Exploring the Bio-Functional Effect of Single Nucleotide Polymorphisms in the Promoter Region of the TNFSF4, CD28, and PDCD1 Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. SNP Selection
2.2. Promoter–Reporter Assay
2.2.1. Constructing the Template of Promoter–Reporter and Plasmid
2.2.2. Construction of Luciferase Expression Vectors and Transformation
2.2.3. Dual-Luciferase Reporter Assay
2.3. Statistical Analysis
3. Results
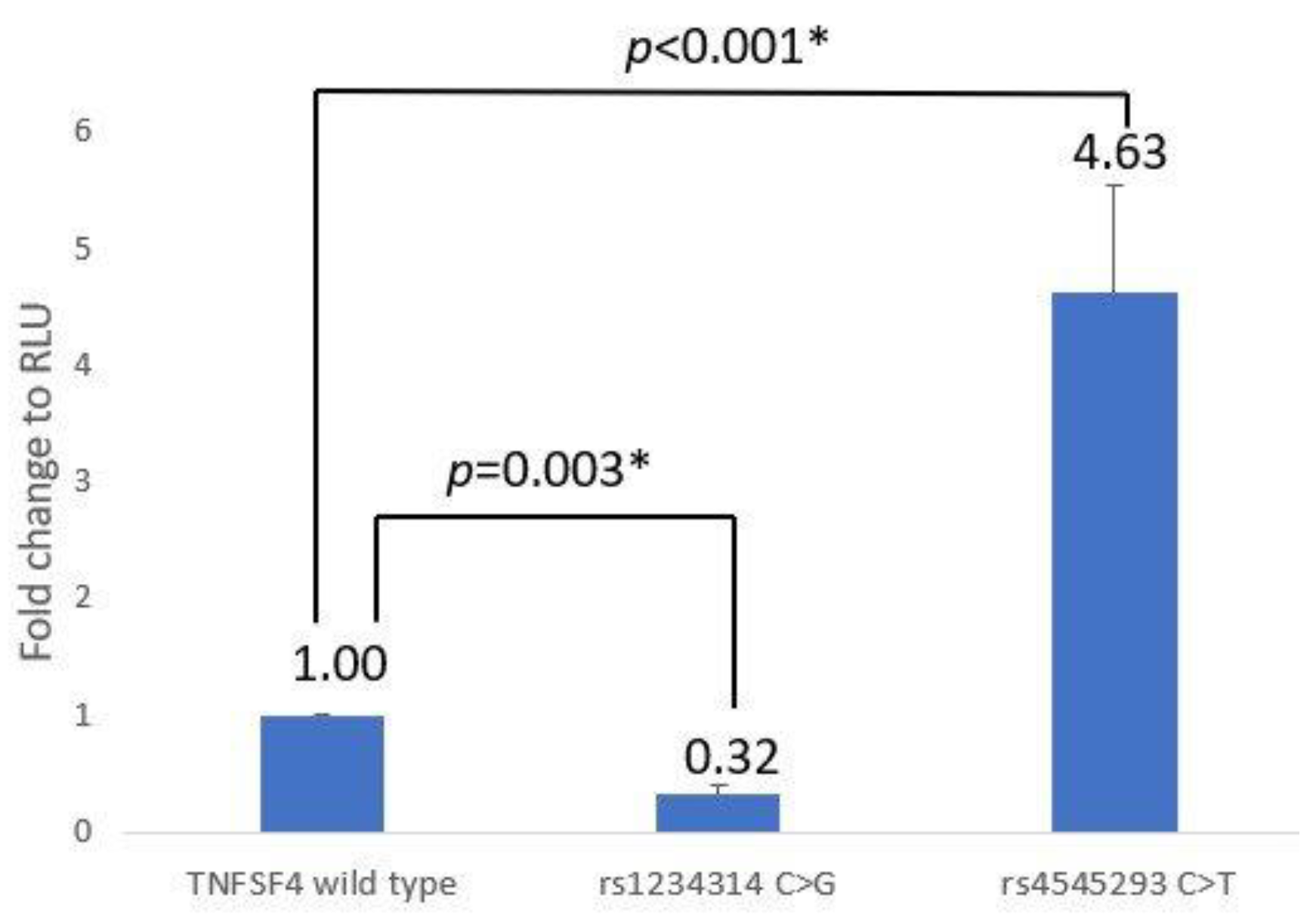
3.1. TNFSF4 Promoter–Reporter Assay
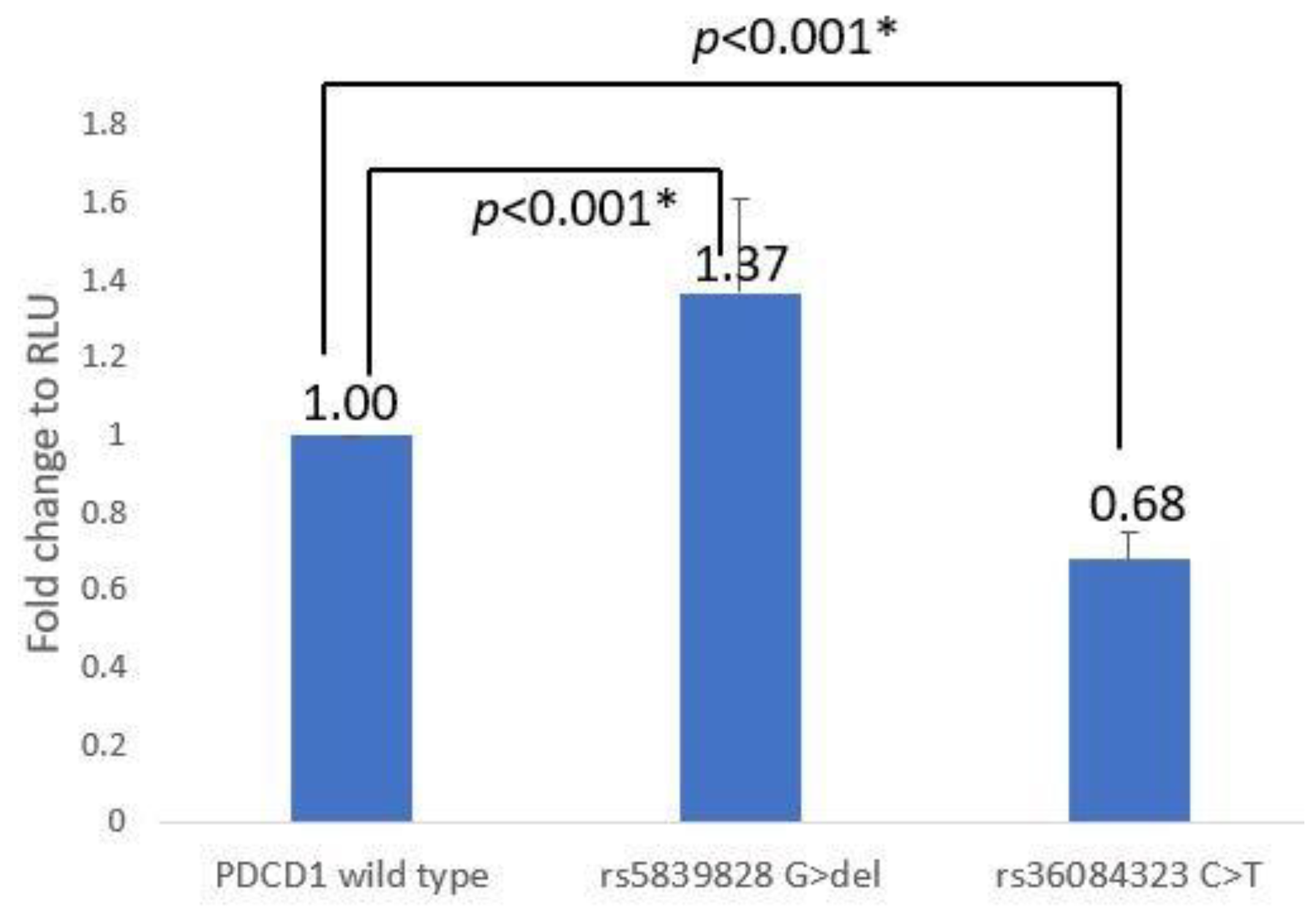
3.2. PDCD-1 Promoter–Reporter Assay
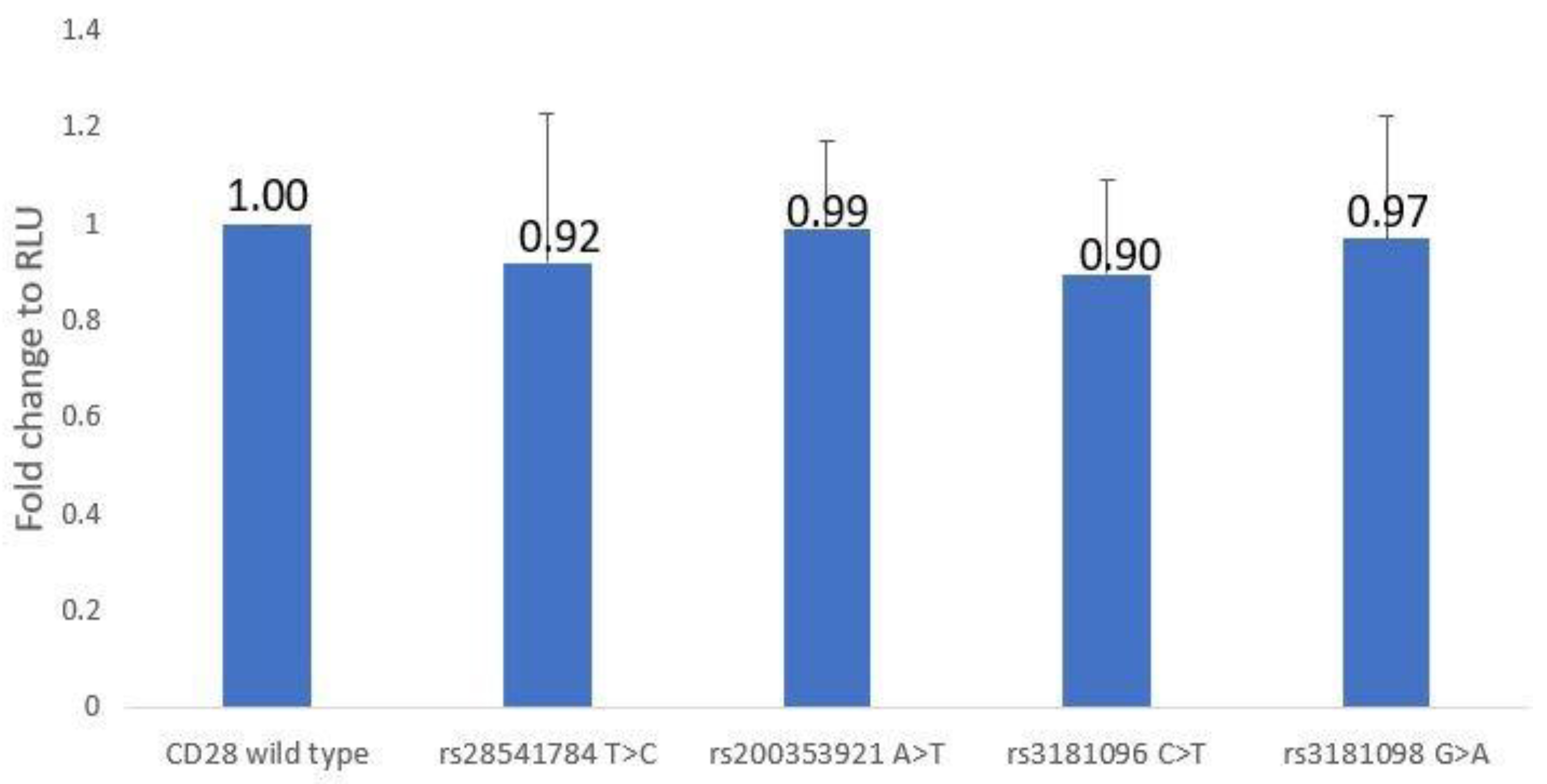
3.3. CD28 Promoter–Reporter Assay
4. Discussion
4.1. About TNFSF4 Analysis
4.2. About PDCD1 Analysis
4.3. About CD28 Analysis
4.4. The Significant and Further Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatia, S.; Francisco, L.; Carter, A.; Sun, C.-L.; Baker, K.S.; Gurney, J.G.; McGlave, P.B.; Nademanee, A.; O’Donnell, M.; Ramsay, N.K.C.; et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: Report from the Bone Marrow Transplant Survivor Study. Blood 2007, 110, 3784–3792. [Google Scholar] [CrossRef] [PubMed]
- Bieri, S.; Roosnek, E.; Ozsahin, H.; Huguet, S.; Ansari, M.; Trombetti, A.; Helg, C.; Chapuis, B.; Miralbell, R.; Passweg, J.; et al. Outcome and risk factors for late onset complications 24 months beyond allogeneic hematopoietic stem cell transplantation. Eur. J. Haematol. 2011, 87, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.A.; Chien, J.W.; Warren, E.H.; Zhao, L.P.; Martin, P.J. Defining genetic risk for graft versus-host disease and mortality following allogeneic hematopoietic stem cell transplantation. Curr. Opin. Hematol. 2010, 17, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.M.; Holler, E. Polymorphisms of cytokine and innate immunity genes and GVHD. Best Pract. Res. Clin. Haematol. 2008, 21, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.E.; Abdi, R. Immunoregulatory gene polymorphisms and graft versus-host disease. Exp. Rev. Clin. Immunol. 2009, 5, 523–534. [Google Scholar] [CrossRef]
