Association between Preoperative 18-FDG PET-CT SUVmax and Next-Generation Sequencing Results in Postoperative Ovarian Malignant Tissue in Patients with Advanced Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- De Jong, D.; Otify, M.; Chen, I.; Jackson, D.; Jayasinghe, K.; Nugent, D.; Thangavelu, A.; Theophilou, G.; Laios, A. Survival and Chemosensitivity in Advanced High Grade Serous Epithelial Ovarian Cancer Patients with and without a BRCA Germline Mutation: More Evidence for Shifting the Paradigm towards Complete Surgical Cytoreduction. Medicina 2022, 58, 1611. [Google Scholar] [CrossRef] [PubMed]
- Harbin, L.M.; Gallion, H.H.; Allison, D.B.; Kolesar, J.M. Next Generation Sequencing and Molecular Biomarkers in Ovarian Cancer-An Opportunity for Targeted Therapy. Diagnostics 2022, 12, 842. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.Y.; Lu, Y. New developments in molecular targeted therapy of ovarian cancer. Discov. Med. 2018, 26, 219–229. [Google Scholar] [PubMed]
- Mittica, G.; Ghisoni, E.; Giannone, G.; Genta, S.; Aglietta, M.; Sapino, A.; Valabrega, G. PARP Inhibitors in Ovarian Cancer. Recent Pat. Anticancer Drug Discov. 2018, 13, 392–410. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef]
- Fonti, R.; Conson, M.; Del Vecchio, S. PET/CT in radiation oncology. Semin. Oncol. 2019, 46, 202–209. [Google Scholar] [CrossRef]
- Song, Z.; Wang, X.; Fu, J.; Wang, P.; Chen, X.; Zhang, D. Copenhagen index (CPH-I) is more favorable than CA125, HE4, and risk of ovarian malignancy algorithm (ROMA): Nomogram prediction models with clinical-ultrasonographic feature for diagnosing ovarian neoplasms. Front. Surg. 2022, 9, 1068492. [Google Scholar] [CrossRef]
- Tanizaki, Y.; Kobayashi, A.; Shiro, M.; Ota, N.; Takano, R.; Mabuchi, Y.; Yagi, S.; Minami, S.; Terada, M.; Ino, K. Diagnostic value of preoperative SUVmax on FDG-PET/CT for the detection of ovarian cancer. Int. J. Gynecol. Cancer 2014, 24, 454–460. [Google Scholar] [CrossRef]
