Cognitive Dysfunctions Measured with the MCCB in Deficit and Non-Deficit Schizophrenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Assessment
- -
- Speed of processing—Trail Making Test (TMT: Part A), Brief Assessment of Cognition in Schizophrenia (BACS), and the Category Fluency Test: Animal Naming (CF);
- -
- Attention/vigilance—Continuous Performance Test—Identical Pairs (CPT-IP);
- -
- Working memory—Letter-Number Span (LNS) and Wechsler Memory Scale-III (WMS III);
- -
- Verbal learning and memory—Hopkins Verbal Learning Test-Revised (HVLT-R);
- -
- Visual learning and memory—Brief Visuospatial Memory Test-Revised (BVMT-R);
- -
- Reasoning and problem solving—Neuropsychological Assessment Battery (NAB): Mazes.
- -
- Social cognition—Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT).
2.3. Clinical Assessment
2.4. Statistical Analysis
3. Results
3.1. Demographic, Psychological and Clinical Characteristics
3.2. Differences in Cognitive Domains
3.3. Relationships between Psychopathological Dimensions and Cognitive Domains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fioravanti, M.; Bianchi, V.; Cinti, M.E. Cognitive deficits in schizophrenia: An updated metanalysis of the scientific evidence. BMC Psychiatry 2012, 12, e64. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, G.; Jin, H.; Lyu, H.; Liu, Y.; Guo, W.; Shi, C.; Meyers, J.; Wang, J.; Zhao, J.; et al. Cognitive deficits in subjects at risk for psychosis, first-episode and chronic schizophrenia patients. Psychiatry Res. 2019, 274, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Akdede, B.; Alptekin, K. Neurocognitive impairment in deficit and non-deficit schizophrenia: A meta-analysis. Psychol. Med. 2017, 47, 2401–2413. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Mucci, A.; Galderisi, S. Primary, enduring negative symptoms: An update on research. Schizophr. Bull. 2017, 43, 730–736. [Google Scholar] [CrossRef]
- Carpenter, W.T.; Heinrichs, D.W.; Wagman, A.M. Deficit and nondeficit forms of schizophrenia: The concept. Am. J. Psychiatry 1998, 145, 578–583. [Google Scholar] [CrossRef]
- Voineskos, A.N.; Foussias, G.; Lerch, J.; Felsky, D.; Remington, G.; Rajji, T.K.; Lobaugh, N.; Pollock, B.G.; Mulsant, B.H. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA Psychiatry 2013, 70, 472–480. [Google Scholar] [CrossRef]
- Podwalski, P.; Szczygieł, K.; Tyburski, E.; Sagan, L.; Misiak, B.; Samochowiec, J. Magnetic resonance diffusion tensor imaging in psychiatry: A narrative review of its potential role in diagnosis. Pharmacol. Rep. 2021, 73, 43–56. [Google Scholar] [CrossRef]