- Ting, C.; Alterovitz, G.; Merlob, A.; Abdi, R. Genomic studies of GVHD lessons learned thus far. Bone Marrow Transpl. 2013, 48, 4–9. [Google Scholar] [CrossRef]
- Bonnefoy-Berard, N.; Besnard, V.; Morel, P.; Hmama, Z.; Verrier, B.; Mandrand, B.; Vincent, C.; Revillard, J.P. Second signal for T lymphocyte activation: Multiple targets for pharmacological modulation. Dev. Biol. Stand. 1992, 77, 41–48. [Google Scholar]
- Linsley, P.S.; Ledbetter, J.A. The role of the CD28 receptor during T cell responses to antigen. Annu. Rev. Immunol. 1993, 11, 191–212. [Google Scholar] [CrossRef]
- Bluestone, J.A. New perspectives of CD28-B7-mediated T cell costimulation. Immunity 1995, 2, 555–559. [Google Scholar] [CrossRef]
- Rothstein, D.M.; Sayegh, M.H. T-cell costimulatory pathways in allograft rejection and tolerance. Immunol. Rev. 2003, 196, 85–108. [Google Scholar] [CrossRef]
- Boenisch, O.; Sayegh, M.H.; Najafian, N. Negative T-cell costimulatory pathways: Their role in regulating alloimmune responses. Curr. Opin. Organ Transpl. 2008, 13, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Jindra, P.T.; Conway, S.E.; Ricklefs, S.M.; Porcella, S.F.; Anzick, S.L.; Haagenson, M.; Wang, T.; Spellman, S.; Milford, E.; Kraft, P.; et al. Analysis of a genetic polymorphism in the costimulatory molecule TNFSF4 with hematopoietic stem cell transplant outcomes. Biol. Blood Marrow Transpl. 2016, 22, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Z.; Martin, P.J.; Anasetti, C. Role of CD28 in acute graft-versus-host disease. Blood 1998, 92, 2963–2970. [Google Scholar] [CrossRef] [PubMed]