- Della Corte, L.; Foreste, V.; Di Filippo, C.; Giampaolino, P.; Bifulco, G. Poly (ADP-ribose) polymerase (PARP) as target for the treatment of epithelial ovarian cancer: What to know. Expert Opin. Investig. Drugs 2021, 30, 543–554. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Gonzalez-Martin, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Rubinstein, M.M.; Makker, V. Optimizing immunotherapy for gynecologic cancers. Curr. Opin. Obstet. Gynecol. 2020, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Matsumoto, K.; Takehara, K.; Kawamura, N.; Hasegawa, K.; Takeshima, N.; Aoki, D.; Kamiura, S.; Arakawa, A.; Kondo, E.; et al. Pembrolizumab monotherapy in Japanese patients with advanced ovarian cancer: Subgroup analysis from the KEYNOTE-100. Cancer Sci. 2020, 111, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Feng, Z.; Wen, H.; Jiang, Z.; Pan, H.; Deng, Y.; Zhang, L.; Ju, X.; Chen, X.; Wu, X. (18)F-FDG PET/CT can predict chemosensitivity and proliferation of epithelial ovarian cancer via SUVmax value. Jpn. J. Radiol. 2018, 36, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.F.; Zhou, S.H.; Yu, Q. Predicting response to radiotherapy in tumors with PET/CT: When and how? Transl. Cancer Res. 2020, 9, 2972–2981. [Google Scholar] [CrossRef]
- Nakamura, K.; Hongo, A.; Kodama, J.; Hiramatsu, Y. The pretreatment of maximum standardized uptake values (SUVmax) of the primary tumor is predictor for poor prognosis for patients with epithelial ovarian cancer. Acta Med. Okayama 2012, 66, 53–60. [Google Scholar] [CrossRef]
- Liu, S.; Ju, X.; Feng, Z.; Wen, H.; Xu, J.; Chen, X.; Wu, X. Tumor-to-background ratios of the maximum standardized uptake value could not indicate the prognosis of advanced high-grade serous ovarian cancer patients. Nucl. Med. Commun. 2018, 39, 319–324. [Google Scholar] [CrossRef]
- Jiang, Y.; Hou, G.; Wu, F.; Zhu, Z.; Zhang, W.; Cheng, W. The maximum standardized uptake value and extent of peritoneal involvement may predict the prognosis of patients with recurrent ovarian cancer after primary treatment: A retrospective clinical study. Medicine 2020, 99, e19228. [Google Scholar] [CrossRef]