- Réthelyi, J.M.; Czobor, P.; Polgár, P.; Mersich, B.; Bálint, S.; Jekkel, E.; Magyar, K.; Mészáros, A.; Fábián, A.; Bitter, I. General and domain-specific neurocognitive impairments in deficit and non-deficit schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 107–115. [Google Scholar] [CrossRef]
- Cohen, A.S.; Saperstein, A.M.; Gold, J.M.; Kirkpatrick, B.; Carpenter, W.T., Jr.; Buchanan, R.W. Neuropsychology of the deficit syndrome: New data and meta-analysis of findings to date. Schizophr. Bull. 2007, 33, 1201–1212. [Google Scholar] [CrossRef]
- Brazo, P.; Marié, R.M.; Halbecq, I.; Benali, K.; Segard, L.; Delamillieure, P.; Langlois-Théry, S.; Van Der Elst, A.; Thibaut, F.; Petit, M.; et al. Cognitive patterns in subtypes of schizophrenia. Eur. Psychiatry 2002, 17, 155–162. [Google Scholar] [CrossRef]
- Buchanan, R.W.; Strauss, M.E.; Kirkpatrick, B.; Holstein, C.; Breier, A.; Carpenter, W.T., Jr. Neuropsychological impairments in deficit vs nondeficit forms of schizophrenia. Arch. Gen. Psychiatry 1994, 51, 804–811. [Google Scholar] [CrossRef]
- Horan, W.P.; Blanchard, J.J. Neurocognitive, social, and emotional dysfunction in deficit syndrome schizophrenia. Schizophr. Res. 2003, 65, 125–137. [Google Scholar] [CrossRef]
- Polgár, P.; Farkas, M.; Nagy, O.; Kelemen, O.; Réthelyi, J.; Bitter, I.; Myers, C.E.; Gluck, M.A.; Kéri, S. How to find the way out from four rooms? The learning of “chaining” associations may shed light on the neuropsychology of the deficit syndrome of schizophrenia. Schizophr. Res. 2008, 99, 200–207. [Google Scholar] [CrossRef]
- Polgár, P.; Réthelyi, J.M.; Bálint, S.; Komlósi, S.; Czobor, P.; Bitter, I. Executive function in deficit schizophrenia: What do the dimensions of the Wisconsin Card Sorting Test tell us? Schizophr. Res. 2010, 122, 85–93. [Google Scholar] [CrossRef]
- Wang, X.; Yao, S.; Kirkpatrick, B.; Shi, C.; Yi, J. Psychopathology and neuropsychological impairments in deficit and nondeficit schizophrenia of Chinese origin. Psychiatry Res. 2008, 158, 195–205. [Google Scholar] [CrossRef]
- Tyburski, E.; Pełka-Wysiecka, J.; Mak, M.; Samochowiec, A.; Bieńkowski, P.; Samochowiec, J. Neuropsychological Profile of Specific Executive Dysfunctions in Patients with Deficit and Non-deficit Schizophrenia. Front. Psychol. 2017, 8, e1459. [Google Scholar] [CrossRef]
- Cohen, A.S.; Docherty, N.M. Deficit versus negative syndrome in schizophrenia: Prediction of attentional impairment. Schizophr. Bull. 2004, 30, 827–835. [Google Scholar] [CrossRef][Green Version]
- Cascella, N.G.; Testa, S.M.; Meyer, S.M.; Rao, V.A.; Diaz-Asper, C.M.; Pearlson, G.D.; Schretlen, D.J. Neuropsychological impairment in deficit vs. non-deficit schizophrenia. J. Psychiatr. Res. 2008, 42, 930–937. [Google Scholar] [CrossRef]