- Wallace, P.M.; Johnson, J.S.; MacMaster, J.F.; Kennedy, K.A.; Gladstone, P.; Linsley, P.S. CTLA4Ig treatment ameliorates the lethality of murine graft-versus-host disease across major histocompatibility complex barriers. Transplantation 1994, 58, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, K. Targeting immune signaling checkpoints in acute myeloid leukemia. J. Clin. Med. 2019, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.E.; Cao, X. Co-stimulatory and co-inhibitory pathways in cancer immunotherapy. Adv. Cancer Res. 2019, 143, 145–194. [Google Scholar]
- Riella, L.V.; Paterson, A.M.; Sharpe, A.H.; Chandraker, A. Role of the PD-1 pathway in the immune response. Am. J. Transpl. 2012, 12, 2575–2587. [Google Scholar] [CrossRef]
- Simonetta, F.; Pradier, A.; Bosshard, C.; Masouridi-Levrat, S.; Dantin, C.; Koutsi, A.; Tirefort, Y.; Roosnek, E.; Chalandon, Y. Dynamics of expression of programmed cell death protein-1 (PD-1) on T cells after allogeneic hematopoietic stem cell transplantation. Front. Immunol. 2019, 10, 1034. [Google Scholar] [CrossRef]
- Baroni, M.L.; Sanchez Martinez, D.; Gutierrez Aguera, F.; Roca Ho, H.; Castella, M.; Zanetti, S.R.; Hernandez, T.V.; de la Guardia, R.D.; Castaño, J.; Anguita, E.; et al. 41BB-based and CD28-based CD123-redirected T-cells ablate human normal hematopoiesis in vivo. J. Immunother. Cancer 2020, 8, e000845. [Google Scholar] [CrossRef]
- Harkensee, C.; Oka, A.; Onizuka, M.; Middleton, P.G.; Inoko, H.; Hirayasu, K.; Kashiwase, K.; Yabe, T.; Nakaoka, H.; Gennery, A.R.; et al. Single nucleotide polymorphisms and outcome risk in unrelated mismatched hematopoietic stem cell transplantation: An exploration study. Blood 2012, 119, 6365–6372. [Google Scholar] [CrossRef]
- Bluestone, J.A.; Bour-Jordan, H.; Cheng, M.; Anderson, M. T cells in the control of organ-specific autoimmunity. J. Clin. Investig. 2015, 125, 2250–2260. [Google Scholar] [CrossRef]
- Dornmair, K.; Goebels, N.; Weltzien, H.U.; Wekerle, H.; Hohlfeld, R. T-cell-mediated autoimmunity: Novel techniques to characterize autoreactive T-cell receptors. Am. J. Pathol. 2003, 163, 1215–1226. [Google Scholar] [CrossRef]
