Treatment of Peritoneal Surface Malignancies by Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Spain: Results of the National Registry of the Spanish Group of Peritoneal Oncologic Surgery (REGECOP)
Abstract
1. Introduction
2. Patients and Methods
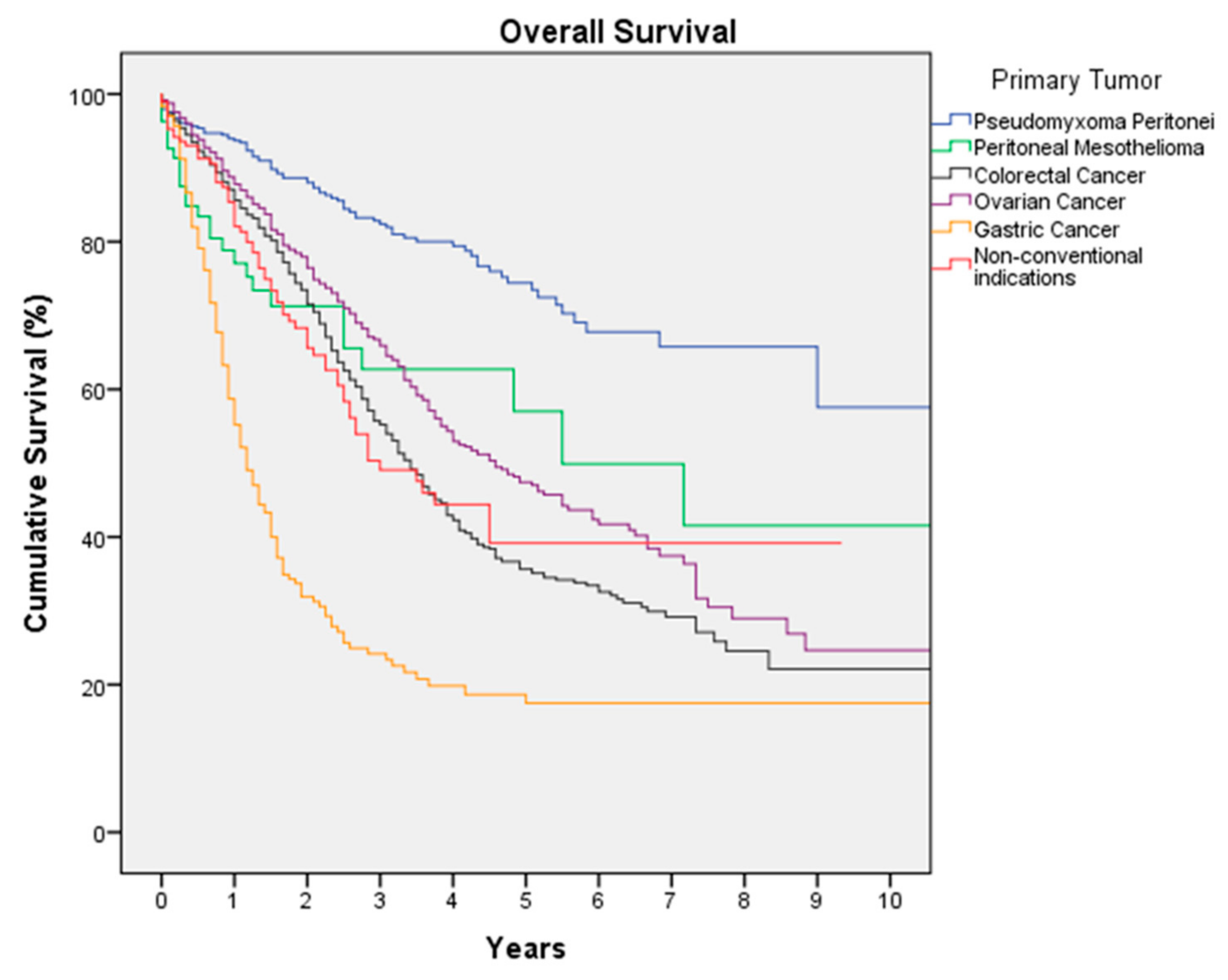
3. Results
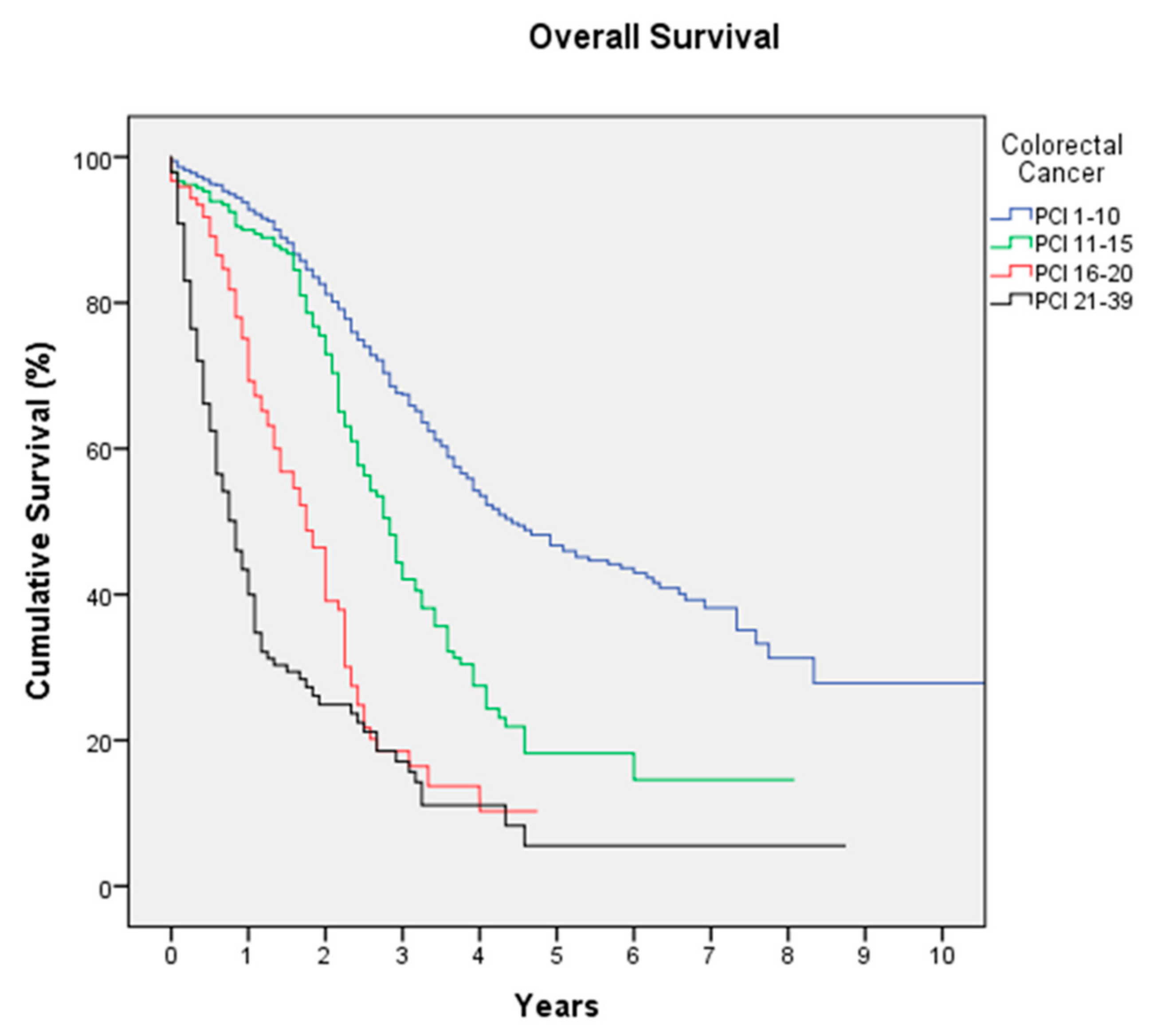
3.1. Colorectal Cancer
3.2. Ovarian Cancer
3.3. Pseudomyxoma Peritonei (PMP)
3.4. Gastric Cancer
3.5. Peritoneal Mesothelioma
3.6. Non-Conventional Indications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brücher, B.L.; Piso, P.; Verwaal, V.; Esquivel, J.; Derraco, M.; Yonemura, Y.; Gonzalez-Moreno, S.; Pelz, J.; Königsrainer, A.; Ströhlein, M.; et al. Peritoneal carcinomatosis: Cytoreductive surgery and HIPEC—Overview and basics. Cancer Investig. 2012, 30, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Glehen, O. Extent of Peritoneal Resection for Peritoneal Metastases: Looking Beyond a Complete Cytoreduction. Ann. Surg. Oncol. 2020, 27, 1458–1470. [Google Scholar] [CrossRef]
- Yonemura, Y.; Canbay, E.; Li, Y.; Coccolini, F.; Glehen, O.; Sugarbaker, P.H.; Morris, D.; Moran, B.; Gonzaletz-Moreno, S.; Deraco, M.; et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur. J. Surg. Oncol. 2016, 42, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Welch, L.; Black, D.; Sugarbaker, P.H. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann. Oncol. 2007, 18, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006, 7, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Pereira, F.; Serrano, A.; Manzanedo, I.; Pérez-Viejo, E.; González-Moreno, S.; González-Bayón, L.; Arjona-Sánchez, A.; Torres, J.; Ramos, I.; Barrios, M.E.; et al. GECOP-MMC: Phase IV randomized clinical trial to evaluate the efficacy of hyperthermic intraperitoneal chemotherapy (HIPEC) with mytomicin-C after complete surgical cytoreduction in patients with colon cancer peritoneal metastases. BMC Cancer 2022, 22, 536. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Yang, X.-J.; Huang, C.-Q.; Suo, T.; Mei, L.-J.; Yang, G.-L.; Cheng, F.-L.; Zhou, Y.-F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef]
- Bonnot, P.-E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.-M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef]