- Khurana, E.; Fu, Y.; Chakravarty, D.; Demichelis, F.; Rubin, M.A.; Gerstein, M. Role of non-coding sequence variants in cancer. Nat. Rev. Genet. 2016, 17, 93–108. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jo, K.; Hwang, S.H.; Jeong, Y.; Lee, J.Y.; Kim, S.; Kim, S.W.; Kim, Y.T.; Kang, W.J. Association between PD-L1 expression and (18)F-FDG uptake in ovarian cancer. Ann. Nucl. Med. 2021, 35, 415–420. [Google Scholar] [CrossRef]
- Kim, T.M.; Laird, P.W.; Park, P.J. The landscape of microsatellite instability in colorectal and endometrial cancer genomes. Cell 2013, 155, 858–868. [Google Scholar] [CrossRef]
- Orimo, H.; Nakajima, E.; Yamamoto, M.; Ikejima, M.; Emi, M.; Shimada, T. Association between single nucleotide polymorphisms in the hMSH3 gene and sporadic colon cancer with microsatellite instability. J. Hum. Genet. 2000, 45, 228–230. [Google Scholar] [CrossRef]
- Chang, G.H.; Kurzrock, R.; Tran, L.; Schwaederle, M.; Hoh, C.K. TP53 mutations and number of alterations correlate with maximum standardized uptake value (SUVmax) determined by positron emission tomography/computed tomography (PET/CT) [(18)F] fluorodeoxyglucose ((18)F-FDG PET). Oncotarget 2018, 9, 14306–14310. [Google Scholar] [CrossRef] [PubMed]
- Haghighat Jahromi, A.; Chang, G.; Kurzrock, R.; Hoh, C.K. Standardized uptake value (SUV(max)) in (18)F-FDG PET/CT is correlated with the total number of main oncogenic anomalies in cancer patients. Cancer Biol. Ther. 2020, 21, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, S.; Silan, F.; Akgun, M.Y.; Araci, N.; Cirpan, I.; Koc Ozturk, F.; Ozdemir, O. Prognostic Prediction of BRCA Mutations by (18)F-FDG PET/CT SUV(max) in Breast Cancer. Mol. Imaging Radionucl. Ther. 2021, 30, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Oh, J.S.; Lee, H.S.; Kim, J.S. Genetic alterations associated with (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in head and neck squamous cell carcinoma. Transl. Oncol. 2021, 14, 100988. [Google Scholar] [CrossRef]