- Gough, S.C.; Simmonds, M.J. The HLA Region and Autoimmune Disease: Associations and Mechanisms of Action. Curr. Genom. 2007, 8, 453–465. [Google Scholar]
- Kobata, T.; Azuma, M.; Yagita, H.; Okumura, K. Role of costimulatory molecules in autoimmunity. Rev. Immunogenet. 2000, 2, 74–80. [Google Scholar]
- Lenschow, D.J.; Walunas, T.L.; Bluestone, J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996, 14, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, C.; Schmitt, N.; Contin-Bordes, C.; Liu, Y.; Narayanan, P.; Seneschal, J.; Maurouard, T.; Dougall, D.; Davizon, E.S.; Dumortier, H.; et al. OX40 ligand contributes to human lupus pathogenesis by promoting T follicular helper response. Immunity 2015, 42, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.P.; Jaing, T.H.; Hour, A.L.; Lin, W.T.; Hsu, F.P. Single-nucleotide polymorphisms within non-HLA regions are associated with engraftment effectiveness for patients with unrelated cord blood transplantation. Front. Immunol. 2022, 13, 888204. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.P.; Chang, S.W.; Wang, P.N.; Lin, W.T.; Hsu, F.P.; Wang, W.T.; Tseng, C.P. The association between single-nucleotide polymorphisms of co-stimulatory genes within non-HLA region and the prognosis of leukemia patients with hematopoietic stem cell transplantation. Front. Immunol. 2021, 12, 730507. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.P.; Lin, W.T.; Yu, K.H. Investigation of the association between the genetic polymorphisms of the co-stimulatory system and systemic lupus erythematosus. Front. Immunol. 2022, 13, 946456. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nature reviews. Immunology 2013, 13, 227–242. [Google Scholar] [PubMed]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef] [PubMed]
