Abstract
To date, the exact pathophysiology of haemorrhoids is poorly understood. The different philosophies on haemorrhoids aetiology may lead to different approaches of treatment. A pathogenic theory involving a correlation between altered anal canal microflora, local inflammation, and muscular dyssynergia is proposed through an extensive review of the literature. Since the middle of the twentieth century, three main theories exist: (1) the varicose vein theory, (2) the vascular hyperplasia theory, and (3) the concept of a sliding anal lining. These phenomena determine changes in the connective tissue (linked to inflammation), including loss of organization, muscular hypertrophy, fragmentation of the anal subepithelial muscle and the elastin component, and vascular changes, including abnormal venous dilatation and vascular thrombosis. Recent studies have reported a possible involvement of gut microbiota in gut motility alteration. Furthermore, dysbiosis seems to represent the leading cause of bowel mucosa inflammation in any intestinal district. The alteration of the gut microbioma in the anorectal district could be responsible for haemorrhoids and other anorectal disorders. A deeper knowledge of the gut microbiota in anorectal disorders lays the basis for unveiling the roles of these various gut microbiota components in anorectal disorder pathogenesis and being conductive to instructing future therapeutics. The therapeutic strategy of antibiotics, prebiotics, probiotics, and fecal microbiota transplantation will benefit the effective application of precision microbiome manipulation in anorectal disorders.
1. Introduction
The exact pathophysiology of haemorrhoids is poorly understood. Currently, the term “haemorrhoids” describes the symptomatic and abnormal downward displacement of normal anal cushions [1]; it is associated with destructive changes in the supporting connective tissue and abnormal blood circulation within anal cushions, as well as abnormal dilation and distortion of the hemorrhoidal plexus with subsequent sliding of mucosa into the anal canal. Studies on morphology and hemodynamic of the arterial supply to the anal canal have highlighted a dysregulation of the vascular tone with hyper perfusion of the hemorrhoidal plexus in patients with haemorrhoids [1,2,3]. Unfortunately, hyperperfusion does not translate into hyperoxygenation. Many studies have well demonstrated the content of inflammatory cells [4] and newly formed micro vessels [5] of hemorrhoidal tissue. Hypoxia could be considered the main responsibility of the inflammatory state characterizing haemorrhoids. Other authors proposed the theory of the internal rectal prolapse in order to explain circumferential prolapsing haemorrhoids [6]. Probably, the true pathophysiology of haemorrhoid development is multifactorial, including sliding anal cushion, hyperperfusion of haemorrhoid plexus, vascular abnormality, tissue inflammation, and internal rectal prolapse [1]. Different etiopathogenetic theories have led to different therapeutic approaches [1,7]. Haemorrhoids are strictly linked to other anorectal conditions, such as anal itching, fissures, and anismus. For most of these conditions, pathophysiology is not entirely clear, but an involvement of the smooth musculature of the internal anal sphincter has been advocated. According to current hypotheses, all anorectal disorders could be considered the direct consequence of constipation [8]. A new pathogenic theory involving a correlation between altered anal canal microflora, local inflammation, and smooth muscle impairment is proposed through an extensive review of the literature.
2. The Current Pathophysiology of Haemorrhoids
The cause of haemorrhoids is not clear, although, over the centuries, several theories have been postulated. Since the middle of the twentieth century, three main theories exist: (1) the varicose vein theory, (2) the vascular hyperplasia theory, and (3) the concept of a sliding anal lining. Since the time of Galen and Hippocrates, haemorrhoids were identified as varicose veins [9]. Later, this theory was disproved by Thomson and other authors who demonstrated the presence of venous dilations already in infants, suggesting that they are part of normal anal anatomy [10]. In 1956, Parks [11] attributed the varicose swellings to a local increase in pressure due to hard stools. Actually, haemorrhoids can be considered a complex system of portosystemic anastomoses [10]; in fact, haemorrhoids are no more common in patients with portal hypertension than the normal population [12]. The associated suggestion that lows fibre intake, constipation, and straining are important causative factors [13,14] is not supported by subsequent works [14,15,16,17]. Other authors, taking a cue by histologic specimens, suggested that haemorrhoids could be considered as a sort of vascular hyperplastic process [10,18] and, in 1963, Stelzner [19] proposed the concept of the corpus cavernosum recti, but this theory was confuted by Thomson [10] and Loder [18], who pointed out that there are no significant differences in the vascular anatomy between normal and pathologic hemorrhoidal tissue. Hemorrhoidal bleeding seems to arise from capillaries in the lamina propria, rather than the venous dilations [10,20]. The last and more recent theory recognizes in the pathologic slippage of the anal canal lining, the primum movens of haemorrhoids [10,18,21]. According to this theory, haemorrhoids are caused by supporting tissue disintegration or deterioration; shearing forces during defecation tend to exacerbate the problem. On microscopy, these phenomena are expressed by loss of connective fibre organization, muscular hypertrophy, fragmentation of the anal subepithelial muscle and the elastin component, and vascular changes, including abnormal venous dilatation and vascular thrombosis [10,21,22]. Ischemia due to traction and constriction of microvascular system leads to severe inflammation of the surrounding connective tissue [4]. Matrix metalloproteinase-9 (MMP-9) overexpression seems to be associated with the breakdown of elastic fibres [23] and, together with MMP-2, promotes the angioproliferative activity of transforming growth factor β (TGF-β) [24], suggesting that neovascularization might be another important phenomenon in hemorrhoidal disease. Furthermore, Chung [5] and Han et al. [23] also demonstrated that there is a higher expression of angiogenesis-related protein such as VEGF. Such growth factor contributes to the increased microvascular density in hemorrhoidal tissue. In Figure 1, a summary of current insights on haemorrhoidal disease is showed.
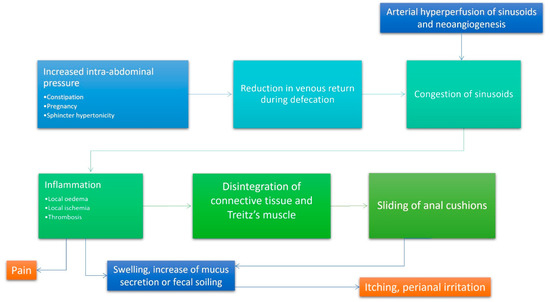
Figure 1.
