Abstract
Background: Cognitive impairment is a frequent consequence of bipolar disorder (BD) that is difficult to prevent and treat. In addition, the quality of the preliminary evidence on the treatment of BD through Cognitive Remediation (CR) with traditional methods is poor. This study aims to evaluate the feasibility of a CR intervention with fully immersive Virtual Reality (VR) as an additional treatment for BD and offers preliminary data on its efficacy. Methods: Feasibility randomized controlled cross-over clinical study, with experimental condition lasting three months, crossed between two groups. Experimental condition: CR fully immersive VR recovery-oriented program plus conventional care; Control condition: conventional care. The control group began the experimental condition after a three months period of conventional care (waiting list). After the randomization of 50 people with BD diagnosis, the final sample consists of 39 participants in the experimental condition and 25 in the control condition because of dropouts. Results: Acceptability and tolerability of the intervention were good. Compared to the waitlist group, the experimental group reported a significant improvement regarding cognitive functions (memory: p = 0.003; attention: p = 0.002, verbal fluency: p = 0.010, executive function: p = 0.003), depressive symptoms (p = 0.030), emotional awareness (p = 0.007) and biological rhythms (p = 0.029). Conclusions: The results are preliminary and cannot be considered exhaustive due to the small sample size. However, the evidence of efficacy, together with the good acceptability of the intervention, is of interest. These results suggest the need to conduct studies with larger samples that can confirm this data. Trial registration: ClinicalTrialsgov NCT05070065, registered in September 2021
1. Background
Cognitive impairment (CI) is associated with social and functional impairment in individuals that suffer from mental health disorders [1,2]. CI could be defined as a complex relationship of selective hypo and hyperactivity networks linked to attention, verbal fluency, memory, and executive function [2,3]. CI is an important target for rehabilitation for people with bipolar disorder (BD) [3,4,5]. BD is a common, chronic disorder and one of the leading causes of disability worldwide [6,7,8,9]. It is associated with a frequent neurocognitive impairment during the manic, depressive and euthymia phases [10,11], with a long-term risk of developing dementia [12,13]. People with BD often display neuropsychological deficits in their attentional capacities and executive functions (flexibility, working memory, and inhibitory control) and processing speed and verbal/memory learning [13,14,15]. These CI aspects are a barrier to achieving clinical, personal, and social improvements that are essential for a good quality of life [16,17,18]. CI is highly prevalent in patients with BD. About 30% were impaired in at least two different cognitive domains [17]. Although psychopharmacological treatments associated with psychosocial interventions [19,20,21] contribute to the improvement of some core symptoms of BD, such as depressive/manic symptoms, cognitive deficits do not improve and get worse over time [10]. Except for the indirect positive lithium effect, most treatments have cognitive side effects caused by their extrapyramidal, sedative, anticholinergic and blunting mechanisms. Furthermore, given the alteration of the circadian rhythm (including social and behavioral rhythms) that occurs with the onset of BD [7,22] and the comorbidity of anxiety symptoms [23,24], cognitive rehabilitation should be an aim of treatment in BD, in order to promote recovery and a human rights-based approach [25]. Different psychiatric rehabilitation interventions of proven effectiveness, such as Cognitive Remediation (CR) programs and physical activity, are currently used to reduce the cognitive and clinical impairment of people with mental and neurodegenerative disorders [26,27,28,29,30]. These interventions also play a role in the primary prevention of cognitive decline and alterations of the social and behavioral rhythms in healthy populations [31,32,33,34,35]. CR programs include behavioral training aiming to improve cognitive functions, social cognition and metacognition, and the generalization of the achieved goals in daily life [36]. They are effective in the treatment of different mental and neurodegenerative disorders [37,38,39,40,41,42,43,44]. In addition to traditional approaches, different methods of CR interventions (computerized, paper and pencil, in individual or group settings) are available [43,45,46]. Preliminary evidence for the use of traditional CR methods for BD is still limited due to the high risk of bias associated with the sample size and dropout rates [47]. In line with the WHO innovation objective in mental health and global digitalization [48], the use of technologies for the assessment and treatment of mental and neurodegenerative disorders is increasing [49,50]. Virtual Reality (VR), which engages people in playful and ecological scenarios and facilitates the generalization of the trained skills [47,51,52,53,54], is considered an effective add-on intervention in psychosocial rehabilitation [50], particularly in social cognition training (e.g., social/occupational skill training in people with schizophrenia or autism) [50,55] and in psychotherapy to treat anxiety, phobia and post-traumatic syndrome disorders [56,57,58]. VR has also proven to be effective in assessing and treating cognitive deficits in people with Mild Cognitive Impairment (MCI) and Alzheimer’s disease and dementia [59,60,61,62]. The use of fully immersive VR-based CR programs is increasing [63], particularly in the treatment of MCI and schizophrenia. To date, the methodological quality of preliminary evidence is poor [64,65,66]. To our knowledge, no studies on the use of fully immersive VR as a CR intervention aiming to improve the cognitive, personal and social functioning of people with BD are available. Our hypothesis is that a fully immersive VR-based CR intervention could be feasible and clinically effective in people that have experienced BD.
