Artificial Liver Support with CytoSorb and MARS in Liver Failure: A Retrospective Propensity Matched Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Analysis of Biochemical Parameters
3.1.1. Single Sessions
3.1.2. Course of Treatment
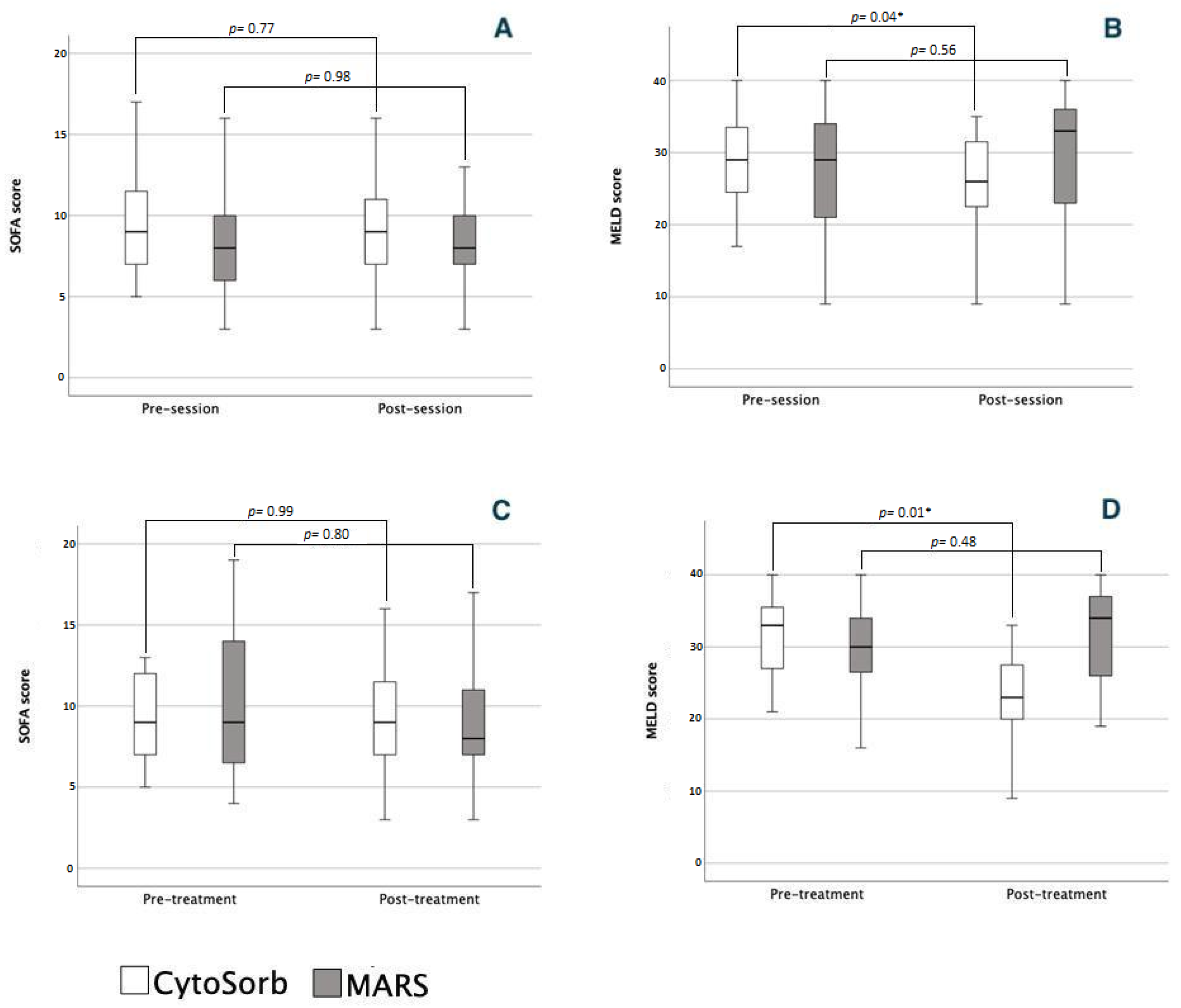
3.2. Severity Scores and Organ Dysfunction
3.2.1. Single Sessions
3.2.2. Course of Treatment
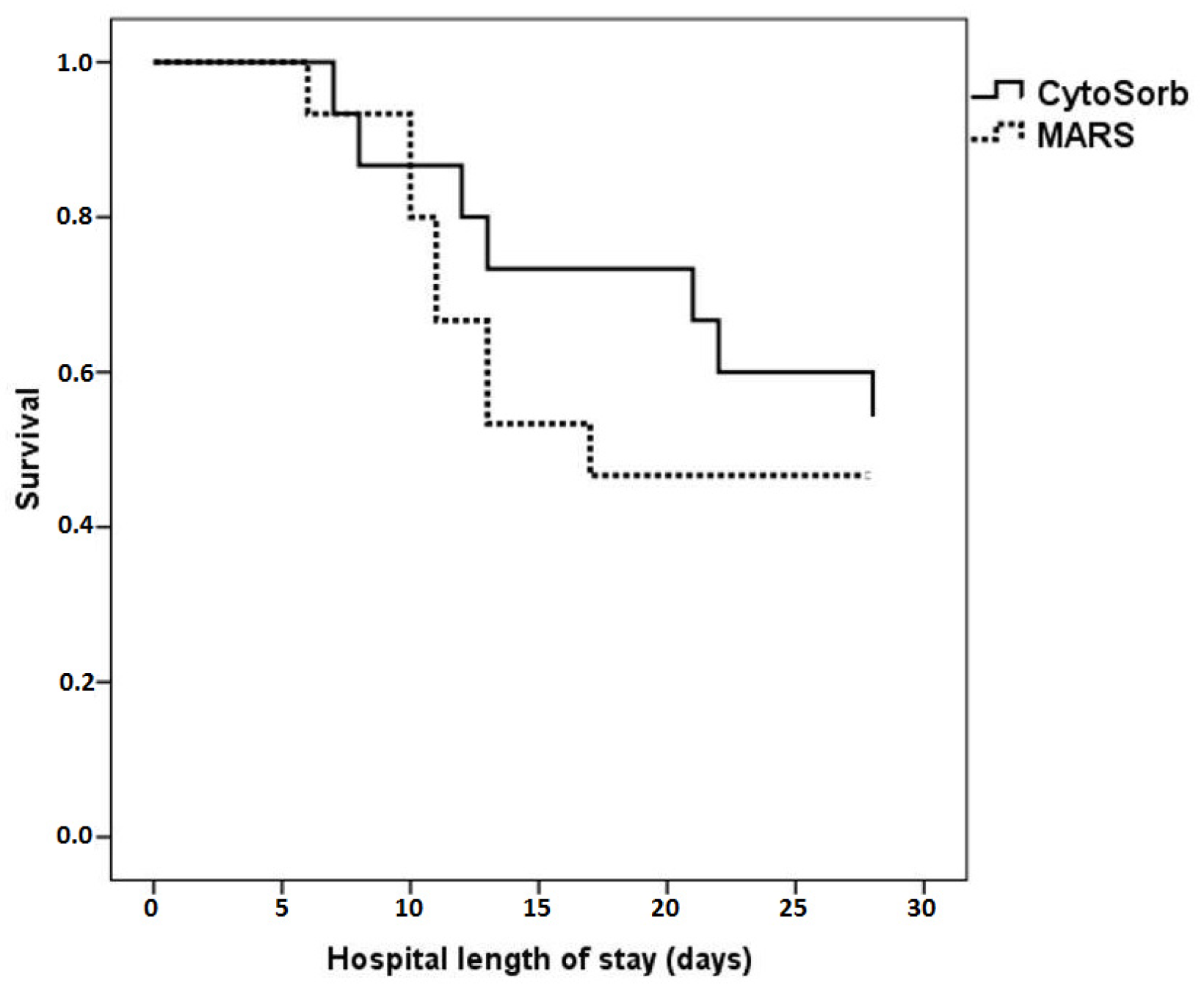
3.3. ICU Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernal, W.; Wendon, J. Acute Liver Failure. N. Engl. J. Med. 2013, 369, 2525–2534. [Google Scholar] [CrossRef]
- Grek, A.; Arasi, L. Acute Liver Failure. AACN Adv. Crit Care 2016, 27, 420–429. [Google Scholar] [CrossRef]
- Reuben, A.; Tillman, H.; Fontana, R.J.; Davern, T.; McGuire, B.; Stravitz, R.T.; Durkalski, V.; Larson, A.M.; Liou, I.; Fix, O.; et al. Outcomes in Adults with Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann. Int. Med. 2016, 164, 724–732. [Google Scholar] [CrossRef]
- Bernal, W.; Hyyrylainen, A.; Gera, A.; Audimoolam, V.K.; McPhail, M.J.; Auzinger, G.; Rela, M.; Heaton, N.; O’Grady, J.G.; Wendon, J.; et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J. Hepatol. 2013, 59, 74–80. [Google Scholar] [CrossRef]
- Ichai, P.; Samuel, D. Etiology and prognosis of fulminant hepatitis in adults. Liver Transplant. 2008, 14 (Suppl. 2), S67–S79. [Google Scholar] [CrossRef]
- Bernal, W.; Jalan, R.; Quaglia, A.; Simpson, K.; Wendon, J.; Burroughs, A. Acute-on-chronic liver failure. Lancet 2015, 386, 1576–1587. [Google Scholar] [CrossRef]
- Clària, J.; Arroyo, V.; Moreau, R. The Acute-on-Chronic Liver Failure Syndrome, or When the Innate Immune System Goes Astray. J. Immunol. 2016, 197, 3755–3761. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.-Y.; Chen, C.; Guan, J.; Zhu, H.-H.; Chen, Z. The immunological roles in acute-on-chronic liver failure: An update. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 403–411. [Google Scholar] [CrossRef]
- Wu, W.; Yan, H.; Zhao, H.; Sun, W.; Yang, Q.; Sheng, J.; Shi, Y. Characteristics of systemic inflammation in hepatitis B-precipitated ACLF: Differentiate it from No-ACLF. Liver Int. 2018, 38, 248–257. [Google Scholar] [CrossRef]
- Clària, J.; Stauber, R.E.; Coenraad, M.J.; Moreau, R.; Jalan, R.; Pavesi, M.; Amorós, À.; Titos, E.; Alcaraz-Quiles, J.; Oettl, K.; et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology 2016, 64, 1249–1264. [Google Scholar] [CrossRef]
- Markwick, L.J.; Riva, A.; Ryan, J.M.; Cooksley, H.; Palma, E.; Tranah, T.H.; Vijay, G.K.M.; Vergis, N.; Thursz, M.; Evans, A.; et al. Blockade of PD1 and TIM3 Restores Innate and Adaptive Immunity in Patients with Acute Alcoholic Hepatitis. Gastroenterology 2015, 148, 590–602.e10. [Google Scholar] [CrossRef]