Integrated algorithm summarizing contemporary thinking regarding the pathophysiology of haemorrhoids. According to the current accepted physiopathological mechanism of hemorrhoidal disease, increased intraabdominal pressure (due to constipation, diarrhoea, pregnancy, or obstructed defecation) and arterial hyperperfusion of the hemorrhoidal plexus represents the most important initiation points. The subsequent reduction of venous return during defecation and stagnation of blood inside the dilated plexus favour inflammation and its epiphenomena (oedema, ischemia and thrombosis). Inflammation is responsible of the impaired quality of collagen and relaxation of the supporting muscles of the internal hemorrhoidal plexus. These events lead to sliding of the anal cushions that is responsible for all symptoms experienced in haemorrhoids.
3. Constipation and Haemorrhoids
Abnormalities of anorectal physiology can be shown in patients with haemorrhoids and altered intestinal motility. Usually, one of the most common intestinal motility alterations is represented by constipation. Most patients affected by constipation have a functional disorder affecting the colon or the anorectum [25]. Constipation can be classified as (a) functional, (b) associated with irritable bowel syndrome, (c) opioid-induced, or (d) associated to functional defecation disorders, including inadequate defecatory propulsion and dyssynergic defecation. Functional constipation is characterized by prolonged delay in the transit of stool through the colon, primarily due to smooth muscle or nerve plexuses degeneration. Constipation associated to functional defecation disorders, also known as obstructive defecation [26], anismus [27], pelvic floor dyssynergia [28], or outlet obstruction [29,30], is characterized by either difficulty or inability with expelling stools from the anorectum [26]. A prolonged colonic transit and a dyssynergic defecation can co-exist. The third subtype is comprised of patients with irritable bowel syndrome and constipation (IBS-C) in whom abdominal pain, with or without bloating, is a prominent symptom, together with altered bowel habit [31].
Interestingly, in patients affected by hemorrhoidal disease, other intestinal dysmotility can be recognized. For instance, in patients with muscular dyssynergia, anal resting pressures is often found to be raised [16,32,33,34,35,36,37,38]. According to some authors, this abnormal finding could represent the result, rather than a cause of the pathology, suggesting a return to normal anal resting pressure within three months after haemorrhoidectomy [33,34]. Some authors have identified, in haemorrhoid hypervascularization, the possible mechanism at the base of increased anal resting pressure [36,37], but, on the other side, rubber band ligation is unable to restore normal blood pressure levels [32,33,37,38]. Recent theories have attributed dyssynergic defecation to acquired behavioural disorder or to defective learning in the childhood [25]. In these cases, a failure of recto-anal coordination due to impaired rectal contraction, paradoxical anal contraction, or inadequate anal relaxation or involuntary anal spasm (anismus), could be associated with dyssynergic defecation [26,39,40,41,42,43]. Another condition supporting the relationship between dyssynergic defecation and haemorrhoids is the demonstration of rectal ultraslow waves in patients suffering from haemorrhoids [44]. It is possible that the ultraslow waves are associated with the high resting pressures and probably originate in the internal sphincter muscle, although their significance is not clear [18]. Other changes have been recorded, but they are less reproducible, including increased external sphincter activity (spike potentials) [44], decreased anal sensation [18], and an increased number of sampling responses [44]. Unfortunately, to date, the temporal relationship between these physiologic findings and the development of haemorrhoids has not yet been explored. However, a significant slowdown of colonic propulsion mechanisms has been proven in patients with slow transit constipation [45,46]. Furthermore, it has been shown that the gastrocolic responses following a meal and the morning waking responses after sleep are also significantly diminished, but the diurnal variation of colonic motor activity is preserved [46]. In contrast, periodic rectal motor activity, a three-cycles-per-minute activity that predominately occurs in the rectum and rectosigmoid region and is invariably seen at night-time [47], significantly increases in patients with slow transit constipation [48]. This excessive uninhibited distal colonic activity may serve as a nocturnal break and retard colonic propulsion of stool [48]. Previous studies have shown that high amplitude, prolonged duration, andpropagated contractions are significantly decreased in patients affected by constipation [49,50]. Furthermore, in patients with constipation, the velocity of propagation is slower, and waves have a greater tendency to abort prematurely, and their amplitude is also decreased [49,51].
4. The Role of Intestinal Microorganisms in Functional Gastrointestinal Disorders
The importance of the gut microbiota in human health is currently well established. Intestinal microbiota seems to be involved in the development of the host immune system, metabolism, and digestion; it imparts specific function in the maintenance of structural integrity of the gut mucosal barrier and protection against pathogens and has a role in brain–gut communication. Tolerance to gut microbiota occurs early in life, and it is crucial to prevent allergic and immune-mediated diseases. Whenever the cross-talk between microbiota and immune system is altered, inflammation of the bowel occurs. For example, IBD (inflammatory bowel diseases) are characterized by an alteration of microbial population equilibrium, with a decrease in “good” bacteria (such as F. prausnitzii or R. hominis) and high concentration of “bad” bacteria (i.e., E. coli). Microbial taxa influence the immune system, hence affecting the inflammatory status of the host [52].
Gut bacteria are not only involved in immune system regulation, but they possibly also correlate with functional gastrointestinal disorders (FGIDs), which has been hypothesized [53]. For example, in irritable bowel syndrome (IBS), a functional disorder characterized by altered bowel transit, which is related to the use of probiotic and antibiotic therapy, has been demonstrated to bring beneficial effects to intestinal motility [54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69].
Another frequent and bothersome functional gastrointestinal disease is represented by chronic constipation. There is increasingly clear evidence supporting an association between the altered mucosal and faecal microbiota and chronic constipation [70,71], although precise pathophysiologic mechanisms remain poorly understood.
In recent years, several studies have been published exploring the gut microbiome in patients with constipation. Cao and colleagues [72] reported an up-regulation of serotonin (5-HT) transporter with a subsequent reduction of 5-HT concentrations in the colonic lumen of germ-free mice that received faecal microbiota from patients with constipation. Low 5-HT levels were associated with slow intestinal transit and, furthermore, the authors highlighted changes in gut microbiota composition, with a reduction of those bacterial strains belonging to the phylum Firmicutes (Clostridium, Lactobacillus, Desulfovibrio, and Methylobacterium) and increased Bacteroidetes and Akkermansia. These findings suggest a potential role for gut microbiota in the pathogenesis of chronic constipation via increased expression of the 5-HT transporter [72]. A higher production of methane gas by metanogenic flora could be another potential mechanism able to impair intestinal muscle contractions [35,73,74,75].