2. Aims
2.1. Primary Aim
To assess the feasibility of a confirmatory trial that evaluates the effectiveness of the use of a VR tool for the treatment of CI among individuals with bipolar disorder.
2.2. Secondary Aim
To evaluate the preliminary efficacy of the trial in terms of intervention’s safety, participants’ satisfaction, and clinical outcomes (regarding cognitive functions and personal and social functioning).
3. Methods
3.1. Study Design
This study is a randomized-controlled (two-arm) cross-over clinical feasibility trial. This study follows the reporting guidelines according to the CONSORT extension for feasibility studies [67]. After the experimental group (A) received the VR-based CR intervention as an add-on to conventional treatment and the control group (B) received only conventional treatment for three months (waiting list), group A underwent a one-month interval period which was followed by a phase of conventional treatment, whereas group B received the VR-based CR intervention as an add-on to conventional treatment and became the experimental group.
The trial [63] was registered in ClinicalTrialsgov (NCT05070065, September 2021).
3.2. Participants
This trial’s target population included people with bipolar disorder recruited at the Consultation and Psychosomatic Psychiatry Center of the University Hospital of Cagliari (San Giovanni di Dio Civil Hospital), who met the following inclusion criteria: (1) age ranging from 18 to 75; (2) diagnosis of bipolar disorder according to DSM-IV [68]; (3) both sexes. The participants that met these inclusion criteria or their guardians were provided with an informed consent form and signed it before the intervention began. Subjects that did not meet the inclusion criteria, or those who showed a current manic/depressive episode, a diagnosis of epilepsy, or serious eye diseases, were excluded due to the risk associated with the excessive stimulation of virtual reality.
After the randomization of 50 people who met the inclusion criteria, the final sample was composed of 39 subjects in the experimental group (due to some dropouts at the follow-up) and 25 in the control group. Of the participants, 33.3% were males, and 66.7% were females. The mean age in the total sample was 47.23 ± 13.37 (Table 1).

Table 1.
Baseline demographic characteristics.
Even if in the registered trial (ClinicalTrialsgov, NCT05070065) we planned to recruit and randomize 60 subjects who met the inclusion criteria, we stopped the enrollment phase early due to the COVID-19 pandemic, which did not allow us to easily involve all the subjects we prevent in the study protocol.
3.3. Randomization
Eligible participants were randomized into two groups. The random allocation sequence was generated using a computer-generated randomization list at the University of Cagliari. Randomization was carried out by a biometrician who was not aware of the participants’ identities and was not involved either in the assessment or in the analysis process.
3.4. Blinding
Neither the participants nor the mental health workers of the project could be blinded due to the nature of the intervention.
3.5. Intervention
The experimental group was involved in a fully immersive VR-based CR recovery-oriented program. We used the “CEREBRUM” software, one of the most recent VR-implemented CR tools in psychiatric rehabilitation, conceived and designed by “PRoMIND-Services for mental health Srls” (Rome) in association with “IDEGO-Virtual Psychology” (Rome). CEREBRUM is a fully immersive Virtual Reality software created by clinicians and experts specialized in cognitive rehabilitation (psychiatric rehabilitation technicians and psychologists).
It is compatible with the “Oculus Go” virtual reality viewer, a CE-marked device developed by Facebook Technologies in partnership with Qualcomm and Xiaomi that was the hardware of this technology for the present study. The “Oculus Go” is an all-in-one headset containing all the components to provide virtual reality experiences and does not need to be tethered to an external device to use.