- Tanhapour, M.; Vaisi-Raygani, A.; Khazaei, M.; Rahimi, Z.; Pourmotabbed, T. Cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) polymorphism, cancer, and autoimmune diseases. AIMS Med. Sci. 2017, 4, 395–412. [Google Scholar] [CrossRef]
- Fu, Y.; Lin, Q.; Zhang, Z.; Zhang, L. Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm. Sin. B 2020, 10, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Redmond, W.L.; Ruby, C.E.; Weinberg, A.D. The role of OX40-mediated co-stimulation in T-cell activation and survival. Crit. Rev. Immunol. 2009, 29, 187–201. [Google Scholar] [CrossRef]
- Tripathi, T.; Yin, W.; Xue, Y.; Zurawski, S.; Fujita, H.; Hanabuchi, S.; Liu, Y.-J.; Oh, S.; Joo, H. Central roles of OX40L-OX40 interaction in the induction and progression of human T cell-driven acute graft-versus-host disease. Immuno Horiz. 2019, 3, 110–120. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, Y.; Wang, X.Q.; Ke, X.; Kang, H.Y.; Hong, S.L. Association between TNFSF4 and BLK gene polymorphisms and susceptibility to allergic rhinitis. Mol. Med. Rep. 2017, 16, 3224–3232. [Google Scholar] [CrossRef]
- Karl, F.; Hudecek, M.; Berberich-Siebelt, F.; Mackensen, A.; Mougiakakos, D. T-cell metabolism in graft versus host disease. Front. Immunol. 2021, 12, 760008. [Google Scholar] [CrossRef]
- Krovi, S.H.; Kuchroo, V.K. Activation pathways that drive CD4+ T cells to break tolerance in autoimmune diseases. Immunol. Rev. 2022, 307, 161–190. [Google Scholar] [CrossRef]
- Riley, J.L. PD-1 signaling in primary T cells. Immunol. Rev. 2009, 229, 114–125. [Google Scholar] [CrossRef]
- Fife, B.T.; Pauken, K.E. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann. N. Y. Acad. Sci. 2011, 1217, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska-Byczkiewicz, K.; Kołacińska-Wow, A. Liquid biopsy in targeting gene polymorphism related to the response within immunocheckpoint inhibitors therapeutic regimen. Med. Res. J. 2021, 6, 245–248. [Google Scholar] [CrossRef]