- Morris, L.G.; Riaz, N.; Desrichard, A.; Senbabaoglu, Y.; Hakimi, A.A.; Makarov, V.; Reis-Filho, J.S.; Chan, T.A. Pan-cancer analysis of intratumor heterogeneity as a prognostic determinant of survival. Oncotarget 2016, 7, 10051–10063. [Google Scholar] [CrossRef]
- Haghighat Jahromi, A.; Zabel, M.; Okamura, R.; Hoh, C.K.; Kurzrock, R. Variant allele fraction of genomic alterations in circulating tumor DNA (%ctDNA) correlates with SUV(max) in PET scan. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 307–312. [Google Scholar]
- Burrell, R.A.; McGranahan, N.; Bartek, J.; Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013, 501, 338–345. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dang, H.; Wang, X.W. The significance of intertumor and intratumor heterogeneity in liver cancer. Exp. Mol. Med. 2018, 50, e416. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number of Patients |
|---|---|
| Age | |
| <50 | 10 (40%) |
| ≥50 | 15 (60%) |
| Stage | |
| IIIC | 18 (72%) |
| IV | 7 (28%) |
| Pelvic LN metastasis | |
| Yes | 10 (40%) |
| No | 15 (60%) |
| Para-aortic LN metastasis | |
| Yes | 12 (48%) |
| No | 13 (52%) |
| Recurrence | |
| Yes | 15 (60%) |
| No | 10 (40%) |
| Death | |
| Yes | 4 (16%) |
| No | 21 (84%) |
| Histopathology | |
| HGSC | 17 (68%) |
| Mucinous | 1 (4%) |
| Endometrioid | 1 (4%) |
| Clear cell | 2 (8%) |
| Carcinosarcoma | 4 (16%) |
| NGS Results (Total 25) | SUVmax (Mean ± SD) | p-Value |
|---|---|---|
| Variant not found (n = 2) | 8.55 ± 4.72 | p = 0.265 |
| Variant found (n = 23) | 12.27 ± 4.41 | |
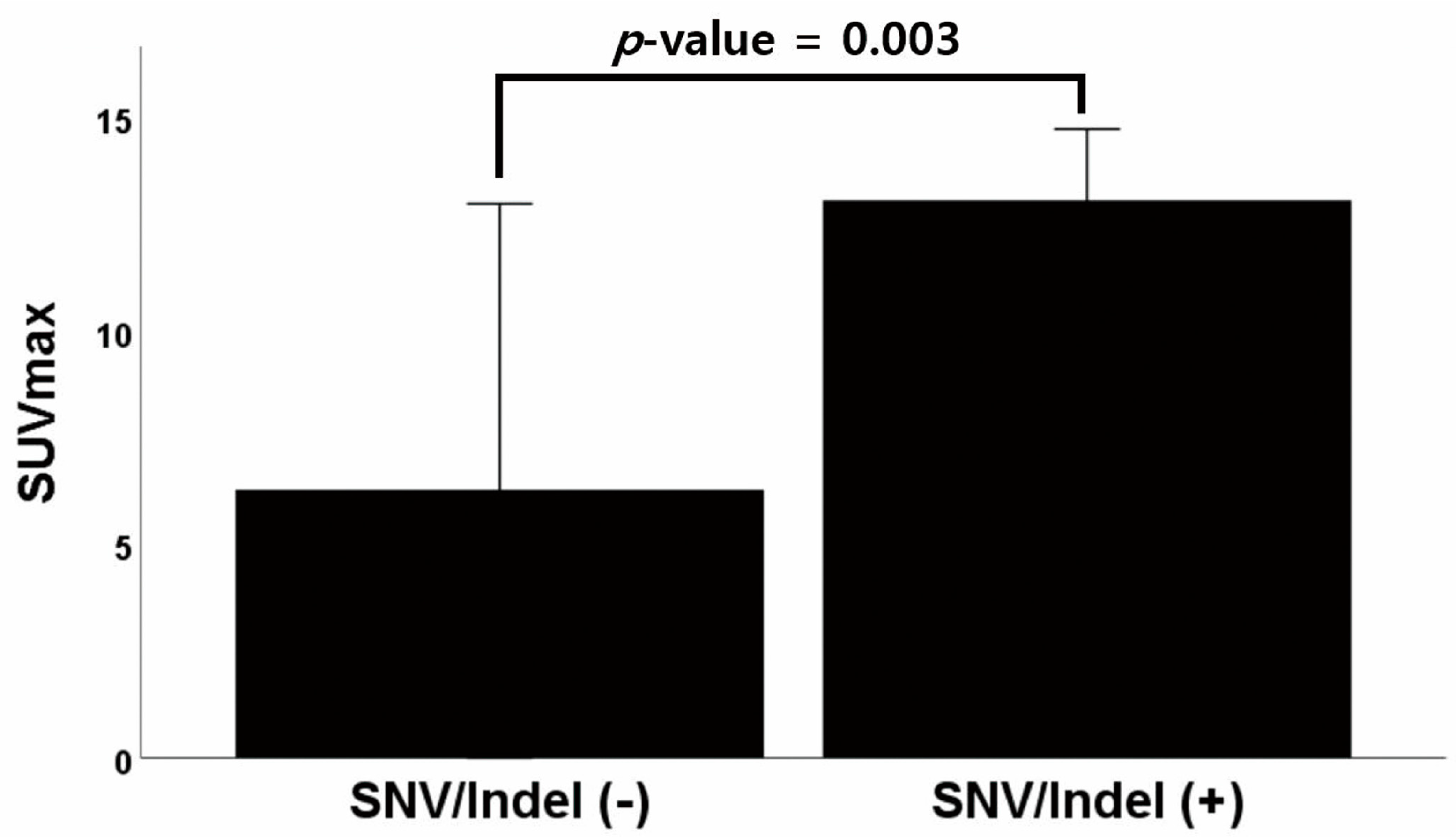
| SNV/Indel not found (n = 4) | 6.28 ± 4.21 | p = 0.003 |
| SNV/Indel found (n = 21) | 13.06 ± 3.66 | |
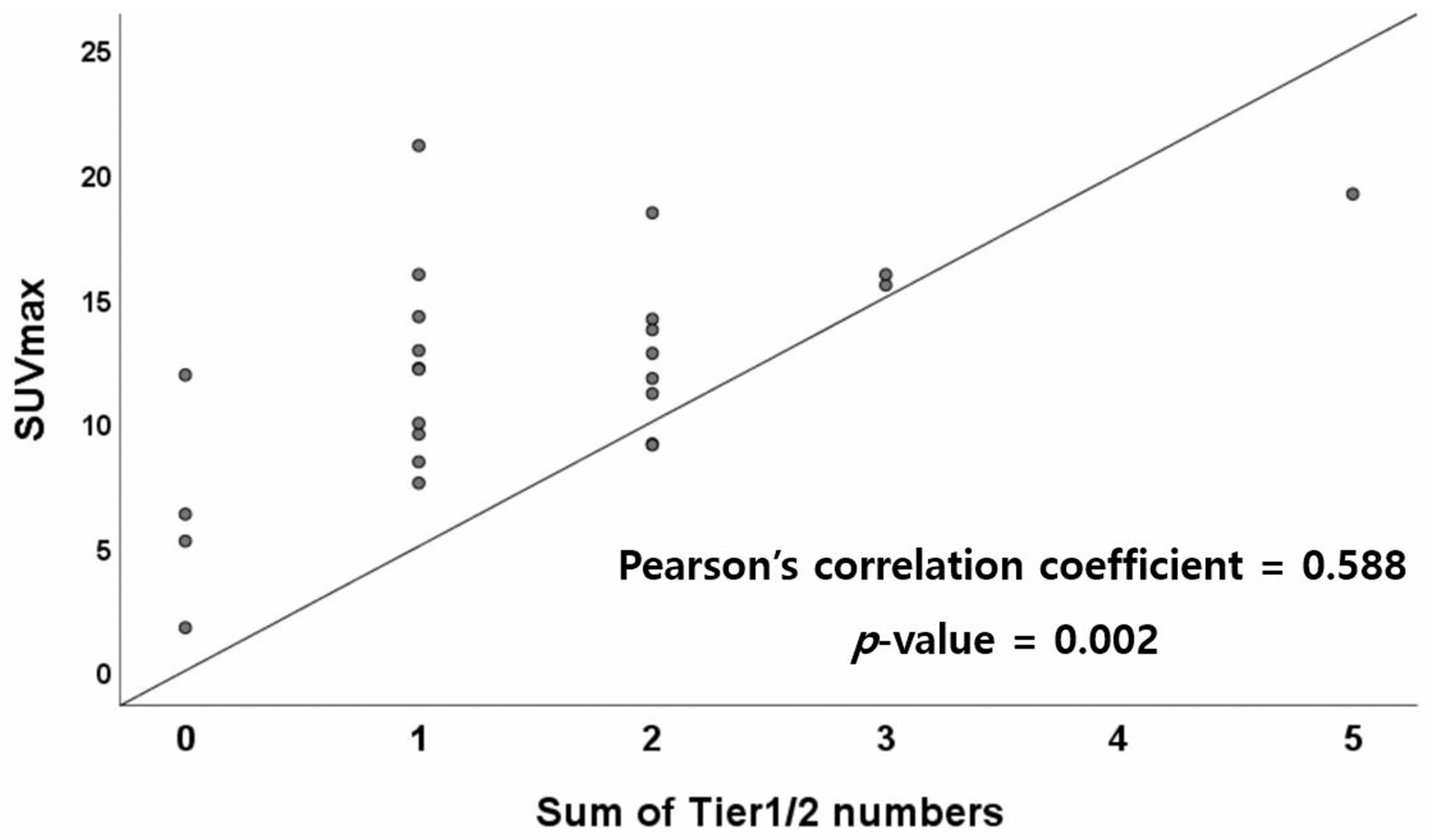
| Tier1 not found (n = 17) | 11.82 ± 5.08 | p = 0.804 |
| Tier1 found (n = 8) | 12.31 ± 3.00 | |
| BRCA 1/2 not found (n = 19) | 11.69 ± 4.83 | p = 0.582 |
| BRCA 1/2 found (n = 6) | 12.87 ± 3.18 | |
| Tier2 not found (n = 5) | 7.45 ± 4.49 | p = 0.008 |
| Tier2 found (n = 20) | 13.11 ± 3.75 | |
| TP53 not found (N = 8) | 9.35 ± 4.35 | p = 0.041 |