- Portilla, A.G.; Shigeki, K.; Dario, B.; Marcello, D. The intraoperative staging systems in the management of peritoneal surface malignancy. J. Surg. Oncol. 2008, 98, 228–231. [Google Scholar] [CrossRef]
- González-Moreno, S.; Kusamura, S.; Baratti, D.; Deraco, M. Postoperative residual disease evaluation in the locoregional treatment of peritoneal surface malignancy. J. Surg. Oncol. 2008, 98, 237–241. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Spiliotis, J.; Halkia, E.; Lianos, E.; Kalantzi, N.; Grivas, A.; Efstathiou, E.; Giassas, S. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: A prospective randomized phase III study. Ann. Surg. Oncol. 2015, 22, 1570–1575. [Google Scholar] [CrossRef]
- Verwaal, V.J.; Bruin, S.; Boot, H.; van Slooten, G.; van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Passot, G.; Villeneuve, L.; Vaudoyer, D.; Bin-Dorel, S.; Boschetti, G.; Piaton, E.; Garofalo, A. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: A randomized and multicenter phase III study. BMC Cancer 2014, 14, 183. [Google Scholar] [CrossRef]
- Noiret, B.; Piessen, G.; Eveno, C. Update of randomized controlled trials evaluating cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in prevention and therapy of peritoneal metastasis: A systematic review. Pleura Peritoneum 2022, 7, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Antonio, C.C.P.; Alida, G.G.; Elena, G.G.; Rocío, G.S.; Jerónimo, M.G.; Luis, A.R.J.; Aníbal, N.D.; Francisco, B.V.; Jesús, G.R.Á.; Pablo, R.R.; et al. Cytoreductive Surgery With or Without HIPEC After Neoadjuvant Chemotherapy in Ovarian Cancer: A Phase 3 Clinical Trial. Ann. Surg. Oncol. 2022, 29, 2617–2625. [Google Scholar] [CrossRef]
- Govaerts, K.; Lurvink, R.J.; De Hingh, I.; Van der Speeten, K.; Villeneuve, L.; Kusamura, S.; Kepenekian, V.; Deraco, M.; Glehen, O.; Moran, J.; et al. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. 2021, 47, 11–35. [Google Scholar] [CrossRef]
- Kusamura, S.; Kepenekian, V.; Villeneuve, L.; Lurvink, R.J.; Govaerts, K.; De Hingh, I.; Moran, B.J.; Van der Speeten, K.; Deraco, M.; Glehen, O.; et al. Peritoneal mesothelioma: PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Eur. J. Surg. Oncol. 2021, 47, 36–59. [Google Scholar] [CrossRef]