- Bernsmeier, C.; Pop, O.T.; Singanayagam, A.; Triantafyllou, E.; Patel, V.; Weston, C.J.; Curbishley, S.; Sadiq, F.; Vergis, N.; Khamri, W.; et al. Patients with Acute-on-Chronic Liver Failure Have Increased Numbers of Regulatory Immune Cells Expressing the Receptor Tyrosine Kinase MERTK. Gastroenterology 2015, 148, 603–615.e14. [Google Scholar] [CrossRef] [PubMed]
- Wasmuth, H.E.; Kunz, D.; Yagmur, E.; Timmer-Stranghöner, A.; Vidacek, D.; Siewert, E.; Bach, J.; Geier, A.; Purucker, E.A.; Gressner, A.M.; et al. Patients with acute on chronic liver failure display ‘sepsis-like’ immune paralysis. J. Hepatol. 2005, 42, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Berres, M.-L.; Schnyder, B.; Yagmur, E.; Inglis, B.; Stanzel, S.; Tischendorf, J.J.W.; Koch, A.; Winograd, R.; Trautwein, C.; Wasmuth, H.E. Longitudinal monocyte Human leukocyte antigen-DR expression is a prognostic marker in critically ill patients with decompensated liver cirrhosis. Liver Int. 2009, 29, 536–543. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.J.; Fullerton, J.; Massey, K.A.; Auld, G.; Sewell, G.; James, S.; Newson, J.; Karra, E.; Winstanley, A.; Alazawi, W.; et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat. Med. 2014, 20, 518–523. [Google Scholar] [CrossRef]
- Huebener, P.; Sterneck, M.R.; Bangert, K.; Drolz, A.; Lohse, A.W.; Kluge, S.; Fischer, L.; Fuhrmann, V. Stabilisation of acute-on-chronic liver failure patients before liver transplantation predicts post-transplant survival. Aliment. Pharmacol. Ther. 2018, 47, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.M.; Saner, F.M.; Asrani, S.K.M.; Biancofiore, G.M.; Blasi, A.; Lerut, J.M.; Durand, F.M.; Fernandez, J.; Findlay, J.Y.M.; Fondevila, C.; et al. When Is a Critically Ill Cirrhotic Patient Too Sick to Transplant? Development of Consensus Criteria by a Multidisciplinary Panel of 35 International Experts. Transplantation 2021, 105, 561–568. [Google Scholar] [CrossRef]
- Arroyo, V.; Moreau, R.; Jalan, R.; Ginès, P. EASL-CLIF Consortium CANONIC Study. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J. Hepatol. 2015, 62 (Suppl. S1), S131–S143. [Google Scholar] [CrossRef]
- García Martínez, J.J.; Bendjelid, K. Artificial liver support systems: What is new over the last decade? Ann. Intensive Care. 2018, 8, 109. [Google Scholar] [CrossRef]
- Kanjo, A.; Ocskay, K.; Gede, N.; Kiss, S.; Szakács, Z.; Párniczky, A.; Mitzner, S.; Stange, J.; Hegyi, P.; Molnár, Z. Efficacy and safety of liver support devices in acute and hyperacute liver failure: A systematic review and network meta-analysis. Sci. Rep. 2021, 11, 4189. [Google Scholar] [CrossRef]