- Pegoraro, L.F.; Dantas, C.R.; Banzato, C.E.; Fuentes, D. Correlation between insight dimensions and cognitive functions in patients with deficit and nondeficit schizophrenia. Schizophr. Res. 2013, 147, 91–94. [Google Scholar] [CrossRef]
- Beck, A.T.; Grant, P.M.; Huh, G.A.; Perivoliotis, D.; Chang, N.A. Dysfunctional attitudes and expectancies in deficit syndrome schizophrenia. Schizophr. Bull. 2013, 39, 43–51. [Google Scholar] [CrossRef]
- Bryson, G.; Whelahan, H.A.; Bell, M. Memory and executive function impairments in deficit syndrome schizophrenia. Psychiatry Res. 2001, 102, 29–37. [Google Scholar] [CrossRef]
- Seckinger, R.A.; Goudsmit, N.; Coleman, E.; Harkavy-Friedman, J.; Yale, S.; Rosenfield, P.J.; Malaspina, D. Olfactory identification and WAIS-R performance in deficit and nondeficit schizophrenia. Schizophr. Res. 2004, 69, 55–65. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, W.; Zhong, N.; Jiang, H.; Du, J.; Li, Y.; Ma, X.; Zhao, M.; Hashimoto, K.; Gao, C. Impaired processing speed and attention in first-eIe drug naive schizophrenia with deficit syndrome. Schizophr. Res. 2014, 159, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Maj, M.; Mucci, A.; Cassano, G.B.; Invernizzi, G.; Rossi, A.; Vita, A.; Dell’Osso, L.; Daneluzzo, E.; Pini, S. Historical, psychopathological, neurological, and neuropsychological aspects of deficit schizophrenia: A multicenter study. Am. J. Psychiatry 2002, 159, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.; Sherman, E.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Ommentary; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Yu, M.; Tang, X.; Wang, X.; Zhang, X.; Zhang, X.; Sha, W.; Shu, N.; Zhang, X.Y.; Zhang, Z. Neurocognitive impairments in deficit and non-deficit schizophrenia and their relationships with symptom dimensions and other clinical variables. PLoS ONE 2015, 10, e0138357. [Google Scholar] [CrossRef] [PubMed]
- Fervaha, G.; Agid, O.; Foussias, G.; Siddiqui, I.; Takeuchi, H.; Remington, G. Neurocognitive impairment in the deficit subtype of schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 397–407. [Google Scholar] [CrossRef]
- Bryson, G.; Bell, M.; Kaplan, E.; Greig, T.; Lysaker, P. Affect recognition in deficit syndrome schizophrenia. Psychiatry Res. 1998, 77, 113–120. [Google Scholar] [CrossRef]
- Strauss, G.P.; Jetha, S.S.; Ross, S.A.; Duke, L.A.; Allen, D.N. Impaired faabellingect labeling and discrimination in patients with deficit syndrome schizophrenia. Schizophr. Res. 2010, 118, 146–153. [Google Scholar] [CrossRef]