- Bernadó, M.I.R.; Maña, O.C.; Martín-Baranera, M.; Sánchez, P.B. Morbimortality after 1321 consecutive CRS + HIPEC procedures: Seeking excellence in surgery for peritoneal surface malignancy. Clin. Transl. Oncol. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Filis, P.; Mauri, D.; Markozannes, G.; Tolia, M.; Filis, N.; Tsilidis, K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: A systematic review and meta-analysis of randomized trials. ESMO Open 2022, 7, 100586. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A.N. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Arjona-Sánchez, A.; Espinosa-Redondo, E.; Gutiérrez-Calvo, A.; Segura-Sampedro, J.J.; Pérez-Viejo, E.; Concepción-Martín, V.; Sánchez-García, S.; García-Fadrique, A.; Prieto-Nieto, I.; Barrios-Sanchez, P.; et al. Efficacy and Safety of Intraoperative Hyperthermic Intraperitoneal Chemotherapy for Locally Advanced Colon Cancer: A Phase 3 Randomized Clinical Trial. JAMA Surg. 2023, e230662. [Google Scholar] [CrossRef]
- Kusamura, S.; Barretta, F.; Yonemura, Y.; Sugarbaker, P.H.; Moran, B.J.; Levine, E.A.; Goere, D.; Baratti, D.; Nizri, E.; Morris, D.L.; et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Pseudomyxoma Peritonei After Cytoreductive Surgery. JAMA Surg. 2021, 156, e206363. [Google Scholar] [CrossRef] [PubMed]
- Manzanedo, I.; Pereira, F.; Pérez-Viejo, E.; Serrano, Á. Gastric Cancer with Peritoneal Metastases: Current Status and Prospects for Treatment. Cancers 2023, 15, 1777. [Google Scholar] [CrossRef]
- Desiderio, J.; Chao, J.; Melstrom, L.; Warner, S.; Tozzi, F.; Fong, Y.; Parisi, A.; Woo, Y. The 30-year experience—A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur. J. Cancer 2017, 79, 1–14. [Google Scholar] [CrossRef]
- Granieri, S.; Bonomi, A.; Frassini, S.; Chierici, A.P.; Bruno, F.; Paleino, S.; Kusamura, S.; Germini, A.; Facciorusso, A.; Deraco, M.; et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2021, 47, 2757–2767. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Lv, L.; Zhao, S.; Zhou, Q.; Jiang, C.-G. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Combined with Surgery: A 12-Year Meta-Analysis of this Promising Treatment Strategy for Advanced Gastric Cancer at Different Stages. Ann. Surg. Oncol. 2022, 29, 3170–3186. [Google Scholar] [CrossRef]