- Ocskay, K.; Kanjo, A.; Gede, N.; Szakács, Z.; Pár, G.; Erőss, B.; Stange, J.; Mitzner, S.; Hegyi, P.; Molnár, Z. Uncertainty in the impact of liver support systems in acute-on-chronic liver failure: A systematic review and network meta-analysis. Ann. Intensive Care 2021, 11, 10. [Google Scholar] [CrossRef]
- Zuccari, S.; Damiani, E.; Domizi, R.; Scorcella, C.; D’Arezzo, M.; Carsetti, A.; Pantanetti, S.; Vannicola, S.; Casarotta, E.; Ranghino, A.; et al. Changes in Cytokines, Haemodynamics and Microcirculation in Patients with Sepsis/Septic Shock Undergoing Continuous Renal Replacement Therapy and Blood Purification with CytoSorb. Blood Purif. 2020, 49, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ocskay, K.; Tomescu, D.; Faltlhauser, A.; Jacob, D.; Friesecke, S.; Malbrain, M.; Kogelmann, K.; Bogdanski, R.; Bach, F.; Fritz, H.; et al. Hemoadsorption in ‘Liver Indication’—Analysis of 109 Patients’ Data from the CytoSorb International Registry. J. Clin. Med. 2021, 10, 5182. [Google Scholar] [CrossRef] [PubMed]
- Mehta, Y.; Mehta, C.; Kumar, A.; George, J.V.; Gupta, A.; Nanda, S.; Kochhar, G.; Raizada, A. Experience with hemoadsorption (CytoSorb®) in the management of septic shock patients. World J. Crit. Care Med. 2020, 9, 1–12. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J. Hepatol. 2017, 66, 1047–1081. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with de-compensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [PubMed]
- Dhokia, V.D.; Madhavan, D.; Austin, A.; Morris, C.G. Novel use of Cytosorb™ haemadsorption to provide biochemical control in liver impairment. J. Intensiv. Care Soc. 2019, 20, 174–181. [Google Scholar] [CrossRef]
- Faltlhauser, A.; Kullmann, F. Use of Hemoadsorption in a Case of Severe Hepatic Failure and Hyperbilirubinemia. Blood Purif. 2017, 44, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Tomescu, D.R.; Dima, S.O.; Ungureanu, D.; Popescu, M.; Tulbure, D.; Popescu, I. First Report of Cytokine Removal using CytoSorb® in Severe Noninfectious Inflammatory Syndrome after Liver Transplantation. Int. J. Artif. Organs 2016, 39, 136–140. [Google Scholar] [CrossRef]
- Donnelly, M.C.; Hayes, P.C.; Simpson, K.J. Role of inflammation and infection in the pathogenesis of human acute liver failure: Clinical implications for monitoring and therapy. World J. Gastroenterol. 2016, 22, 5958–5970. [Google Scholar] [CrossRef]