Independently of transit time, patients with constipation show characteristic variations of microbially derived metabolites [71]. In one study, antibiotic-treated mice showed altered short-chain fatty acids (SCFA) and bile acid profiles after transfer of faecal microbiota from patients with slow-transit constipation [76]. Taxonomic profiling of the faecal microbiome from patients with functional constipation and healthy volunteers has shown decreased abundance of Bacteroides, Roseburia, and Coprococcus in the first group. Furthermore, healthy volunteers were found to have a gut microbiome enriched in genes involved in carbohydrate, fatty acid, and lipid metabolism, whereas patients with functional constipation harboured a high abundance of genes involved in methanogenic pathways, hydrogen production, and glycerol [77]. Analysis of functional gene targets in constipated and healthy females also has shown increased abundance of hydrogenogenic (hydrogen-producing) and hydrogenotrophic (hydrogen-utilizing) genes in the colonic mucosa of individuals with constipation [78]. In a cross-sectional study of eight obese children suffering from constipation and 14 obese children without constipation, functional constipation was associated with a decreased abundance of the phylum Bacteroidetes, including a significant reduction of the genus Prevotella, as well as an increased abundance of multiple genera within the phylum Firmicutes, including Blautia, Coprococcus, and Ruminococcus [79]. A recent systematic review, including seven randomized controlled trials and enrolling a total of 515 children, investigated the effects of probiotics in pediatric functional constipation. Two of the included studies, those evaluating L. reuteri DSM 17938 and B. longum [80,81], reported significantly increased defecation frequency in the treatment arm, and the meta-analysis concluded that, currently, there is insufficient evidence to support the use of probiotics for pediatric functional constipation [82]. Finally, although a low-fiber diet is a known risk factor for functional constipation in children [83], there is currently little evidence to support the use of fiber for pediatric functional constipation. Multiple systematic reviews note the sparse data and high risk of bias among the current evidence base [84,85,86,87].
Up to now, microbiota has been identified with gut bacterial flora alone, but thanks to the recent advancements, our interests in intestinal microbic flora composition have been expanded to fungi (mycobiota), viruses (virobiota), and helminthes [88]. Already in 1985, 174 patients with anal itching were submitted to perianal mycoculture. Infection by C. albicans was observed in all groups studied, independent of the presence of disease or anal pruritus, whereas the presence of dermatophytes was always associated with pruritus ani [89]. Actually, pruritus ani is common in those cases of stool leakage and subsequent perianal soiling, conditions that could favour local fungal flora overgrowth. Typical is the case of endurance cycling athletes who often report pain and disorders in the anal region, including inflammatory processes and functional defecation problems. Sharma [90] already underlined the role of microclimatic condition on fungal overgrowth. Subsequent inflammatory state and altered smooth muscle dysmotility with high pressure values could be a direct effect of this overgrowth. In addition, permanent microtrauma originating from constant saddle vibration leads to anal fissure and chronic inflammation, which could lead to anal pain and, as a consequence, to high sphincter pressure. The high sphincter pressure, in turn, could result in muscle hypertrophy, leading to defecation problems and diarrhoea with partial anal incontinence [91].
5. An Innovative Alternative Treatment for Anorectal Disfunction and Haemorrhoids
Currently, there are many conservative options to treat haemorrhoids and the other anorectal disorders, but, up to now, no high-quality clinical trials have shown any long-term benefit. Common local medications contain low-dose anaesthetics, corticosteroids, keratolytic, protectants, or antiseptics; the prolonged use of some of these therapies could be even detrimental and should be avoided [92]. Among conservative treatments, an important role has been played by micronized purified flavonoid fractions. These compounds may possibly improve venous tone and lymphatic outflow and may help to control local inflammation [93]. Although there is a widespread use of these supplements, their use could be justified by their mild anti-inflammatory action, but additional trials are required [92,94,95].
Up to now, faecal microbiota in ano-rectal diseases has not yet received the right attention, despite the recent insights, above cited, on its crucial role on biological intestinal homeostasis and physiological process regulation. The use of a Saccharomyces cerevisiae-based ointment for haemorrhoids has been quite diffuse in the proctologist community for several years. One study reported the beneficial effects of S. cerevisiae against Clostridium difficile infections [96]. In another study on pigs, S. cerevisiae was able to reduce the translocation of enterotoxigenic E. coli (ETEC), strengthening mucosal immunity [97]. Very recently, Gaziano et al. [98] proposed S. cerevisiae as a possible treatment of vulvovaginal candidiasis and bacterial vaginosis, thanks to its immunobiotic properties. The authors demonstrated that the yeast has a major role in reducing the colonization of C. albicans and/or G. vaginalis on human mucosal surfaces and enhancing the antimicrobial effect of standard therapeutic approaches. Furthermore, dietary supplementation of S. cerevisiae significantly decreases enteric methane production via several interrelated mechanisms, including: (i) increasing of O2 utilization to allow for a more favourable environment for anaerobic fermentation, which in turn leads to higher volatile fatty acids production and lower pH, which restrains the growth of methanogens and protozoa, (ii) induction of a shift in fermentation pattern toward propionogenesis, which compete with methanogens for the free H2 within the system, and (iii) increasing of acetogenic bacteria growth, which use H2 to produce acetate, which serves as another alternative sink for the free H2 in the system [99].
In 2017, Hager et al. [100] deepened the role of the mycobiota in the gastrointestinal tract. They found that patients with Crohn’s disease (CD) tend to have much higher levels of the fungus Candida tropicalis compared to their healthy family members, as well as two bacteria, Escherichia coli and Serratia marcescens. These three organisms work together to form robust biofilms capable of exacerbating intestinal inflammation. Candida colonization has been also highlighted in patients suffering from ulcerative colitis (UC), as well as gastric and duodenal ulcers [101]. Likely, Candida colonization seems to have a role in delaying reparative processes, while inflammation promotes its growth. These effects may create a vicious cycle in which low-level inflammation promotes fungal colonization, and fungal colonization promotes further inflammation. Both inflammatory bowel disease and gastrointestinal Candida colonization are associated with elevated levels of the pro-inflammatory cytokine IL-17. Therefore, effects on IL-17 levels may underlie the ability of Candida colonization to enhance inflammation.