- Tang, X.W.; Yu, M.; Duan, W.W.; Zhang, X.R.; Sha, W.W.; Wang, X.; Zhang, X.B. Facial emotion recognition and alexithymia in Chinese male patients with deficit schizophrenia. Psychiatry Res. 2016, 246, 353–359. [Google Scholar] [CrossRef]
- Eack, S.M.; Greeno, C.G.; Pogue-Geile, M.F.; Newhill, C.E.; Hogarty, G.E.; Keshavan, M.S. Assessing social-cognitive deficits in schizophrenia with the Mayer-Salovey-Caruso Emotional Intelligence Test. Schizophr. Bull. 2010, 36, 370–380. [Google Scholar] [CrossRef]
- Harvey, P.D.; Koren, D.; Reichenberg, A.; Bowie, C.R. Negative symptoms and cognitive deficits: What is the nature of their relationship? Schizophr. Bull. 2006, 32, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Dibben, C.R.; Rice, C.; Laws, K.; McKenna, P.J. Is executive impairment associated with schizophrenic syndromes? A meta-analysis. Psychol. Med. 2009, 39, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenstein, M.R.; Aleman, A.; De Haan, E.H. Relationship between symptom dimensions and neurocognitive functioning in schizophrenia: A meta-analysis of WCST and CPT studies. J. Psychiatr. Res. 2001, 35, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.; Crawford, J. A meta-analytic review of verbal fluency deficits in schizophrenia relative to other neurocognitive deficits. Cogn. Neuropsychiatry 2005, 10, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Jędrasik-Styła, M.; Ciołkiewicz, A.; Styła, R.; Linke, M.; Parnowska, D.; Gruszka, A.; Denisiuk, M.; Jarema, M.; Green, M.F.; Wichniak, A. The Polish academic version of the MATRICS Consensus Cognitive Battery (MCCB): Evaluation of psychometric properties. Psychiatr. Q. 2015, 8, 435–447. [Google Scholar] [CrossRef]
- Nuechterlein, K.H.; Green, M.F.; Kern, R.S.; Baade, L.E.; Barch, D.M.; Cohen, J.D.; Essock, S.; Fenton, W.S.; Frese, F.J., 3rd; Gold, J.M.; et al. The MATRICS Consensus Cognitive Battery, part 1: Test selection, reliability, and validity. Am. J. Psychiatry 2008, 165, 203–213. [Google Scholar] [CrossRef]
- Shafer, A.; Dazzi, F. Meta-analysis of the positive and Negative Syndrome Scale (PANSS) factor structure. J. Psychiatr. Res. 2019, 115, 113–120. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar]
- Podwalski, P.; Tyburski, E.; Szczygieł, K.; Waszczuk, K.; Rek-Owodziń, K.; Mak, M.; Plichta, P.; Bielecki, M.; Rudkowski, K.; Kucharska-Maalr, J.; et al. White Matter Integrity of the Corpus Callosum and Psychopathological Dimensions in Deficit and Non-Deficit Schizophrenia Patients. J. Clin. Med. 2021, 10, e2225. [Google Scholar] [CrossRef]