- Novelli, G.; Rossi, M.; Pretagostini, M.; Pugliese, F.; Ruberto, F.; Novelli, L.; Nudo, F.; Bussotti, A.; Corradini, S.; Martelli, S.; et al. One Hundred Sixteen Cases of Acute Liver Failure Treated With MARS. Transplant. Proc. 2005, 37, 2557–2559. [Google Scholar] [CrossRef] [PubMed]
- Donati, G.; La Manna, G.; Cianciolo, G.; Grandinetti, V.; Carretta, E.; Cappuccilli, M.; Panicali, L.; Iorio, M.; Piscaglia, F.; Bolondi, L.; et al. Extracorporeal detoxification for hepatic failure using molecular adsorbent recirculating system: Depurative efficiency and clinical results in a long-term fol-low-up. Artif. Organs. 2014, 38, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.E.; Sørensen, V.R.; Svendsen, L.B.; Hansen, B.A.; Larsen, F.S. Hemodynamic changes during a single treatment with the molecular adsorbents recirculating system in patients with acute-on-chronic liver failure. Liver Transpl. 2001, 7, 1034–1039. [Google Scholar] [CrossRef]
- Steiner, C.; Mitzner, S. Experiences with MARS liver support therapy in liver failure: Analysis of 176 patients of the International MARS Registry. Liver Int. 2002, 22 (Suppl. 2), 20–25. [Google Scholar]
- Catalina, M.-V.; Barrio, J.; Anaya, F.; Salcedo, M.; Rincón, D.; Clemente, G.; Bañares, R. Hepatic and systemic haemodynamic changes after MARS in patients with acute on chronic liver failure. Liver Int. 2003, 23 (Suppl. 3), 39–43. [Google Scholar] [CrossRef]
- Sen, S.; Davies, N.A.; Mookerjee, R.P.; Cheshire, L.M.; Hodges, S.J.; Williams, R.; Jalan, R. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: A randomized controlled study. Liver Transpl. 2004, 10, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Krisper, P.; Aigner, R.; Haditsch, B.; Jung, A.; Lackner, C.; E Stauber, R. Effect of extracorporeal liver support by MARS and Prometheus on serum cytokines in acute-on-chronic liver failure. Crit. Care 2006, 10, R169. [Google Scholar] [CrossRef]
- Dominik, A.; Stange, J. Similarities, Differences, and Potential Synergies in the Mechanism of Action of Albumin Dialysis Using the MARS Albumin Dialysis Device and the CytoSorb Hemoperfusion Device in the Treatment of Liver Failure. Blood Purif. 2021, 50, 119–128. [Google Scholar] [CrossRef]
- Alharthy, A.; Faqihi, F.; Memish, Z.A.; Balhamar, A.; Nasim, N.; Shahzad, A.; Tamim, H.; Alqahtani, S.A.; Brindley, P.G.; Karakitsos, D. Continuous renal replacement therapy with the addition of CytoSorb cartridge in critically ill patients with COVID-19 plus acute kidney injury: A case-series. Artif. Organs 2021, 45, E101–E112. [Google Scholar] [CrossRef]
- Paul, R.; Sathe, P.; Kumar, S.; Prasad, S.; Aleem, M.; Sakhalvalkar, P. Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb®) in patients with sepsis and septic shock. World J. Crit. Care Med. 2021, 10, 22–34. [Google Scholar] [CrossRef]