In their work, Panpetch and colleagues demonstrated the correlation between C. albicans and inflammation in a dextran-sulfate solution (DSS) induced-colitis mouse model (DSS + Candida). Higher concentration of C. albicans in murine intestinal lumen seems to favour translocation of LPS, BG, and bacteria (not fungemia) from the gut into systemic circulation and causes higher mortality, more severe colon histology, and enhanced gut-leakage. The study highlighted also the presence of Pseudomonas aeruginosa in blood and faecal samples. The administration of L. rhamnosus L34 attenuated gut local inflammation, gut-leakage severity, faecal dysbiosis, and systemic inflammation.
Other studies have reported the effects of antifungal treatment on patients affected by ulcerative colitis, observing that a reduction in fungal colonization could be beneficial for colonized patients. Data from some studies reported beneficial effects on human patients with UC after administration of Lactobacillus acidophilus [102], whereas acetic-acid treated rats who received C. albicans and an inhibitor of gastric acid secretion [103,104] showed reduced ulcer size. These findings suggest that, by antagonizing Candida colonization, modulation of the bacterial microbiota could provide beneficial effects for patients.
Further studies to discern the mechanisms for the effect of inflammation on Candida colonization and the effect of Candida on inflammatory lesions represent exciting directions for future research.
From depicted data, anorectal inflammation could be the direct effect of altered bacterial and fungal intestinal flora, representing the primum movens of all anorectal disorders. Chronic constipation, dyssynergic defecation, anal fissures, and haemorrhoids could be the lowest common denominator of the same condition: dysbiosis. Microbioma composition alteration promotes inflammation and dysmotility in the whole intestinal tract, including the anal canal and rectum. Figure 2 shows a possible physiopathological hypothesis of haemorrhoids and other anorectal disorders, involving dysbiosis as the main starting point. Taking into account this new insight, an effort should be made on deepening the mechanisms that link dysbiosis to anorectal disorders in order to shift therapy from current surgical approaches to the next generation of precise mechanism-based interventions. To date, although new surgical techniques allow quicker recovery and promise to be less painful, higher recurrence rates and potentially serious complications remain an issue [105]. A deeper knowledge of the gut microbiota in anorectal disorders lays the basis for unveiling the roles of these various gut microbiota components in anorectal disorders pathogenesis, being conductive to instructing on future therapeutics. The therapeutic strategy of antibiotics, prebiotics, probiotics, and faecal microbiota transplantation will benefit the effective application of precision microbiome manipulation in anorectal disorders [106,107]. Further studies are needed in order to assess possible new non-invasive microbiota-based approaches to anorectal diseases, avoiding surgical interventions and improving patients’ satisfaction. Certainly, in the next few years, new insights on micro-/mico-/virobiota will lead to new therapeutic strategies, which are less invasive and cheaper than current surgical approaches. Local administration of “good microorganisms” against “bad microorganisms” could be a promising strategy in order to turn off inflammation and normalize internal sphincter activity, restoring a complete anal canal functionality and favouring more physiologic intestinal movements.
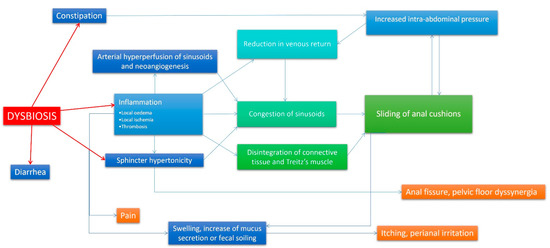
Figure 2.
A new theory on haemorrhoidal disease pathophysiology. In this flow-chart, a new model of haemorrhoidal disease pathophysiology mechanism is presented. In this case, dysbiosis is the primum movens of the disease, and events that lead to symptoms are the direct effect of the local inflammatory state caused by altered rectal microbiota/mycobiota. vascular congestion, neoangiogenesis and arterial hyperperfusion, oedema, and connective disruption due to inflammation are responsible of swelling and cushion prolapse. Inflammation and a possible direct involvement of bacteria (producing substances able affect intestinal muscular layer) could be involved also in internal sphincter hypertonicity and subsequent increased intra-abdominal pressure. The reported mechanism is established and perpetuated with the assistance of many vicious cycles, and it is continuously auto-reinforced. In other words, haemorrhoidal disease becomes worse over time.
Author Contributions
Conceptualization, V.D.P., M.M. and A.I.L.M. and G.G.; methodology, R.T.; validation, R.T., M.S., P.V., C.N. and G.L.S.; formal analysis, C.P. and E.M.; data curation, G.B.; writing—original draft preparation, V.D.P.; writing—review and editing, R.T., G.B. and S.B.; supervision, A.I.L.M.; project administration, V.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lohsiriwat, V. Hemorrhoids: From basic pathophysiology to clinical management. World J. Gastroenterol. 2012, 18, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Lohsiriwat, V. Approach to haemorrhoids. Curr. Gastroenterol. Rep. 2013, 15, 332. [Google Scholar] [CrossRef] [PubMed]
- Aigner, F.; Gruber, H.; Conrad, F.; Eder, J.; Wedel, T.; Zelger, B.; Engelhardt, V.; Lametschwandtner, A.; Wienert, V.; Böhler, U.; et al. Revised morphology and hemodynamics of the anorectal vascular plexus: Impact on the course of hemorrhoidal disease. Int. J. Color. Dis. 2009, 24, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Morgado, P.J.; Suárez, J.A.; Gómez, L.G. Histoclinical basis for a new classification of hemorrhoidal disease. Dis. Colon Rectum. 1988, 31, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Hou, Y.; Pan, A.C. Endoglin (CD105) expression in the development of haemorrhoids. Eur. J. Clin. Investig. 2004, 34, 107–112. [Google Scholar] [CrossRef]