- Brzeziński, J.; Gaul, M.; Hornowska, E.; Jaworowska, A.; Machowski, A.; Zakrzewska, M. Wechsler Adult Intelli–Ence Scale-Revised. Polish Normalization; Psychological Test Laboratory of the Polish Psychological Association: Warsaw, Poland, 2004. [Google Scholar]
- Khandaker, G.M.; Barnett, J.H.; White, I.R.; Jones, P.B. A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr. Res. 2011, 132, 220–227. [Google Scholar] [CrossRef]
- Missar, C.D.; Gold, J.M.; Goldberg, T.E. WAIS-R short forms in chronic schizophrenia. Schizophr. Res. 1994, 12, 247–250. [Google Scholar] [CrossRef]
- Russell, A.J.; Munro, J.; Jones, P.B.; Hayward, P.; Hemsley, D.R.; Murray, R.M. The National Adult Reading Test as a measure of premorbid IQ in schizophrenia. Br. J. Clin. Psychol. 2000, 39, 297–305. [Google Scholar] [CrossRef]
- Blyler, C.R.; Gold, J.M.; Iannone, V.N.; Buchanan, R.W. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr. Res. 2000, 46, 209–215. [Google Scholar] [CrossRef]
- Miller, H.R.; Streiner, D.L.; Goldberg, J.O. Short, shorter, shortest: The efficacy of WAIS-R short forms with mixed psychiatric patients. Assessment 1996, 3, 165–169. [Google Scholar] [CrossRef]
- Christensen, B.K.; Girard, T.A.; Bagby, R.M. Wechsler Adult Intelligence Scale-short form for index and IQ scores in a psychiatric population. Psychol. Assess. 2007, 19, 236–240. [Google Scholar] [CrossRef]
- Sumiyoshi, C.; Fujino, H.; Sumiyoshi, T.; Yasuda, Y.; Yamamori, H.; Ohi, K.; Fujimoto, M.; Takeda, M.; Hashimoto, R. Usefulness of the Wechsler Intelligence Scale short form for assessing functional outcomes in patients with schizophrenia. Psychiatry Res. 2016, 245, 371–378. [Google Scholar] [CrossRef]
- Bulzacka, E.; Meyers, J.E.; Boyer, L.; Le Gloahec, T.; Fond, G.; Szöke, A.; Leboyer, M.; Schürhoff, F. WAIS-IV seven-subtest short form: Validity and clinical use in schizophrenia. Arch. Clin. Neuropsychol. 2016, 31, 915–925. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Rzewuska, M. Validity and reliability of the Polish version of the Positive and Negative Syndrome Scale (PANSS). Int. J. Methods Psychiatr. Res. 2002, 11, 27–32. [Google Scholar] [CrossRef]
- Tatsumi, K.; Kirkpatrick, B.; Strauss, G.P.; Opler, M. The Brief Negative Symptom Scale in Translation: A Review of Psychometric Properties and Beyond. Eur. Neuropsychopharmacol. 2020, 33, 36–44. [Google Scholar] [CrossRef]