- Scharf, R.E. Thrombocytopenia and Hemostatic Changes in Acute and Chronic Liver Disease: Pathophysiology, Clinical and Laboratory Features, and Management. J. Clin. Med. 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Droege, C.A.; Ernst, N.E.; Messinger, N.J.; Burns, A.M.; Mueller, E.W. Evaluation of Thrombocytopenia in Critically Ill Patients Re-ceiving Continuous Renal Replacement Therapy. Ann. Pharmacother. 2018, 52, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Tomescu, D.; Popescu, M.; David, C.; Sima, R.; Dima, S. Haemoadsorption by CytoSorb® in patients with acute liver failure: A case series. Int. J. Artif. Organs 2021, 44, 560–564. [Google Scholar] [CrossRef]
- Al-Dorzi, H.M.; Alhumaid, N.A.; Alwelyee, N.H.; Albakheet, N.M.; Nazer, R.; Aldakhil, S.K.; AlSaif, S.A.; Masud, N. Anemia, Blood Transfusion, and Filter Life Span in Critically Ill Patients Requiring Continuous Renal Replacement Therapy for Acute Kidney Injury: A Case-Control Study. Crit. Care Res. Pract. 2019, 2019, 3737083. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Vela, R.; Andrés-Esteban, E.M.; Gea-Caballero, V.; Sánchez-González, J.L.; Marcos-Neira, P.; Serrano-Lázaro, A.; Tirado-Anglés, G.; Ruiz-Rodríguez, J.C.; Durante, Á.; Santolalla-Arnedo, I.; et al. Related Factors of Anemia in Critically Ill Patients: A Prospective Multicenter Study. J. Clin. Med. 2022, 11, 1031. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Wang, F.-L.; Wu, M.-S.; Jiang, B.-Y.; Kao, W.-L.; Chao, H.-Y.; Wu, J.-Y.; Lee, C.-C. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: A systematic review and meta-analysis. Diagn. Microbiol. Infect. Dis. 2014, 80, 72–78. [Google Scholar] [CrossRef]
- Guo, L.-M.; Liu, J.-Y.; Xu, D.-Z.; Li, B.-S.; Han, H.; Wang, L.-H.; Zhang, W.-Y.; Lu, L.-H.; Guo, X.; Sun, F.-X.; et al. Application of Molecular Adsorbents Recirculating System to remove NO and cytokines in severe liver failure patients with multiple organ dysfunction syndrome. Liver Int. 2003, 23 (Suppl. 3), 16–20. [Google Scholar] [CrossRef]
- Chen, B.-H.; Tseng, H.-J.; Chen, W.-T.; Chen, P.-C.; Ho, Y.-P.; Huang, C.-H.; Lin, C.-Y. Comparing Eight Prognostic Scores in Predicting Mortality of Patients with Acute-On-Chronic Liver Failure Who Were Admitted to an ICU: A Single-Center Experience. J. Clin. Med. 2020, 9, 1540. [Google Scholar] [CrossRef]
- Schmidt, L.E.; Wang, L.P.; Hansen, B.A.; Larsen, F.S. Systemic hemodynamic effects of treatment with the molecular adsorbents recirculating system in patients with hyperacute liver failure: A prospective controlled trial. Liver Transpl. 2003, 9, 290–297. [Google Scholar] [CrossRef]