- Martins, M.; Santos-Sousa, H.; Araújo, F.; Nogueiro, J.; Sousa-Pinto, B. Impact of Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy in the Treatment of Gastric Cancer with Peritoneal Carcinomatosis: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2022, 29, 7528–7537. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; Elias, D.; et al. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [CrossRef]
- Chia, C.S.; You, B.; Decullier, E.; Vaudoyer, D.; Lorimier, G.; Abboud, K.; Bereder, J.-M.; Arvieux, C.; Boschetti, G.; Glehen, O.; et al. Patients with Peritoneal Carcinomatosis from Gastric Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Is Cure a Possibility? Ann. Surg. Oncol. 2016, 23, 1971–1979. [Google Scholar] [CrossRef]
- Baratti, D.; Kusamura, S.; Cabras, A.D.; Bertulli, R.; Hutanu, I.; Deraco, M. Diffuse malignant peritoneal mesothelioma: Long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Eur. J. Cancer 2013, 49, 3140–3148. [Google Scholar] [CrossRef]
- Helm, J.H.; Miura, J.T.; Glenn, J.A.; Marcus, R.K.; Larrieux, G.; Jayakrishnan, T.T.; Donahue, A.E.; Gamblin, T.C.; Turaga, K.K.; Johnston, F.M. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: A systematic review and meta-analysis. Ann. Surg. Oncol. 2015, 22, 1686–1693. [Google Scholar] [CrossRef]
- Rajha, A.; Piso, P.; Halmy, L.; Panczel, I.; Nedelcut, D.-S.; Herold, Z.; Szasz, A.M.; Acs, M. Rare Histologies and Infrequent Indications for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Anticancer. Res. 2022, 42, 3681–3692. [Google Scholar] [CrossRef]
- Kusamura, S.; Bhatt, A.; Hubner, M.; Villeneuve, L.; Deraco, M.; Bakrin, N.; Van Der Speeten, K.; Glehen, O. The 2022 PSOGI International Consensus on HIPEC Regimens for Peritoneal Malignancies: Methodology. Ann. Surg. Oncol. 2023, 30, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- van Stein, R.M.; Lok, C.A.; Aalbers, A.G.; de Hingh, I.H.; Houwink, A.P.; Stoevelaar, H.J.; Sonke, G.S.; van Driel, W.J. Standardizing HIPEC and perioperative care for patients with ovarian cancer in the Netherlands using a Delphi-based consensus. Gynecol. Oncol. Rep. 2022, 39, 100945. [Google Scholar] [CrossRef] [PubMed]