The CEREBRUM App allows users to immerse themselves in virtual scenarios that simulate everyday reality, home and urban scenarios (Figures in supplementary material). It offers 52 exercises of varying difficulty: 22 exercises are part of the Memory and Learning Module, 10 exercises are part of the Cognitive Estimates Module, and 20 exercises are part of the Attention and Working Memory Module. During the VR exposure, while exploring the 360° scenario, the participants, who could not directly interact with the virtual environments, answered the health worker’s questions. The increasing degrees of difficulty allowed the clinician to adapt the intervention to the participants’ functional diagnosis and to their residual abilities, creating a stimulating learning context in which the exercises were neither too easy nor too difficult. The intervention consisted of 24 sessions of 45 min, divided into two sessions per week over three months. Each session was structured as follows:
- ➢
- Reception, psychoeducation and orientation to the tool;
- ➢
- Exercise psychoeducation;
- ➢
- Psychoeducation to the function to be learned during the exercise;
- ➢
- Generalization phase, in which the function and its importance were explained in the participants’ life context (a bio-psycho-socio-cultural approach based on cognition);
- ➢
- Execution of the exercise in VR with positive and corrective feedback;
- ➢
- Post-exercise comment;
- ➢
- Second exercise that used the same method mentioned above (the maximum duration of the exposure to Virtual Reality was 15–20 min);
- ➢
- Final comment;
- ➢
- Homework, intended as practical suggestions to be implemented by the patients in their daily life. Sessions included an Attention and Working Memory exercise plus one Memory/Learning exercise or one Cognitive Estimation exercise. In some sessions, depending on the participant, the session, and the operator’s assessment, an extra exercise of any type could also be done.
A multidisciplinary team (psychiatric rehabilitation technicians, psychologists, and a psychiatrist) was involved in the intervention. According to the new framework for developing complex interventions [69], the methods used to structure the sessions were replicable and allowed for the promotion of a human-centered approach [70], the accomplishment of cognitive outcomes and improvements in clinical and personal functioning, and the achievement of the generalization of the skills trained. Indeed, we selected this app thanks to the heterogeneity of trained domains (in line with the health need in BD) as a result of a person-centered and recovery-oriented rehabilitation intervention [71], in which the participants could enhance their skills and obtain a global improvement in their health and wellbeing.
3.6. Control
The control group consisted of patients put on a waiting list that received conventional treatment, consisting of psychiatric consultations with psychopharmacological drugs administration and/or without psychotherapy.
3.7. Outcomes
The primary outcomes regard the feasibility of the study in terms of acceptability and tolerability (dropout rate). They were measured, respectively, as the proportion of patients recruited among those considered eligible and as the proportion of patients completing the trial intervention among those included.
Secondary outcomes regarding the efficacy of the trial included: (1) the intervention’s safety (frequencies of adverse events and severe adverse events); (2) patients’ satisfaction; (3) cognitive functions (visuospatial, attention, memory, verbal and semantic fluency, and executive function); (4) personal and social functioning (anxiety and depression symptoms, quality of life, emotional awareness, social functioning, and biological rhythms regulation).
3.8. Data Collection
Patients were screened and enrolled at the Consultation and Psychosomatic Psychiatry Center of the University Hospital of Cagliari (San Giovanni di Dio Civil Hospital). Ad hoc data sheets were created to collect sociodemographic data, level of satisfaction with the program and side effects.
Cognitive functions were evaluated by:
- -
- Rey Figure Test [72] for the visuospatial function,
- -
- Matrix test [73] and Rey’s Words Test [74] for the immediate recall,
- -
- Rey’s Words Test Delayed recall [74], Test of the Tale [75], and Backward Digit Span [76,77] for the memory function,
- -
- Forward Digit Span [76,77] and Trail Making Test, part A [73,78] for the attention function,
- -
- Phonological and Semantic Verbal Fluency Test, both versions [75,79] for the language function,
- -
- Digital Symbol Substitution Test [80,81], Trail Making Test, part B [81], Stroop Test [82], Frontal Assessment Battery—FAB [83] and Cognitive Estimates Test (CET), both versions [84,85] for the executive function.
Personal and social functioning were evaluated by:
- -
- SF-12, Short Form Health Survey, 12 items [86], a self-administered questionnaire that investigates the following dimensions of quality of life and wellbeing: vitality, physical function, physical pain, perception of general health, mental health, physical and emotional health, work functioning and social role;
- -
- TAS-20, Toronto Alexithymia Scale-20 item [87], a self-administered questionnaire that evaluates the level of emotional awareness;
- -
- SAS, Zung Self-Rating Anxiety Scale [88], a self-administered questionnaire that evaluates perceived anxiety levels;
- -
- PHQ-9, Patient Health Questionnaire [89], a self-administered questionnaire that evaluates depressive symptoms;
- -
- HoNOS, Health of The Nation Outcome Scale [90], a clinical scale to evaluate general, personal and social functioning;
- -
- BRIAN, Biological Rhythms Interview of Assessment in Neuropsychiatry [91], a clinical interview consisting of 18 items that investigates four main areas related to the dysregulation of circadian rhythms (sleep, activity, social rhythms and nutrition).