- Praktiknjo, M.; Monteiro, S.; Grandt, J.; Kimer, N.; Madsen, J.L.; Werge, M.P.; William, P.; Brol, M.J.; Turco, L.; Schierwagen, R.; et al. Cardiodynamic state is associated with systemic inflammation and fatal acute-on-chronic liver failure. Liver Int. 2020, 40, 1457–1466. [Google Scholar] [CrossRef]
- Garg, H.; Kumar, A.; Garg, V.; Kumar, M.; Kumar, R.; Sharma, B.C.; Sarin, S.K. Hepatic and systemic hemodynamic derangements predict early mortality and recovery in patients with acute-on-chronic liver failure. J. Gastroenterol. Hepatol. 2013, 28, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.S.; Abraldes, J.G.; Sy, E.; Ronco, J.J.; Bagulho, L.; Mcphail, M.J.; Karvellas, C.J. Lactate and number of organ failures predict intensive care unit mortality in patients with acute-on-chronic liver failure. Liver Int. 2019, 39, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Drolz, A.; Horvatits, T.; Rutter, K.; Landahl, F.; Roedl, K.; Meersseman, P.; Wilmer, A.; Kluwe, J.; Lohse, A.W.; Kluge, S.; et al. Lactate Improves Prediction of Short-Term Mortality in Critically Ill Patients with Cirrhosis: A Multinational Study. Hepatology 2019, 69, 258–269. [Google Scholar] [CrossRef] [PubMed]


| Parameter | CytoSorb Group (n = 15) | MARS Group (n = 15) | p Value |
|---|---|---|---|
| Age (years) | 37.4 ± 14.2 | 39.8 ± 14.8 | 0.72 |
| MELD Score | 31.9 ± 5.8 | 30.0 ± 5.4 | 0.31 |
| SOFA Score | 9.2 ± 2.8 | 8.2 ± 3.1 | 0.97 |
| APACHE II Score | 14.6 ± 7.4 | 14.5 ± 4.4 | 0.17 |
| Bilirubin (mg/dL) | 17.0 ± 9.7 | 20.8 ± 16.5 | 0.33 |
| Albumin (mg/dL) | 2.7 ± 0.5 | 2.9 ± 0.5 | 0.72 |
| INR | 3.2 ± 2.3 | 2.8 ± 1.1 | 0.32 |
| Lactate (mmol/L) | 2.8 ± 0.9 | 2.6 ± 1.3 | 0.30 |
| Grade IV HE | 33% (n = 5) | 40% (n = 6) | 0.79 |
| Need for MV (%) | 27% (n = 4) | 40% (n = 6) | 0.70 |
| CytoSorb Group | MARS Group | p Value between Groups | |||||
|---|---|---|---|---|---|---|---|
| Before Session | After Session | p Value | Before Session | After Session | p Value | ||
| Lactate (µmol/L) | 2.2 (0.1, 4.2) | 2.1 (0.8, 3.7) | 0.07 | 1.7 (0.8, 5.8) | 2.1 (0.8, 19.0) | 0.83 | 0.11 |
| Creatinine (mg/dL) | 0.8 (0.2, 6.0) | 0.7 (0.2, 5.1) | 0.38 | 0.5 (0.1, 3.3) | 0.5 (0.2, 2.1) | 0.47 | 0.98 |
| BUN (mg/dL) | 30 (3, 276) | 25 (2, 201) | 0.45 | 29 (11, 188) | 29 (7, 136) | 0.35 | 0.79 |
| Sodium (mmol/L) | 138 (125, 147) | 139 (123, 150) | 0.39 | 137 (121, 150) | 136 (124, 145) | 0.83 | 0.42 |
| INR | 2.6 (1.3, 11.2) | 2.4 (1.2, 5.0) | 0.46 | 2.3 (1.2, 5.4) | 2.8 (1.2, 13.2) | 0.24 | 0.02 |
| Albumin (g/dL) | 2.7 (1.7, 4.0) | 2.8 (1.8, 4.0) | 0.46 | 2.9 (1.17, 3.8) | 2.7 (1.9, 3.8) | 0.50 | 0.29 |