- Dollfus, S.; Mach, C.; Morello, R. Self-Evaluation of Negative Symptoms. Schizophr. Bull. 2016, 42, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.C. Global Assessment of Functioning: A Modified Scale. Psychosomatics 1995, 36, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson Educational International: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Sakia, R.M. The Box-Cox transformation technique: A review. J. R. Stat. Soc. 1992, 41, 169–178. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Perugini, M.; Gallucci, M.; Costantini, G. A practical primer to power analysis for simple experimental designs. Int. Rev. Soc. Psychol. 2018, 31, 1–23. [Google Scholar] [CrossRef]
- Leucht, S.; Samara, M.; Heres, S.; Davis, J.M. Dose equivalents for antipsychotic drugs: The DDD method. Schizophr. Bull. 2016, 42, 90–94. [Google Scholar] [CrossRef]
- Buczylowska, D.; Petermann, F. Age-related differences and heterogeneity in executive functions: Analysis of NAB executive functions module scores. Arch. Clin. Neuropsychol. 2016, 31, 254–262. [Google Scholar] [CrossRef]
- Li, W.; Zhou, F.C.; Zhang, L.; Ng, C.H.; Ungvari, G.S.; Li, J.; Xiang, Y.T. Comparison of cognitive dysfunction between schizophrenia and bipolar disorder patients: A meta-analysis of comparative studies. J. Affect. Disord. 2020, 274, 652–661. [Google Scholar] [CrossRef]
- Tiryaki, A.; Yazıcı, M.K.; Anıl, A.E.; Kabakçı, E.; Karaağaoğlu, E.; Göğüş, A. Reexamination of the characteristics of the deficit schizophrenia patients. Eur. Arch. Psychiatry Clin. Neurosci. 2003, 253, 221–227. [Google Scholar] [CrossRef]
- Girard, R.; Météreau, E.; Thomas, J.; Pugeat, M.; Qu, C.; Dreher, J.C. Hormone therapy at early post-menopause increases cognitive control-related prefrontal activity. Sci. Rep. 2017, 7, e44917. [Google Scholar] [CrossRef]
| Deficit Schizophrenia Patients (DS) (n = 29) | Non-Deficit Schizophrenia Patients (NDS) (n = 45) | Healthy Controls (HC) (n = 39) | F/χ2/t | ɳ2/V/d | |
|---|---|---|---|---|---|
| Age: M (SD) | 38.59 (6.17) | 39.16 (7.21) | 37.08 (7.94) | 0.90 c | 0.02 f |
| Years of education: M (SD) | 12.66 (3.24) i * | 13.53 (2.64) | 14.59 (2.62) | 4.06 c * | 0.07 f |
| Sex: female/male | 7/22 | 24/21 | 23/16 | 9.01 d * | 0.28 g |
| Premorbid IQ in WAIS-R-IV: | |||||
| Picture Completion: M (SD) | 17.86 (7.60)/20.52 (13.35) b,i, ***, j * | 22.56 (6.13)/29.53 (13.24) b,k *** | 29.62 (3.63)/47.46 (10.34) b | 43.27 c *** | 0.44 f |
| Vocabulary: M (SD) | 33.97 (14.47) ) i ***, j ** | 43.40 (10.18) k *** | 56.18 (6.55) | 38.81 c *** | 0.41 f |
| Antipsychotic medications: | |||||
| Atypical: n (%) | 20 (68.97) | 29 (64.44) | - | 2.09 d | 0.17 g |
| Atypical and typical: n (%) | 8 (27.58) | 12 (26.67) | - | ||
| Typical: n (%) | 0 (0.00) | 3 (6.67) | - | ||
| No medications: n (%) | 1 (3.45) | 1 (2.22) | - | ||
| Chlorpromazine equivalent (mg): M (SD) | 695.86 (311.57) | 644.04 (309.71) | - | 0.71 e | 0.17 h |
| Duration of illness: M (SD) | 16.97 (5.73) | 14.00 (5.14) | - | 2.32 e | 0.55 h |
