. A technique of experimental and numerical analysis of influence of defects in the intraocular lens on the retinal image quality. Appl. Digit. Image Process. XXXIX 2016, 9971, 997125. [Google Scholar] [CrossRef]
- Weindler, J.N.; Łabuz, G.; Yildirim, T.M.; Tandogan, T.; Khoramnia, R.; Auffarth, G.U. The impact of glistenings on the optical quality of a hydrophobic acrylic intraocular lens. J. Cataract Refract. Surg. 2019, 45, 1020–1025. [Google Scholar] [CrossRef]
- DeHoog, E.; Doraiswamy, A. Evaluation of the impact of light scatter from glistenings in pseudophakic eyes. J. Cataract Refract. Surg. 2014, 40, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Werner, L.; Thatthamla, I.; Ong, M.; Schatz, H.; Garcia-Gonzalez, M.; Gros-Otero, J.; Cañones-Zafra, R.; Teus, M.A. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J. Cataract Refract. Surg. 2019, 45, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Rajan, M.; Ligabue, E.; Heiner, P. Clinical properties of a novel, glistening-free, single-piece, hydrophobic acrylic IOL. Clin. Ophthalmol. 2014, 8, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Honbo, M.; Otani, S.; Nejima, R.; Minami, K. Effect on visual acuity of increased surface light scattering in intraocular lenses. J. Cataract Refract. Surg. 2012, 38, 221–226. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value |
|---|---|
| Age (years) | 70.2 ± 1 1.3 (55–92) |
| Sex (female/male, %) | 54/46 |
| Eye (right/left) | 51.5/48.5 |
| Time since surgery (days) | 1843 ± 843 (603–3617) |
| IOL power (diopters) | 20.3 ± 4.1 (8–27) |
| IOL model and material | SN60WF (acrylic hydrophobic) |
| Parameter | Values | Parameter | Values | |
|---|---|---|---|---|
| Intraobserver reproducibility | Measurement 1 | 11.62 ± 20.01 (0–90) | Severity scale 1 | 0.82 ± 0.94 (0–3) |
| Measurement 2 | 11.32 ± 19.30 (0–85) | Severity scale 2 | 0.82 ± 0.94 (0–3) | |
| ICC | 0.994 (0.990–0.997) | ICC | 0.977 (0.961–0.987) | |
| Interobserver reproducibility | Observer 1 | 11.62 ± 20.01 (0–90) | Severity scale observer 1 | 0.82 ± 0.94 (0–3) |
| Observer 2 | 11.54 ± 18.87 (0–87) | Severity scale observer 2 | 0.84 ± 0.98 (0–3) | |
| ICC | 0.996 (0.993–0.998) | ICC | 0.967 (0.943–0.981) | |
| Repeatability | Exploration 1 | 11.10 ± 18.5 (0–82) | Exploration 1 | 0.78 ± 1.07 |
| Exploration 2 | 9.63 ± 17.11 (0–87) | Exploration 2 | 0.75 ± 1.06 | |
| ICC | 0.958 (0.927–0.975) | ICC | 0.955 (0.926–0.973) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Vigo, J.I.; Burgos-Blasco, B.; De-Pablo-Gómez-de-Liaño, L.; Sánchez-Guillén, I.; Albitre-Barca, V.; Fernández-Aragón, S.; Fernández-Vigo, J.Á.; Macarro-Merino, A. Objective Classification of Glistening in Implanted Intraocular Lenses Using Optical Coherence Tomography: Proposal for a New Classification and Grading System. J. Clin. Med. 2023, 12, 2351. https://doi.org/10.3390/jcm12062351
Fernández-Vigo JI, Burgos-Blasco B, De-Pablo-Gómez-de-Liaño L, Sánchez-Guillén I, Albitre-Barca V, Fernández-Aragón S, Fernández-Vigo JÁ, Macarro-Merino A. Objective Classification of Glistening in Implanted Intraocular Lenses Using Optical Coherence Tomography: Proposal for a New Classification and Grading System. Journal of Clinical Medicine. 2023; 12(6):2351. https://doi.org/10.3390/jcm12062351
Chicago/Turabian StyleFernández-Vigo, José Ignacio, Bárbara Burgos-Blasco, Lucía De-Pablo-Gómez-de-Liaño, Inés Sánchez-Guillén, Virginia Albitre-Barca, Susana Fernández-Aragón, José Ángel Fernández-Vigo, and Ana Macarro-Merino. 2023. "Objective Classification of Glistening in Implanted Intraocular Lenses Using Optical Coherence Tomography: Proposal for a New Classification and Grading System" Journal of Clinical Medicine 12, no. 6: 2351. https://doi.org/10.3390/jcm12062351
APA StyleFernández-Vigo, J. I., Burgos-Blasco, B., De-Pablo-Gómez-de-Liaño, L., Sánchez-Guillén, I., Albitre-Barca, V., Fernández-Aragón, S., Fernández-Vigo, J. Á., & Macarro-Merino, A. (2023). Objective Classification of Glistening in Implanted Intraocular Lenses Using Optical Coherence Tomography: Proposal for a New Classification and Grading System. Journal of Clinical Medicine, 12(6), 2351. https://doi.org/10.3390/jcm12062351