Participants were assessed before the treatment, after the end of the intervention and six and 12 months after the end of the intervention by the same evaluators who were blinded to the groups that they had been assigned to. In the present study, we report findings regarding pre- and post-evaluation.
3.9. Data Analyses
The statistical data were analyzed using the software SPSS (version 21). Frequencies (percentages) or mean ± standard deviation were used for descriptive statistics about sociodemographic variables such as “sex” and “age”, as well as about the level of satisfaction with the experimental intervention and the occurrence of side effects.
Chi-square test and one-way ANOVA were used to test the homogeneity between experimental and control groups regarding “sex” and “age” distributions.
A series of repeated-measure ANOVA was performed, one for each of the outcomes considered, to compare means between the intervention and non-intervention groups over time (pre- and post-intervention) with Bonferroni’s correction. The normality assumption of the dependent variables was tested as sphericity (i.e., variances of the differences between all combinations of related groups must be equal) with Mauchly’s test.
3.10. Sample Size Considerations
In the registered trial, we planned to recruit and randomize 60 subjects who met the inclusion criteria. However, we stopped the enrollment phase early due to the COVID-19 pandemic, which did not allow us to easily involve all the subjects we planned to in the study protocol. Therefore, 50 people were randomized to assess the feasibility outcomes. To date, an effective methodology in terms of sample size cannot be established yet, as the evidence in this field of research is limited [38,92,93]. Therefore, the aim of this study is to verify the feasibility and preliminary efficacy of a VR tool for the treatment of CI in people with BD.
4. Results
4.1. Primary Outcome: Feasibility of the Trial (Acceptability and Tolerability)
As shown in the flow diagram (Figure 1), 119 subjects were contacted, with 62 people becoming enrolled and 12 excluded after evaluation (three did not meet the inclusion criteria, and nine declined to participate). Fifty of the participants were randomized: 25 of them were assigned to group A and received the intervention, and 25 participants were assigned to the control group (group B) and were initially put on a waiting list. They later crossed over and received the experimental intervention. Thanks to the cross-over method, we could include 50 subjects in the experimental group and 25 in the control group. Participants who did not complete at least 50% of the sessions were considered dropouts. Out of the 25 participants of group A, 18 completed all the sessions, and 7 dropped out due to work commitments interfering with the schedule of the interventions or the long distance from their residence and the health service. Out of the 25 participants of group B, 21 completed all the sessions after the cross-over, and 4 dropped out for the same reasons as those dropping out of group A. No one dropped out while on the waiting list. Overall, at the end of the intervention, the experimental and the control groups consisted of 39 and 25 participants, respectively (Figure 1). As shown in Table 1, the experimental and control groups were homogeneous regarding the distributions of the variables “sex” and “age”. Regarding tolerability (dropout rate) and acceptability, around 20% of subjects dropped out, and less than 45% of the people that were contacted did not respond or refused to participate in this trial.
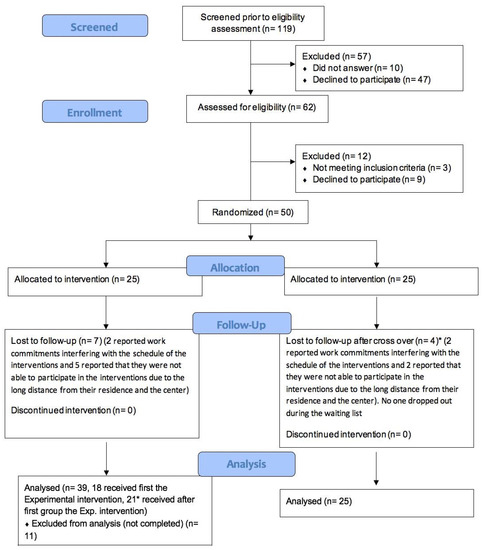
Figure 1.
CONSORT flow diagram extension for the feasibility study.