- Chicago Consensus Working Group; Izquierdo, F.J.; Schuitevoerder, D.; Plana, A.; Eng, O.S.; Sherman, S.; Badgwell, B.; Johnston, F.M.; Abdel-Misih, S.; Blazer, D.G.; et al. The Chicago Consensus on Peritoneal Surface Malignancies: Management of Gastric Metastases. Cancer 2020, 126, 2541–2546. [Google Scholar] [CrossRef]

| Hospital | Region | Number of Procedures |
|---|---|---|
| H. Universitario de Fuenlabrada | Madrid | 735 |
| H. Virgen de la Arrixaca | Murcia | 369 |
| H. Virgen del Rocío | Sevilla | 271 |
| H. Río Hortega | Valladolid | 210 |
| H. Torrecárdenas | Almería | 202 |
| H. Infanta Cristina | Badajoz | 147 |
| H. General de Elche | Alicante | 136 |
| H. Príncipe de Asturias | Madrid | 135 |
| H. La Fe | Valencia | 129 |
| MD Anderson | Madrid | 119 |
| H. Regional de Málaga | Málaga | 115 |
| H. Insular | Gran Canaria | 102 |
| Variable | Procedures (n = 4159) |
|---|---|
| Sex (%) | |
| Female | 65.8 |
| Male | 34.2 |
| Median age (years (range)) | 59 (18–86) |
| Primary tumor (%) | |
| Colorectal cancer | 41.4 |
| Ovarian cancer | 31.9 |
| Pseudomyxoma peritonei | 13.3 |
| Gastric cancer | 6 |
| Peritoneal mesothelioma | 2.6 |
| Non-conventional indications | 4.8 |
| Neoadjuvant SCT (%) | 63.2 |
| Laparoscopic surgery (%) | 2.7 |
| Median surgical PCI (range) | 9 (0–39) |
| High-complexity surgery (%) | 41.4 |
| CCS (%) | |
| CCS-0 | 81.7 |
| CCS-1 | 7.5 |
| CCS-2 | 2.2 |
| CCS-3 | 8.6 |
| HIPEC technique (%) | |
| Open or coliseum | 69.2 |
| Close | 3.5 |
| Close with CO2 recirculation | 27.3 |
| HIPEC drug (%) | |
| MMC | 34.3 |
| Oxaliplatin | 24.5 |
| Paclitaxel | 18 |
| Cisplatin | 10.8 |
| Cisplatin + Doxorubicin | 8.4 |
| Cisplatin + MMC | 2.5 |
| Others | 1.6 |
| Postoperative complications (%) | |
| No complication | 49.9 |
| Minor (I–II) | 30.3 |
| Severe complications (III–IV) | 17.7 |
| Grade V | 2.1 |
| Surgical reintervention (%) | 11.9 |
| Median hospital stay (days (range)) | 11 (0–259) |
| Variable | High-Volume Center | Low-Volume Center | p |
|---|---|---|---|
| Median surgical PCI (range) | 9 (0–39) | 8 (0–39) | 0.001 |
| CCS (%) | |||
| CCS-0 or CCS-1 | 89.4 | 88.9 | 0.36 |
| CCS-2 or CCS-3 | 10.6 | 11.1 | |
| Postoperative complications (%) | |||
| No complication | 52.8 | 44.8 | |
| Minor (I–II) | 27.5 | 35.3 | 0.0001 |