| AST (U/L) | 97 (24, 8219) | 90 (21, 3942) | 0.26 | 139 (35, 8219) | 130 (38, 5207) | 0.83 | 0.91 |
| ALT (U/L) | 142 (16, 8332) | 91 (16, 3735) | 0.38 | 114 (7, 7550) | 103 (18, 6412) | 0.71 | 0.93 |
| GGT (U/L) | 43 (17, 489) | 38 (13, 361) | 0.18 | 38 (16, 200) | 35 (8, 157) | 0.36 | 0.81 |
| Hemoglobin (g/dL) | 9.8 (6.7, 14.2) | 9.7 (7.2, 14.1) | 0.24 | 8.0 (6.9, 11.8) | 7.8 (7.0, 11.7) | 0.52 | 0.85 |
| WBC (×103/μL) | 10.0 (2.9, 34.2) | 9.9 (2.9, 25.3) | 0.48 | 8.2 (1.6, 16.7) | 9.1 (2.1, 27.1) | 0.90 | 0.38 |
| PCT (ng/mL) | 0.8 (0.0, 5.8) | 0.5 (0.0, 4.7) | 0.22 | 0.6 (0.1, 2.5) | 0.5 (0.2, 2.3) | 0.95 | 0.47 |
| CRP (mg/L) | 7.0 (0.5, 137.0) | 5.2 (0.5, 91.0) | 0.59 | 14 (2, 75) | 21 (1, 112) | 0.83 | 0.53 |
| Fibrinogen (mg/dL) | 141 (60, 387) | 142 (73, 232) | 0.94 | 125 (60, 286) | 108 (48, 123) | 0.07 | 0.16 |
| HCO3 (mmol/L) | 23 (17, 27) | 23 (21, 27) | 0.13 | 24 (14, 32) | 24 (11, 29) | 0.47 | 0.73 |
| SOFA CV | 0 (0, 4) | 0 (0, 4) | 0.75 | 0 (0, 4) | 0 (0, 4) | 0.62 | 0.91 |
| SOFA Resp | 1 (0, 3) | 1 (0, 3) | 0.95 | 2 (0, 3) | 1 (0, 3) | 0.80 | 0.85 |
| SOFA Coag | 2 (0, 3) | 2 (0, 3) | 0.10 | 2 (0, 3) | 2 (0, 4) | 0.45 | 0.45 |
| SOFA Liver | 3 (2, 4) | 3 (1, 4) | 0.10 | 4 (0, 4) | 4 (0, 4) | 0.84 | 0.72 |
| SOFA Renal | 0 (0, 4) | 0 (0, 4) | 0.37 | 0 (0, 3) | 0 (0, 3) | 0.84 | 0.21 |
| SOFA CNS | 1 (0, 4) | 1 (0, 4) | 0.65 | 0 (0, 4) | 0 (0, 4) | 0.85 | 0.72 |
| GCS (points) | 12 (3, 15) | 13 (3, 15) | 0.59 | 15 (3, 15) | 15 (2, 15) | 0.98 | 0.09 |
| MAP (mmHg) | 75 (68, 97) | 75 (68, 92) | 0.94 | 83 (68, 104) | 81 (61, 105) | 0.77 | 0.32 |
| HR (bpm) | 83 (52, 125) | 82 (52, 115) | 0.34 | 87 (52, 109) | 86 (56, 128) | 0.74 | 0.64 |
| CytoSorb Group | MARS Group | p Value between Groups | |||||
|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | p Value | Before Treatment | After Treatment | p Value | ||
| Creatinine (mg/dL) | 1.0 (0.2, 6.0) | 0.7 (0.4, 2.1) | 0.18 | 0.7 (0.2, 3.3) | 0.6 (0.2, 1.8) | 0.48 | 0.55 |
| BUN (mg/dL) | 60 (3, 276) | 27 (2, 94) | 0.11 | 29 (11, 188) | 32 (7, 109) | 0.50 | 0.15 |
| Sodium (mmol/L) | 138 (125, 141) | 140 (126, 150) | 0.21 | 137 (126, 150) | 137 (124, 145) | 0.95 | 0.18 |
| INR | 2.5 (1.5, 11.2) | 2.1 (1.2, 3.4) | 0.12 | 2.6 (1.4, 5.4) | 2.8 (1.2, 13.2) | 0.49 | 0.04 |
| Albumin (g/dL) | 2.7 (1.7, 3.6) | 3.0 (2.0, 4.0) | 0.10 | 3.1 (2.1, 3.7) | 2.7 (1.9, 3.8) | 0.53 | 0.08 |
| AST (U/L) | 98 (46, 8219) | 56 (21, 3942) | 0.05 | 133 (35, 8219) | 123 (38, 2703) | 0.59 | 0.85 |
| ALT (U/L) | 257 (31, 8332) | 85 (20, 1043) | 0.12 | 108 (7, 7550) | 94 (18, 2639) | 0.56 | 0.71 |