| TP53 found (n = 17) | 13.21 ± 4.06 | |
| Tier3 not found (N = 22) | 11.56 ± 4.46 | p = 0.220 |
| Tier3 found (n = 3) | 14.98 ± 3.69 | |
| CNV not found (n = 15) | 11.77 ± 3.72 | p = 0.789 |
| CNV found (n = 10) | 12.28 ± 5.59 | |
| Fusion not found (n = 23) | 11.79 ± 4.35 | p = 0.487 |
| Fusion found (n = 2) | 14.13 ± 7.10 |
| Gene Variants | n |
|---|---|
| Tier 1 | |
| KRAS | 2 (8%) |
| BRCA1 | 3 (12%) |
| BRCA2 | 3 (12%) |
| Tier 2 | |
| TP53 | 17 (68%) |
| PIK3CA | 2 (8%) |
| PTEN | 1 (4%) |
| FBXW7 | 1 (4%) |
| ESR1 | 1 (4%) |
| FGFR2 | 1 (4%) |
| SPOP | 1 (4%) |
| NOTCH3 | 1 (4%) |
| MSH3 | 1 (4%) |
| FGFR4 | 1 (4%) |
| NF1 | 1 (4%) |
| CREBBP | 1 (4%) |
| ARID1A | 1 (4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, J.M.; Jeong, Y.Y.; Lee, S.-J.; Choi, B.W.; Choi, Y.S. Association between Preoperative 18-FDG PET-CT SUVmax and Next-Generation Sequencing Results in Postoperative Ovarian Malignant Tissue in Patients with Advanced Ovarian Cancer. J. Clin. Med. 2023, 12, 2287. https://doi.org/10.3390/jcm12062287
Ryu JM, Jeong YY, Lee S-J, Choi BW, Choi YS. Association between Preoperative 18-FDG PET-CT SUVmax and Next-Generation Sequencing Results in Postoperative Ovarian Malignant Tissue in Patients with Advanced Ovarian Cancer. Journal of Clinical Medicine. 2023; 12(6):2287. https://doi.org/10.3390/jcm12062287
Chicago/Turabian StyleRyu, Jung Min, Yoon Young Jeong, Sun-Jae Lee, Byung Wook Choi, and Youn Seok Choi. 2023. "Association between Preoperative 18-FDG PET-CT SUVmax and Next-Generation Sequencing Results in Postoperative Ovarian Malignant Tissue in Patients with Advanced Ovarian Cancer" Journal of Clinical Medicine 12, no. 6: 2287. https://doi.org/10.3390/jcm12062287
APA StyleRyu, J. M., Jeong, Y. Y., Lee, S.-J., Choi, B. W., & Choi, Y. S. (2023). Association between Preoperative 18-FDG PET-CT SUVmax and Next-Generation Sequencing Results in Postoperative Ovarian Malignant Tissue in Patients with Advanced Ovarian Cancer. Journal of Clinical Medicine, 12(6), 2287. https://doi.org/10.3390/jcm12062287