- Sasaki, H.; Tatemaysu, T.; Okuda, K.; Moriyama, S.; Yano, M.; Fujii, Y. PD-1 gene promoter polymorphisms correlate with a poor prognosis in non-small cell lung cancer. Mol. Clin. Oncol. 2014, 2, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, Y.; Yukaya, N.; Kusuhara, K.; Kira, R.; Torisu, H.; Ihara, K.; Sakai, Y.; Sanefuji, M.; Pipo-Deveza, J.R.; Silao, C.L.T.; et al. PD1 as a common candidate susceptibility gene of subacute sclerosing panencephalitis. Hum. Genet. 2010, 127, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.F.; Wang, Y.Q.; Luo, H.R. Drug-related genomics in cancer and immunological diseases. Int. J. Genom. 2014, 2014, 306980. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Semple, K.; Suh, W.-K.; Liu, C.; Chen, F.; Blazar, B.R.; Yu, X.-Z. Roles of CD28, CTLA4, and inducible costimulator in acute graft-versus-host disease in mice. Biology of blood and marrow transplantation. Biol. Blood Marrow Transpl. 2011, 17, 962–969. [Google Scholar] [CrossRef]
- Wen, Y.H.; Lin, W.T.; Wang, W.T.; Hen, D.P. CD28 Gene Polymorphisms in the Promoter Region Are Associated with Transfusion Reactions: A Functional Study. J. Clin. Med. 2021, 10, 871. [Google Scholar] [CrossRef]
| Primer | Sequence | NCBI Position |
|---|---|---|
| SacI-TNFSF4F | 5′-GGCG GAGCTC CT CAA CAC CAG TAT GTT CTC C-3′ | 173208582 |
| EcoRV-TNFSF4R | 5-GGCG GATATC AA TAG GCA AAG GTC CCA GGG C-3′ | 173207292 |
| Rs1234314CF | 5′-TAC ATC ACA TGA GCC TGG CAC TGT ACT GGA-3′ | 173208253 |
| Rs1234314CR | 5′-TCC AGT ACA GTG CCA G GC TCA TGT GAT GTA | |
| Rs4545293TF | 5′-CTT TCT TTG AGG TTG TGG CTG GCC TCA GAA-3′ | 173208097 |
| Rs4545293TR | 5′-TTC TGA GGC CAG CCA CAA CCT CAA AGA AAG-3′ | |
| SacI-PD1F | 5′-ACTG GAGCTC CA ACC AAC AGT TCT CCA GCC C-3′ | 241858839 |
| EcoRV-PD1R | 5-TTATC GATATC G CCT GGA GCA GCC CCA CCA G-3′ | 241860275 |
| Rs36084323TF | 5′-AAG GGG GAT GGG CCA GGA AGG CAG AGG CCA-3′ | 241859444 |
| Rs36084323TR | 5′-TGG CCT CTG CCT TCC TGG CCC ATC CCC CTT-3′ | |