| Exacerbation: M (SD) | 5.69 (2.44)/1.64 (0.48) a | 6.49 (5.01)/1.65 (0.64) a | - | −0.11 e | −0.03 h |
| Global functioning in GAF: M (SD) | 50.93 (14.34) | 58.40 (14.21) | - | −2.20 e | −0.52 h |
| Psychopathological symptoms in PANSS: | |||||
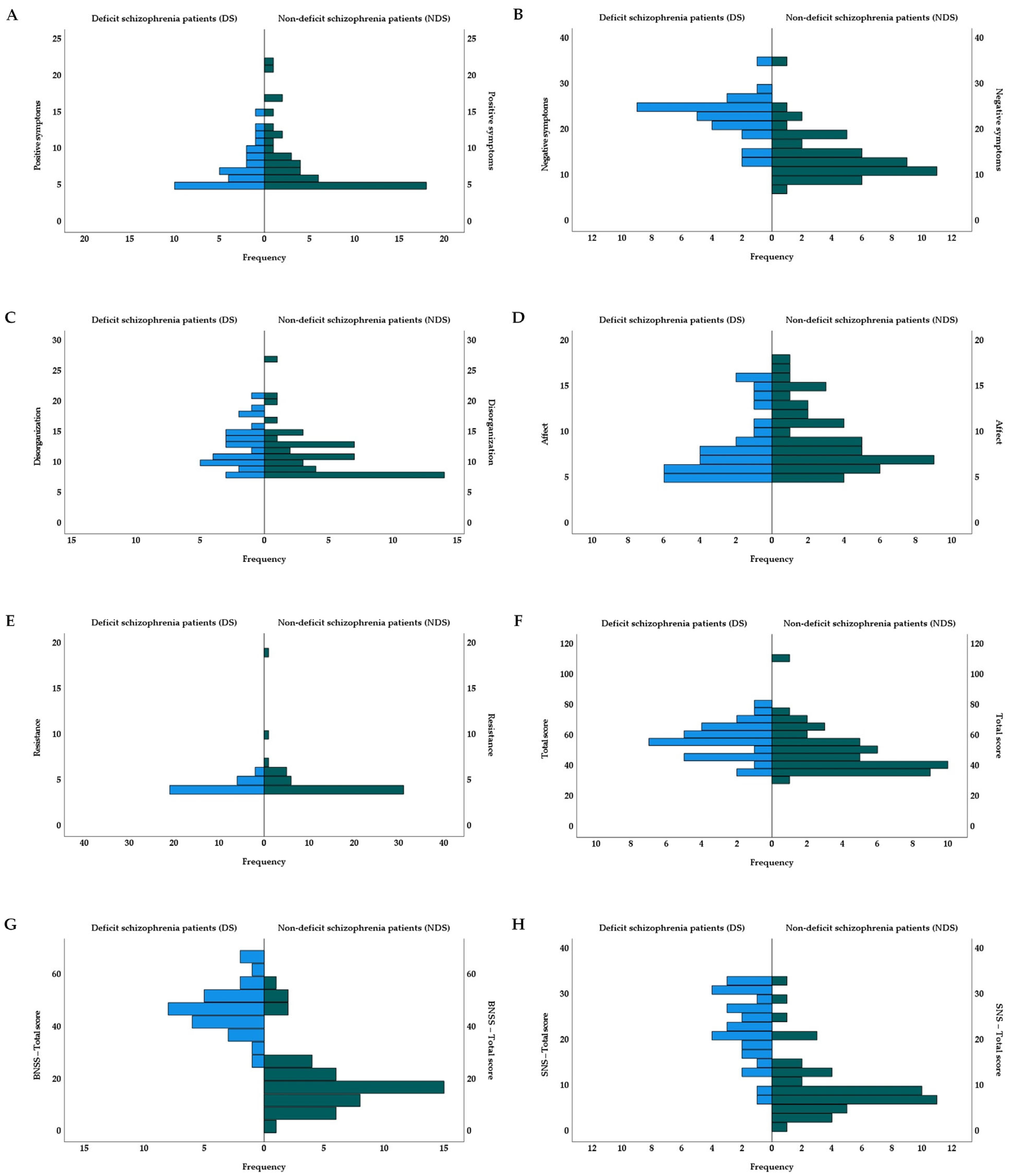
| Positive symptoms: M (SD) | 7.38 (2.73)/5.28 (0.06) b | 8.07 (4.37)/5.28 (0.06) b | - | 0.00 e | 0.00 h |
| Negative symptoms: M (SD) | 22.24 (4.66)/5.85 (0.01) b | 13.80 (5.19)/5.80 (0.03) b | - | 7.45 e *** | 1.51 h |
| Disorganization: M (SD) | 12.62 (3.48)/5.36 (0.02) b | 11.42 (3.98)/5.34 (0.03) b | - | 1.93 e | 0.46 h |
| Affect: M (SD) | 8.24 (3.45)/5.29 (0.06) b | 9.29 (3.53)/5.31 (0.05) b | - | −1.68 e | −0.40 h |
| Resistance: M (SD) | 4.34 (0.61)/5.04 (0.04) b | 4.89 (2.43)/5.05 (0.06) b | - | −1.07 e | −0.23 h |
| Total score: M (SD) | 56.83 (11.17)/5.40 (0.01) b | 49.33 (14.68)/5.40 (0.01) b | - | 3.31 e * | 0.73 h |
| Negative symptoms in BNSS: | |||||
| Total score: M (SD) | 47.07 (9.28)/2.63 (0.43) b | 20.07 (12.68)/1.27 (0.66) b | - | 9.87 e *** | 2.35 h |
| Negative symptoms in SNS: | |||||
| Total score: M (SD) | 22.28 (7.38)/3.03 (0.41) a | 9.71 (6.89)/2.05 (0.70) a | - | −7.63 e *** | 1.63 h |
| Deficit Schizophrenia Patients (DS) (n = 29) | Non-Deficit Schizophrenia Patients (NDS) (n = 45) | Healthy Control (HC) (n = 39) | F | ɳ2 | |
|---|---|---|---|---|---|
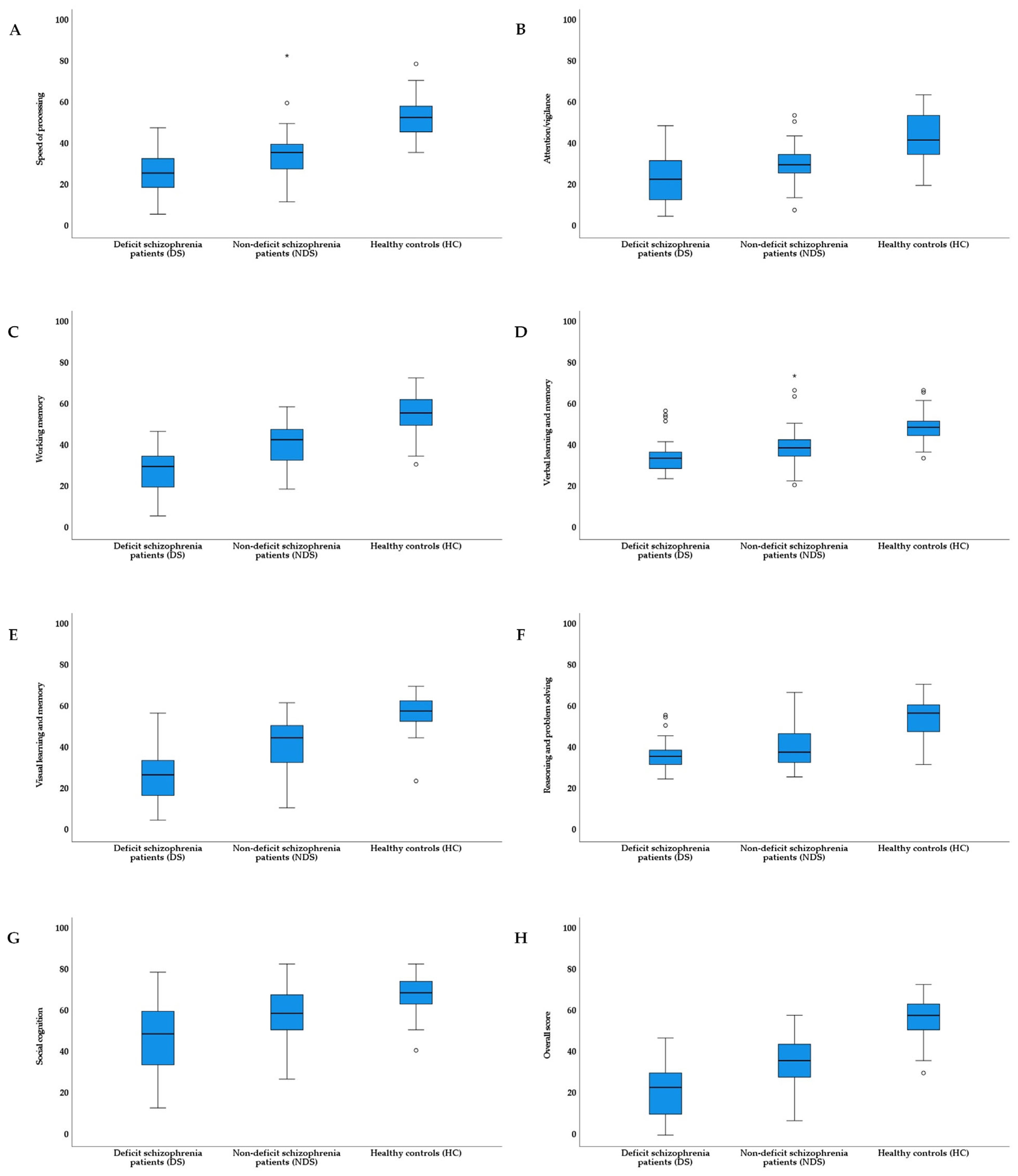
| Speed of processing | 25.93 (11.53)/3.19 (0.49) a,c ***,d *** | 34.62 (12.40)/3.51 (0.36) a,e *** | 53.15 (9.66)/3.97 (0.18) a | 43.25 *** | 0.44 |
| Attention/vigilance | 23.69 (11.58) c ***,d * | 30.22 (8.76) e *** | 42.90 (11.55) | 30.12 *** | 0.35 |
| Working memory | 27.72 (11.40) c ***,d *** | 40.67 (10.95) e *** | 54.51 (9.57) | 53.66 *** | 0.49 |
| Verbal learning and memory | 34.76 (9.24)/3.54 (0.24) a,c ***,d * | 40.09 (10.11)/3.68 (0.24) a,e *** | 48.95 (7.50)/3.90 (0.15) a | 24.27 *** | 0.31 |
| Visual learning and memory | 28.00 (13.78)/5.12 (4.56) b,c ***,d *** | 41.71 (12.65)/9.90 (5.05) b,e *** | 56.90 (8.21)/17.08 (4.29) b | 57.23 *** | 0.51 |
| Reasoning and problem solving | 37.24 (7.85) c *** | 41.49 (11.15) e *** | 54.44 (9.20) | 30.50 *** | 0.36 |
| Social cognition | 48.24 (16.42) c ***,d ** | 58.00 (12.40) e ** | 67.87 (9.33) | 20.20 *** | 0.27 |
| Overall score | 20.31 (12.88) c ***,d *** | 34.87 (11.78) e *** | 56.69 (9.13) | 91.29 *** | 0.62 |
| Deficit Schizophrenia Patients (DS) (n = 29) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Speed of Processing | Attention/Vigilance | Working Memory | Verbal Learning and Memory | Visual Learning and Memory | Reasoning and Problem Solving | Social Cognition | Overall Score | |