- Corman, M.L.; Gravie, J.-F.; Hager, T.; Loudon, M.A.; Mascagni, D.; Nystrom, P.-O.; Seow-Choen, F.; Abcarian, H.; Marcello, P.; Weiss, E.; et al. Stapled haemorrhoidopexy: A consensus position paper by an international working party-indications, contra-indications and technique. Color. Dis. 2003, 5, 304–310. [Google Scholar] [CrossRef]
- Janicke, D.M.; Pundt, M.R. Anorectal disorders. Emerg. Med. Clin. N. Am. 1996, 14, 757–788. [Google Scholar] [CrossRef]
- Bunni, J.; Laugharne, M.J. Pathophysiological basis, clinical assessment, investigation and management of patients with obstruction defecation syndrome. Langenbecks Arch. Surg. 2023, 408, 75. [Google Scholar] [CrossRef]
- Parks, A.G. De haemorrhois; a study in surgical history. Guy’s Hosp. Rep. 1955, 104, 135–156. [Google Scholar]
- Thomson, W.H.F. The nature of haemorrhoids. Br. J. Surg. 1975, 62, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.G. The surgical treatment of hæmorrhoids. Br. J. Surg. 1956, 43, 337–351. [Google Scholar] [CrossRef]
- Jacobs, D.M.; Bubrick, M.P.; Onstad, G.R.; Hitchcock, C.R. The relationship of haemorrhoids to portal hypertension. Dis. Colon Rectum. 1980, 23, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, D.P. Varicose Veins, Deep Vein Thrombosis, and Haemorrhoids: Epidemiology and Suggested Aetiology. BMJ 1972, 2, 556–561. [Google Scholar] [CrossRef][Green Version]
- Broader, J.H.; Gunn, I.F.; Alexander-Williams, J. Evaluation of a bulk-forming evacuant in the management of haemorrhoids. Br. J. Surg. 1974, 61, 142–144. [Google Scholar] [CrossRef]
- Dennison, A.R.; Whiston, R.J.; Rooney, S.; Morris, D.L. The Management of Hemorrhoids. Am. J. Gastroenterol. 1989, 84, 475–481. [Google Scholar] [PubMed]
- Gibbons, C.P.; Bannister, J.J.; Read, N.W. Role of constipation and anal hypertonia in the pathogenesis of haemorrhoids. Br. J. Surg. 1988, 75, 656–660. [Google Scholar] [CrossRef]
- Johanson, J.F.; Sonnenberg, A. Temporal changes in the occurrence of haemorrhoids in the United States and England. Dis. Colon Rectum. 1991, 34, 585–593. [Google Scholar] [CrossRef]
- Loder, P.B.; Kamm, M.A.; Nicholls, R.J.; Phillips, R.K.S. Haemorrhoids: Pathology, pathophysiology and aetiology. Br. J. Surg. 1994, 81, 946–954. [Google Scholar] [CrossRef]
- Stelzner, F. Die Hämorrhoiden und andere Krankheiten des Corpus cavernosum recti und des Analkanals. DMW Dtsch. Med. Wochenschr. 1963, 88, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Graham-Stewart, C.W. Injection Treatment of Haemorrhoids. BMJ 1962, 1, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Gass, O.C.; Adams, J. Hemorrhoids: Etiology and pathology. Am. J. Surg. 1950, 79, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Haas, P.A.; Fox, T.A.; Haas, G.P. The pathogenesis of Hemorrhoids. Dis. Colon Rectum 1984, 27, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wang, Z.; Zhao, B.; Yang, X.Q.; Wang, D.; Wang, J.P.; Tang, X.Y.; Zhao, F.; Hung, Y.T. Pathologic change of elastic fibers with difference of microvessel density and expression of angiogenesis-related proteins in internal haemorrhoid tissues. Zhonghua Wei Chang Wai Ke Za Zhi 2005, 8, 56–59. [Google Scholar]
- Yoon, S.-O.; Park, S.-J.; Yun, C.-H.; Chung, A.-S. Roles of Matrix Metalloproteinases in Tumor Metastasis and Angiogenesis. BMB Rep. 2003, 36, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S. Constipation: Evaluation and Treatment of Colonic and Anorectal Motility Disorders. Gastroenterol. Clin. N. Am. 2007, 36, 687–711. [Google Scholar] [CrossRef]
- Rao, S.S.C. Dyssynergic defecation. Gastroenterol Clin. N. Am. 2001, 30, 97–114. [Google Scholar] [CrossRef]
- Preston, D.M.; Lennard-Jones, J.E. Anismus in chronic constipation. Dig. Dis. Sci. 1985, 30, 413–418. [Google Scholar] [CrossRef]
- Heitmann, P.T.; Vollebregt, P.F.; Knowles, C.H.; Lunniss, P.J.; Dinning, P.G.; Scott, S.M. Understanding the physiology of human defaecation and disorders of continence and evacuation. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 751–769. [Google Scholar] [CrossRef]
- Kawimbe, B.M.; Papachrysostomou, M.; Binnie, N.R.; Clare, N.; Smith, A.N. Outlet obstruction constipation (anismus) managed by biofeedback. Gut 1991, 32, 1175–1179. [Google Scholar] [CrossRef]
- Martelli, H.; Devroede, G.; Arhan, P.; Duguay, C. Mechanisms of Idiopathic Constipation: Outlet Obstruction. Gastroenterology 1978, 75, 623–631. [Google Scholar] [CrossRef]
- Mertz, H.; Naliboff, B.; Mayer, E. Physiology of Refractory Chronic Constipation. Am. J. Gastroenterol. 1999, 94, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, A.A.; Moshkovitz, M.; Nudelman, I.; Dinari, G.; Reiss, R. Anal pressure measurements in the study of haemorrhoid etiology and their relation to treatment. Dis. Colon Rectum. 1987, 30, 855–857. [Google Scholar] [CrossRef]
- El-Gendi, M.A.F.; Abdel-Baky, N. Anorectal pressure in patients with symptomatic haemorrhoids. Dis. Colon Rectum. 1986, 29, 388–391. [Google Scholar] [CrossRef]
- Ho, Y.H.; Seow-Choen, F.; Goh, H.S. Haemorrhoidectomy and disordered rectal and anal physiology in patients with prolapsed haemorrhoids. Br. J. Surg. 1995, 82, 596–598. [Google Scholar] [CrossRef]
- Pimentel, M.; Lin, H.C.; Enayati, P.; Burg, B.V.D.; Lee, H.-R.; Chen, J.H.; Park, S.; Kong, Y.; Conklin, J. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am. J. Physiol. Liver Physiol. 2006, 290, G1089–G1095. [Google Scholar] [CrossRef]