| GGT (U/L) | 58 (28, 489) | 33 (13, 309) | 0.02 | 38 (16, 200) | 35 (8, 157) | 0.43 | 0.31 |
| Hemoglobin (g/dL) | 10.6 (6.7, 14.2) | 9.0 (7.2, 12.1) | 0.03 | 8.6 (6.9, 11.4) | 8.3 (7.0, 11.5) | 0.32 | 0.01 |
| WBC (×103/μL) | 12.3 (6.8, 34.2) | 10.9 (2.9, 20.4) | 0.22 | 9.0 (1.6, 13.7) | 9.1 (2.1, 27.1) | 0.91 | 0.14 |
| PCT (ng/mL) | 0.9 (0.1, 5.8) | 0.4 (0.0, 4.0) | 0.12 | 0.6 (0.1, 2.5) | 0.5 (0.2, 2.2) | 0.74 | 0.42 |
| CRP (mg/L) | 12.0 (0.5, 137.0) | 5.3 (1.2, 90.0) | 0.49 | 14.0 (2.1, 56.0) | 23.0 (1.8, 112.0) | 0.64 | 0.62 |
| Fibrinogen (mg/dL) | 145 (83, 387) | 146 (74, 232) | 0.74 | 125 (82, 286) | 86 (48, 229) | 0.01 | 0.38 |
| HCO3 (mmol/L) | 21 (17, 27) | 23 (21, 27) | 0.02 | 23 (14, 32) | 24 (11, 29) | 0.50 | 0.35 |
| SOFA CV | 0 (0, 0) | 0 (0, 4) | 0.90 | 0 (0, 4) | 0 (0, 4) | 0.99 | 0.38 |
| SOFA Resp | 1 (0, 3) | 2 (0, 2) | 0.93 | 2 (0, 3) | 1 (0, 3) | 0.30 | 0.57 |
| SOFA Coag | 1 (0, 3) | 3 (1, 3) | 0.01 | 2 (0, 3) | 2 (1, 4) | 0.38 | 0.12 |
| SOFA Liver | 4 (2, 4) | 3 (1, 4) | 0.02 | 4 (2, 4) | 4 (0, 4) | 0.96 | 0.13 |
| SOFA Renal | 0 (0, 4) | 0 (0, 4) | 0.23 | 0 (0, 3) | 0 (0, 3) | 0.80 | 0.56 |
| SOFA CNS | 1 (1, 4) | 1 (0, 4) | 0.74 | 1 (0, 4) | 0 (0, 4) | 0.80 | 0.93 |
| GCS (points) | 13 (3, 14) | 14 (3, 15) | 0.62 | 14 (3, 15) | 15 (3, 15) | 0.87 | 0.85 |
| MAP (mmHg) | 72 (68, 97) | 75 (68, 87) | 0.64 | 85 (70, 104) | 81 (61, 85) | 0.08 | 0.12 |
| HR (bpm) | 83 (65, 125) | 82 (55, 95) | 0.28 | 87 (67, 109) | 86 (56, 124) | 0.87 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, M.; David, C.; Marcu, A.; Olita, M.R.; Mihaila, M.; Tomescu, D. Artificial Liver Support with CytoSorb and MARS in Liver Failure: A Retrospective Propensity Matched Analysis. J. Clin. Med. 2023, 12, 2258. https://doi.org/10.3390/jcm12062258
Popescu M, David C, Marcu A, Olita MR, Mihaila M, Tomescu D. Artificial Liver Support with CytoSorb and MARS in Liver Failure: A Retrospective Propensity Matched Analysis. Journal of Clinical Medicine. 2023; 12(6):2258. https://doi.org/10.3390/jcm12062258
Chicago/Turabian StylePopescu, Mihai, Corina David, Alexandra Marcu, Mihaela Roxana Olita, Mariana Mihaila, and Dana Tomescu. 2023. "Artificial Liver Support with CytoSorb and MARS in Liver Failure: A Retrospective Propensity Matched Analysis" Journal of Clinical Medicine 12, no. 6: 2258. https://doi.org/10.3390/jcm12062258
APA StylePopescu, M., David, C., Marcu, A., Olita, M. R., Mihaila, M., & Tomescu, D. (2023). Artificial Liver Support with CytoSorb and MARS in Liver Failure: A Retrospective Propensity Matched Analysis. Journal of Clinical Medicine, 12(6), 2258. https://doi.org/10.3390/jcm12062258