| r | r | r | r | r | r | r | r | |
| Positive symptoms | 0.08 | 0.02 | −0.10 | 0.13 | −0.16 | −0.34 | 0.01 | −0.11 |
| Negative symptoms | 0.04 | −0.13 | −0.05 | 0.13 | −0.26 | 0.01 | −0.02 | −0.08 |
| Disorganization | −0.02 | 0.10 | 0.07 | 0.26 | −0.04 | −0.24 | 0.04 | 0.02 |
| Affect | 0.17 | −0.29 | 0.17 | 0.08 | −0.05 | 0.26 | 0.11 | 0.11 |
| Resistance | −0.31 | −0.01 | −0.35 | −0.07 | −0.25 | −0.30 | −0.14 | −0.34 |
| Non-Deficit Schizophrenia Patients (NDS) (n = 45) | ||||||||
| Speed of Processing | Attention/Vigilance | Working Memory | Verbal Learning and Memory | Visual Learning and Memory | Reasoning and Problem Solving | Social Cognition | Overall Score | |
| r | r | r | r | r | r | r | r | |
| Positive symptoms | −0.33 * | −0.10 | −0.25 | −0.15 | −0.28 | −0.31 * | −0.25 | −0.41 ** |
| Negative symptoms | −0.30 * | −0.03 | 0.06 | 0.20 | −0.04 | −0.12 | 0.00 | −0.10 |
| Disorganization | −0.41 ** | −0.03 | −0.22 | −0.08 | −0.18 | −0.39 ** | −0.12 | −0.35 * |
| Affect | −0.22 | −0.10 | −0.18 | 0.08 | −0.11 | −0.13 | −0.05 | −0.20 |
| Resistance | −0.46 ** | −0.01 | −0.01 | −0.13 | −0.11 | −0.27 | −0.13 | −0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plichta, P.; Tyburski, E.; Bielecki, M.; Mak, M.; Kucharska-Mazur, J.; Podwalski, P.; Rek-Owodziń, K.; Waszczuk, K.; Sagan, L.; Michalczyk, A.; et al. Cognitive Dysfunctions Measured with the MCCB in Deficit and Non-Deficit Schizophrenia. J. Clin. Med. 2023, 12, 2257. https://doi.org/10.3390/jcm12062257
Plichta P, Tyburski E, Bielecki M, Mak M, Kucharska-Mazur J, Podwalski P, Rek-Owodziń K, Waszczuk K, Sagan L, Michalczyk A, et al. Cognitive Dysfunctions Measured with the MCCB in Deficit and Non-Deficit Schizophrenia. Journal of Clinical Medicine. 2023; 12(6):2257. https://doi.org/10.3390/jcm12062257
Chicago/Turabian StylePlichta, Piotr, Ernest Tyburski, Maksymilian Bielecki, Monika Mak, Jolanta Kucharska-Mazur, Piotr Podwalski, Katarzyna Rek-Owodziń, Katarzyna Waszczuk, Leszek Sagan, Anna Michalczyk, and et al. 2023. "Cognitive Dysfunctions Measured with the MCCB in Deficit and Non-Deficit Schizophrenia" Journal of Clinical Medicine 12, no. 6: 2257. https://doi.org/10.3390/jcm12062257
APA StylePlichta, P., Tyburski, E., Bielecki, M., Mak, M., Kucharska-Mazur, J., Podwalski, P., Rek-Owodziń, K., Waszczuk, K., Sagan, L., Michalczyk, A., Misiak, B., & Samochowiec, J. (2023). Cognitive Dysfunctions Measured with the MCCB in Deficit and Non-Deficit Schizophrenia. Journal of Clinical Medicine, 12(6), 2257. https://doi.org/10.3390/jcm12062257