- Sun, W.M.; Peck, R.J.; Shorthouse, A.J.; Read, N.W. Haemorrhoids are associated not with hypertrophy of the internal anal sphincter, but with hypertension of the anal cushions. Br. J. Surg. 1992, 79, 592–594. [Google Scholar] [CrossRef]
- Sun, W.M.; Read, N.W.; Shorthouse, A.J. Hypertensive anal cushions as a cause of the high anal canal pressures in patients with haemorrhoids. Br. J. Surg. 1990, 77, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K. Anal manometric studies in haemorrhoids and anal fissures. Dis. Colon Rectum. 1989, 32, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Bleijenberg, G.; Kuijpers, H.C. Treatment of the spastic pelvic floor syndrome with biofeedback. Dis. Colon Rectum. 1987, 30, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Hatfield, R.; Soffer, E.; Rao, S.; Beaty, J.; Conklin, J.L. Manometric tests of anorectal function in healthy adults. Am. J. Gastroenterol. 1999, 94, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Duthie, G.S.; Bartolo, D.C.C. Anismus: The cause of constipation? Results of investigation and treatment. World J. Surg. 1992, 16, 831–835. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Kavlock, R.; Rao, S. Influence of body position and stool characteristics on defecation in humans. Am. J. Gastroenterol. 2006, 101, 2790–2796. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Welcher, K.D.; Leistikow, J.S. Obstructive defecation: A failure of rectoanal coordination. Am. J. Gastroenterol. 1998, 93, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Waldron, D.J.; Kumar, D.; Hallan, R.I.; Williams, N.S. Prolonged ambulant assessment of anorectal function in patients with prolapsing haemorrhoids. Dis. Colon Rectum. 1989, 32, 968–974. [Google Scholar] [CrossRef]
- Bassotti, G.; Betti, C.; Imbimbo, B.; Pelli, M.A.; Morelli, A. Colonic motor response to eating: A manometric investigation in proximal and distal portions of the viscus in man. Am. J. Gastroenterol. 1989, 84, 118–122. [Google Scholar]
- Rao, S.S.; Sadeghi, P.; Beaty, J.; Kavlock, R. Ambulatory 24-Hour Colonic Manometry in Slow-Transit Constipation. Am. J. Gastroenterol. 2004, 99, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Welcher, K. Periodic rectal motor activity: The intrinsic colonic gatekeeper? Am. J. Gastroenterol. 1996, 91, 890–897. [Google Scholar]
- Rao, S.S.C.; Sadeghi, P.; Batterson, K.; Beaty, J. Altered periodic rectal motor activity: A mechanism for slow transit constipation. Neurogastroenterol. Motil. 2001, 13, 591–598. [Google Scholar] [CrossRef]
- Corsetti, M.; Pagliaro, G.; Demedts, I.; Deloose, E.; Gevers, A.; Scheerens, C.; Rommel, N.; Tack, J. Pan-Colonic Pressurizations Associated with Relaxation of the Anal Sphincter in Health and Disease: A New Colonic Motor Pattern Identified Using High-Resolution Manometry. Am. J. Gastroenterol. 2017, 112, 479–489. [Google Scholar] [CrossRef]
- Bassotti, G.; Gaburri, M. Manometric investigation of high-amplitude propagated contractile activity of the human colon. Am. J. Physiol. Liver Physiol. 1988, 255, G660–G664. [Google Scholar] [CrossRef] [PubMed]
- Milkova, N.; Parsons, S.P.; Ratcliffe, E.; Huizinga, J.D.; Chen, J.-H. On the nature of high-amplitude propagating pressure waves in the human colon. Am. J. Physiol. Liver Physiol. 2020, 318, G646–G660. [Google Scholar] [CrossRef] [PubMed]
- Aldars-García, L.; Marin, A.C.; Chaparro, M.; Gisbert, J.P. The Interplay between Immune System and Microbiota in Inflammatory Bowel Disease: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 3076. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Preidis, G.A.; Shulman, R.; Kashyap, P.C. The Gut Microbiome in Adult and Pediatric Functional Gastrointestinal Disorders. Clin. Gastroenterol. Hepatol. 2019, 17, 256–274. [Google Scholar] [CrossRef]
- Shin, S.P.; Choi, Y.M.; Kim, W.H.; Hong, S.P.; Park, J.-M.; Kim, J.; Kwon, O.; Lee, E.H.; Hahm, K.B. A double blind, placebo-controlled, randomized clinical trial that breast milk derived-Lactobacillus gasseri BNR17 mitigated diarrhea-dominant irritable bowel syndrome. J. Clin. Biochem. Nutr. 2018, 62, 179–186. [Google Scholar] [CrossRef]
- Dior, M.; Delagrèverie, H.; Duboc, H.; Jouet, P.; Coffin, B.; Brot, L.; Humbert, L.; Trugnan, G.; Seksik, P.; Sokol, H.; et al. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol. Motil. 2016, 28, 1330–1340. [Google Scholar] [CrossRef]
- Le Nevé, B.; Brazeilles, R.; Derrien, M.; Tap, J.; Guyonnet, D.; Ohman, L.; Törnblom, H.; Simrén, M. Lactulose Challenge Determines Visceral Sensitivity and Severity of Symptoms in Patients with Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2016, 14, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Labus, J.S.; Hollister, E.B.; Jacobs, J.; Kirbach, K.; Oezguen, N.; Gupta, A.; Acosta, J.; Luna, R.A.; Aagaard, K.; Versalovic, J.; et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome 2017, 5, 49. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, F.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar Fecal Microbiota Signatures in Patients with Diarrhea-Predominant Irritable Bowel Syndrome and Patients with Depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611.e5. [Google Scholar] [CrossRef]
- Azpiroz, F.; Dubray, C.; Bernalier-Donadille, A.; Cardot, J.-M.; Accarino, A.; Serra, J.; Wagner, A.; Respondek, F.; Dapoigny, M. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: A randomized, double blind, placebo controlled study. Neurogastroenterol. Motil. 2017, 29, e12911. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, G.; Noor, S.O.; Ridgway, K.; Scovell, L.; Jamieson, C.; Johnson, I.T.; Colquhoun, I.J.; Kemsley, E.K.; Narbad, A. Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome. J. Proteome Res. 2011, 10, 4208–4218. [Google Scholar] [CrossRef]