| Severe complications (III–IV) | 17.7 | 17.5 | |
| Grade V | 2 | 2.4 | |
| Median disease-free survival (months) | 16 | 15 | 0.49 |
| Median overall survival (months) | 47 | 49 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzanedo, I.; Pereira, F.; Cascales-Campos, P.; Muñoz-Casares, C.; Asensio, E.; Torres-Melero, J.; Prada-Villaverde, A.; Caravaca-García, I.; Gutiérrez-Calvo, A.; Vaqué, J.; et al. Treatment of Peritoneal Surface Malignancies by Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Spain: Results of the National Registry of the Spanish Group of Peritoneal Oncologic Surgery (REGECOP). J. Clin. Med. 2023, 12, 3774. https://doi.org/10.3390/jcm12113774
Manzanedo I, Pereira F, Cascales-Campos P, Muñoz-Casares C, Asensio E, Torres-Melero J, Prada-Villaverde A, Caravaca-García I, Gutiérrez-Calvo A, Vaqué J, et al. Treatment of Peritoneal Surface Malignancies by Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Spain: Results of the National Registry of the Spanish Group of Peritoneal Oncologic Surgery (REGECOP). Journal of Clinical Medicine. 2023; 12(11):3774. https://doi.org/10.3390/jcm12113774
Chicago/Turabian StyleManzanedo, Israel, Fernando Pereira, Pedro Cascales-Campos, Cristobal Muñoz-Casares, Enrique Asensio, Juan Torres-Melero, Arancha Prada-Villaverde, Ibán Caravaca-García, Alberto Gutiérrez-Calvo, Javier Vaqué, and et al. 2023. "Treatment of Peritoneal Surface Malignancies by Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Spain: Results of the National Registry of the Spanish Group of Peritoneal Oncologic Surgery (REGECOP)" Journal of Clinical Medicine 12, no. 11: 3774. https://doi.org/10.3390/jcm12113774
APA StyleManzanedo, I., Pereira, F., Cascales-Campos, P., Muñoz-Casares, C., Asensio, E., Torres-Melero, J., Prada-Villaverde, A., Caravaca-García, I., Gutiérrez-Calvo, A., Vaqué, J., Ortega, G., Titos-García, A., González-Sánchez, L., Pérez-Viejo, E., Serrano, Á., Martínez-Torres, B., & REGECOP Group. (2023). Treatment of Peritoneal Surface Malignancies by Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Spain: Results of the National Registry of the Spanish Group of Peritoneal Oncologic Surgery (REGECOP). Journal of Clinical Medicine, 12(11), 3774. https://doi.org/10.3390/jcm12113774