| Rs5839828delF | 5′-ACCGCCCCAGCCCCCCGTCAGGCTGTTGCAGGCAT-3′ | 241859601 |
| Rs5839828delR | 5′-ATGCCTGCAACAGCCTGACGGGGGGCTGGGGCGGT-3′ | |
| SacI-CD28F | 5′-TAT GAGCTC AGC AGT TGG CCG TGC TGG TGG AAT-3′ | 203705243 |
| HindIII-CD28P | 5′-TTA T AAGCTT GG GTT CCA GCC CCT CCT CCC CGA-3′ | 203706675 |
| Rs28541784CF | 5′-CCTTCCCTCCCTCCCTCTCTCTTTCTTTCCATCTT-3′ | 203705806 |
| Rs28541784CR | 5′-AAGATGGAAAGAAAGAGAGAGGGAGGGAGGGAAGG-3′ | |
| Rs200353921TF | 5′-CCCTCCCTCTTTCTTTCTTTCCTTCTTTCTTTCTTTC-3′ | 203705818 |
| Rs200353921TR | 5′-GAAAGAAAGAAAGAAGGAAAGAAAGAAAGAGGGAGGG-3′ | |
| Rs3181096TF | 5′-CTCCTTTTGTGCCCTATTATTTAACCTTGAGGG-3′ | 203705369 |
| Rs3181096TR | 5′-CCCTCAAGGTTAAATAATAGGGCACAAAAGGAG-3′ | |
| Rs3181098AF | 5′-GTAACTCCTTTAAACATTTATGCAGATGTTTCCC-3′ | 203705655 |
| Rs3181098AR | 5′-GGGAAACATCTGCATAAATGTTTAAAGGAGTTAC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.-P.; Wen, Y.-H.; Wang, W.-T.; Lin, W.-T. Exploring the Bio-Functional Effect of Single Nucleotide Polymorphisms in the Promoter Region of the TNFSF4, CD28, and PDCD1 Genes. J. Clin. Med. 2023, 12, 2157. https://doi.org/10.3390/jcm12062157
Chen D-P, Wen Y-H, Wang W-T, Lin W-T. Exploring the Bio-Functional Effect of Single Nucleotide Polymorphisms in the Promoter Region of the TNFSF4, CD28, and PDCD1 Genes. Journal of Clinical Medicine. 2023; 12(6):2157. https://doi.org/10.3390/jcm12062157
Chicago/Turabian StyleChen, Ding-Ping, Ying-Hao Wen, Wei-Ting Wang, and Wei-Tzu Lin. 2023. "Exploring the Bio-Functional Effect of Single Nucleotide Polymorphisms in the Promoter Region of the TNFSF4, CD28, and PDCD1 Genes" Journal of Clinical Medicine 12, no. 6: 2157. https://doi.org/10.3390/jcm12062157
APA StyleChen, D.-P., Wen, Y.-H., Wang, W.-T., & Lin, W.-T. (2023). Exploring the Bio-Functional Effect of Single Nucleotide Polymorphisms in the Promoter Region of the TNFSF4, CD28, and PDCD1 Genes. Journal of Clinical Medicine, 12(6), 2157. https://doi.org/10.3390/jcm12062157