- Heitkemper, M.M.; Cain, K.C.; Shulman, R.J.; Burr, R.L.; Ko, C.; Hollister, E.B.; Callen, N.; Zia, J.; Han, C.J.; Jarrett, M.E. Stool and urine trefoil factor 3 levels: Associations with symptoms, intestinal permeability, and microbial diversity in irritable bowel syndrome. Benef. Microbes 2018, 9, 345–355. [Google Scholar] [CrossRef]
- Bednarska, O.; Walter, S.A.; Casado-Bedmar, M.; Ström, M.; Salvo-Romero, E.; Vicario, M.; Mayer, E.A.; Keita, V. Vasoactive Intestinal Polypeptide and Mast Cells Regulate Increased Passage of Colonic Bacteria in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 948–960.e3. [Google Scholar] [CrossRef] [PubMed]
- Valentin, N.; Camilleri, M.; Carlson, P.; Harrington, S.C.; Eckert, D.; O’Neill, J.; Burton, D.; Chen, J.; Shaw, A.L.; Acosta, A. Potential mechanisms of effects of serum-derived bovine immunoglobulin/protein isolate therapy in patients with diarrhea-predominant irritable bowel syndrome. Physiol. Rep. 2017, 5, e13170. [Google Scholar] [CrossRef]
- Ko, S.-J.; Han, G.; Kim, S.-K.; Seo, J.-G.; Chung, W.-S.; Ryu, B.; Kim, J.; Yeo, I.; Lee, B.-J.; Lee, J.-M.; et al. Effect of Korean Herbal Medicine Combined with a Probiotic Mixture on Diarrhea-Dominant Irritable Bowel Syndrome: A Double-Blind, Randomized, Placebo-Controlled Trial. Evid. Based Complement. Altern. Med. 2013, 2013, 824605. [Google Scholar] [CrossRef]
- Compare, D.; Rocco, A.; Coccoli, P.; Angrisani, D.; Sgamato, C.; Iovine, B.; Salvatore, U.; Nardone, G. Lactobacillus casei DG and its postbiotic reduce the inflammatory mucosal response: An ex-vivo organ culture model of post-infectious irritable bowel syndrome. BMC Gastroenterol. 2017, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2017, 29, e12969. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Sundin, J.; Rangel, I.; Fuentes, S.; Jong, I.H.-D.; Hultgren-Hörnquist, E.; de Vos, W.M.; Brummer, R.J. Altered faecal and mucosal microbial composition in post-infectious irritable bowel syndrome patients correlates with mucosal lymphocyte phenotypes and psychological distress. Aliment. Pharmacol. Ther. 2015, 41, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Sundin, J.; Rangel, I.; Repsilber, D.; Brummer, R.-J. Cytokine Response after Stimulation with Key Commensal Bacteria Differ in Post-Infectious Irritable Bowel Syndrome (PI-IBS) Patients Compared to Healthy Controls. PLoS ONE 2015, 10, e0134836. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.; O’Toole, P.; Öhman, L.; Claesson, M.; Deane, J.; Quigley, E.M.M.; Simrén, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Chen, J.; Chen, X.; Chia, N.; O’Connor, H.M.; Wolf, P.G.; Gaskins, H.R.; Bharucha, A.E. Relationship between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients with Chronic Constipation. Gastroenterology 2016, 150, 367–379.e1. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, X.; An, Y.; Zhou, G.; Liu, Y.; Xu, M.; Dong, W.; Wang, S.; Yan, F.; Jiang, K.; et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci. Rep. 2017, 7, 10322. [Google Scholar] [CrossRef]
- Pimentel, M.; Chatterjee, S.; Chow, E.J.; Park, S.; Kong, Y. Neomycin Improves Constipation-Predominant Irritable Bowel Syndrome in a Fashion That Is Dependent on the Presence of Methane Gas: Subanalysis of a Double-Blind Randomized Controlled Study. Dig. Dis. Sci. 2006, 51, 1297–1301. [Google Scholar] [CrossRef]
- Pimentel, M.; Mayer, A.G.; Park, S.; Chow, E.J.; Hasan, A.; Kong, Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig. Dis. Sci. 2003, 48, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Attaluri, A.; Jackson, M.; Valestin, J.; Rao, S.S. Methanogenic Flora Is Associated with Altered Colonic Transit but Not Stool Characteristics in Constipation without IBS. Am. J. Gastroenterol. 2010, 105, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zhao, W.; Ding, C.; Tian, H.; Xu, L.; Wang, H.; Ni, L.; Jiang, J.; Gong, J.; Zhu, W.; et al. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Turroni, F.; Mangifesta, M.; Viappiani, A.; Ticinesi, A.; Nouvenne, A.; Meschi, T.; van Sinderen, D.; et al. Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Sci. Rep. 2017, 7, 9879. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.G.; Parthasarathy, G.; Chen, J.; O’Connor, H.M.; Chia, N.; Bharucha, A.E.; Gaskins, H.R. Assessing the colonic microbiome, hydrogenogenic and hydrogenotrophic genes, transit and breath methane in constipation. Neurogastroenterol. Motil. 2017, 29, e13056–e13059. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, W.; Alkhouri, R.; Baker, R.D.; Bard, J.E.; Quigley, E.M.; Baker, S.S. Structural changes in the gut microbiome of constipated patients. Physiol. Genom. 2014, 46, 679–686. [Google Scholar] [CrossRef]
- Coccorullo, P.; Strisciuglio, C.; Martinelli, M.; Miele, E.; Greco, L.; Staiano, A. Lactobacillus reuteri (DSM 17938) in Infants with Functional Chronic Constipation: A Double-Blind, Randomized, Placebo-Controlled Study. J. Pediatr. 2010, 157, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.V.P.; Lima, L.N.; Souza, T.C.; Mazochi, V.; Penna, F.J.; Silva, A.M.; Nicoli, J.R.; Guimarães, E.V. Pediatric functional constipation treatment with bifidobacterium-containing yogurt: A crossover, double-blind, controlled trial. WJG 2011, 17, 3916–3921. [Google Scholar] [CrossRef]
- Wojtyniak, K.; Szajewska, H. Systematic review: Probiotics for functional constipation in children. Eur. J. Pediatr. 2017, 176, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- de Morais, M.B.; Vitolo, M.R.; Aguirre, A.N.C.; Fagundes-Neto, U. Measurement of Low Dietary Fiber Intake As a Risk Factor for Chronic Constipation in Children. J. Craniofacial Surg. 1999, 29, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Pijpers, M.A.M.; Tabbers, M.M.; Benninga, M.A.; Berger, M.Y. Currently recommended treatments of childhood constipation are not evidence based: A systematic literature review on the effect of laxative treatment and dietary measures. Arch. Dis. Child. 2009, 94, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Tabbers, M.M.; Benninga, M.A. Constipation in children: Fibre and probiotics. BMJ Clin. Évid. 2015, 2015, 0303. [Google Scholar] [PubMed]
- Tabbers, M.M.; Boluyt, N.; Berger, M.Y.; Benninga, M.A. Nonpharmacologic Treatments for Childhood Constipation: Systematic Review. Pediatrics 2011, 128, 753–761. [Google Scholar] [CrossRef]
- Gordon, M.; Naidoo, K.; Akobeng, A.K.; Thomas, A.G. Cochrane Review: Osmotic and stimulant laxatives for the management of childhood constipation (Review). Evid. Based Child Health A Cochrane Rev. J. 2013, 8, 57–109. [Google Scholar] [CrossRef]
- Mishra, K.; Bukavina, L.; Ghannoum, M. Symbiosis and Dysbiosis of the Human Mycobiome. Front. Microbiol. 2021, 12, 636131. [Google Scholar] [CrossRef]
- Dodi, G.; Pirone, E.; Bettin, A.; Veller, C.; Infantino, A.; Pianon, P.; Mortellaro, L.M.; Lise, M. The mycotic flora in proctological patients with and without pruritus ani. Br. J. Surg. 1985, 72, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Nonzom, S. Superficial mycoses, a matter of concern: Global and Indian scenario-an updated analysis. Mycoses 2021, 64, 890–908. [Google Scholar] [CrossRef]
- Sauper, T.; Lanthaler, M.; Biebl, M.; Weiss, H.; Nehoda, H. Impaired anal sphincter function in professional cyclists. Wien. Klin. Wochenschr. 2007, 119, 170–173. [Google Scholar] [CrossRef]
- Chong, P.S.; Bartolo, D.C.C. Hemorrhoids and Fissure in Ano. Gastrointest. Endosc. Clin. N. Am. 2008, 37, 627–644. [Google Scholar] [CrossRef]
- Perera, N.; Liolitsa, D.; Iype, S.; Croxford, A.; Yassin, M.; Lang, P.; van Issum, C. Phlebotonics for haemorrhoids. Cochrane. Database. Syst. Rev. 2012, 8, CD004322. [Google Scholar] [CrossRef] [PubMed]
- Madoff, R.D.; Fleshman, J.W. American gastroenterological association technical review on the diagnosis and treatment of hemorrhoids. Gastroenterology 2004, 126, 1463–1473. [Google Scholar] [CrossRef]
- Ganz, R.A. The evaluation and treatment of haemorrhoids: A guide for the gastroenterologist. Clin. Gastroenterol. Hepatol. 2013, 11, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Massot, J.; Sanchez, O.; Couchy, R.; Astoin, J.; Parodi, A.L. Bacterio-pharmacological activity of Saccharomyces boulardii in clindamycin-induced colitis in the hamster. Arzneimittelforschung 1984, 34, 794–797. [Google Scholar]
- Lessard, M.; Dupuis, M.; Gagnon, N.; Nadeau, E.; Matte, J.J.; Goulet, J.; Fairbrother, J.M. Administration of Pediococcus acidilactici or Saccharomyces cerevisiae boulardii modulates development of porcine mucosal immunity and reduces intestinal bacterial translocation after Escherichia coli challenge1,2. J. Anim. Sci. 2009, 87, 922–934. [Google Scholar] [CrossRef]
- Gaziano, R.; Sabbatini, S.; Roselletti, E.; Perito, S.; Monari, C. Saccharomyces cerevisiae-Based Probiotics as Novel Antimicrobial Agents to Prevent and Treat Vaginal Infections. Front. Microbiol. 2020, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.L.; Liang, J.B.; Jahromi, M.F.; Wu, Y.B.; Wright, A.G.; Liao, X.D. Mode of action of Saccharomyces cerevisiae in enteric methane mitigation in pigs. Animal 2018, 12, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Hager, C.L.; Ghannoum, M.A. The mycobiome: Role in health and disease, and as a potential probiotic target in gastrointestinal disease. Dig. Liver Dis. 2017, 49, 1171–1176. [Google Scholar] [CrossRef]
- Kumamoto, C.A. Inflammation and gastrointestinal Candida colonization. Curr. Opin. Microbiol. 2011, 14, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Zwolinska-Wcislo, M.; Brzozowski, T.; Budak, A.; Kwiecień, S.; Sliwowski, Z.; Drozdowicz, D.; Trojanowska, D.; Rudnicka-Sosin, L.; Mach, T.; Konturek, S.J.; et al. Effect of Candida colonization on human ulcerative colitis and the healing of inflammatory changes of the colon in the experimental model of colitis ulcerosa. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60, 107–118. [Google Scholar]
- Zwolińska-Wcisło, M.; Brzozowski, T.; Mach, T.; Budak, A.; Trojanowska, D.; Konturek, P.C.; Pajdo, R.; Drozdowicz, D.; Kwiecień, S. Are probiotics effective in the treatment of fungal colonization of the gastrointestinal tract? Experimental and clinical studies. J. Physiol. Pharmacol Off. J. Pol. Physiol. Soc. 2006, 57 (Suppl. S9), 35–49. [Google Scholar]
- Brzozowski, T.; Zwolinska-Wcislo, M.; Konturek, P.C.; Kwiecien, S.; Drozdowicz, D.; Konturek, S.J.; Stachura, J.; Budak, A.; Bogdal, J.; Pawlik, W.W.; et al. Influence of gastric colonization with Candida albicans on ulcer healing in rats: Effect of ranitidine, aspirin and probiotic therapy. Scand. J. Gastroenterol. 2005, 40, 286–296. [Google Scholar] [CrossRef]
- Ng, K.S.; Holzgang, M.; Young, C. Still a Case of “No Pain, No Gain”? An Updated and Critical Review of the Pathogenesis, Diagnosis, and Management Options for Hemorrhoids in 2020. Ann. Coloproctol. 2020, 36, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Ng, S.C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Panpetch, W.; Hiengrach, P.; Nilgate, S.; Tumwasorn, S.; Somboonna, N.; Wilantho, A.; Chatthanathon, P.; Prueksapanich, P.; Leelahavanichkul, A. Additional Candida albicans administration enhances the severity of dextran sulfate solution induced colitis mouse model through leaky gut-enhanced systemic inflammation and gut-dysbiosis but attenuated by Lactobacillus rhamnosus L34. Gut Microbes 2020, 11, 465–480. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).