4.2. Secondary Outcome: Efficacy of the Trial (Satisfaction with the Trial; Adverse Effects; Cognitive Functions; Personal and Social Functioning)
In the experimental group, 48.7% (19/39) of the participants considered the intervention “excellent” regarding experience, whereas 28.2% and 23.1% considered it “great” and “good”, respectively (Table 2).

Table 2.
Frequencies of Satisfaction.
Some 76.9% (30/39) of participants did not report any side effects during the first fully immersive VR exposure session. The remaining 23.1% reported the following side effects: emptiness/disorientation, nausea/feeling of emptiness, headache, disorientation, dizziness, tremors/nausea/blurred vision/dizziness, nausea, vertigo and sense of unreality (Table 3).

Table 3.
Frequencies of Side_Effects_T0.
At the end of the intervention, 87.2% of participants did not report any side effects. As shown in Table 4, the remaining 12.8% reported the following side effects: nausea (two participants), daze (two participants) and a feeling of emptiness/unreality (one participant). Overall, most participants did not experience any side effects.

Table 4.
Frequencies of Side Effects T1.
Regarding cognitive functions and personal and social functioning, we tested the differences reported over time (pre- and post-intervention) between the experimental group that received the VR-based CR intervention as an add-on to conventional treatment and the control group that received conventional treatment (waiting list).
As reported in Table 5, there was an overall improvement in cognitive functions after the VR-based CR intervention. Specifically, we found a statistically significant change after the VR-based CR intervention in the experimental group compared to the control group (Table 5) regarding attention (Matrix, p = 0.002; Rey’s Words immediate recall, p = 0.019), memory (Rey’s Words delayed recall, p = 0.003), verbal function (Verbal Semantic Test, p = 0.010), and executive function (CET, p = 0.003).

Table 5.
Cognitive Test-Descriptive Analysis (Mean and Standard Deviation): Repeated Measures ANOVA Analyses.
As reported in Table 6, overall personal and social functioning improved after the VR-based CR intervention. In particular, we found a statistically significant improvement in the experimental group compared to the control group regarding depressive symptoms (PHQ-9, p = 0.30), biological rhythms regulation (BRIAN, p = 0.029) and emotional awareness (TAS-20, p = 0.007).

Table 6.
Personal and Social functioning: Test-Descriptive Analysis (Mean) Test-Repeated Measures ANOVA Analyses.
5. Discussion
This study showed that a fully immersive VR-based CR intervention for people with BD has good acceptability and tolerability (primary outcome), according to previous psychosocial treatments [94,95,96,97]. The participants also declared great satisfaction with the intervention received, and the majority did not have any side effects. Furthermore, individuals in the experimental group showed an overall improvement in all cognitive, personal, and social functioning after the fully immersive VR-based CR intervention compared to the control group. Particularly, we found a statistically significant improvement (p < 0.05) regarding attention, memory, verbal function, executive function, depressive symptoms, biological rhythms and emotional awareness.
To our knowledge, no studies on the use of fully immersive VR as a CR intervention aiming to improve the cognitive, personal and social functioning of people with BD are available. It should be noted that higher dropout rates (around 30%) are usually found in traditional CR interventions (without VR) for people with BD, often carried out with small samples [47]. This data could be interpreted considering the behavioral characteristics of this disorder. As BD is associated with a hyperthymic and exploratory temperament [98], traditional methods (i.e., paper and pencil or computerized) are not actually engaging, particularly in an ecological setting. A dropout rate of around 20% is a notable result in the treatment of CI in BD and suggests that the implementation of innovative interventions such as VR-based allows the achievement of the engagement goal in the treatment of CI, as well as improvements in terms of personal and social functioning. Furthermore, the use of the cross-over method is undoubtedly another strength of this study, as it ensures low statistical variance and has the ethical advantage of including all randomized participants in the clinical intervention. Overall, studies that evaluate the efficacy of VR/CR-based programs in other diseases seem to be effective for the improvement of CI. Even fewer studies examined the functional outcomes related to cognitive improvement [26,27,63].
In using a person-centered method, the VR-based CR intervention is an innovative intervention that allows the participants to learn about their personal resources and to develop strategies for their daily life, thanks to the generalization of the improved skills through personal objectives-focused homework. This, in turn, contributes to the achievement of a global impact in improving their clinical, personal, and social functioning. Additionally, professionals should follow a logical framework to develop complex interventions in mental health and ensure that the outcomes are consistent with the skills trained and with functional improvement [69]. Mental health is a fundamental resource that allows people to achieve daily life goals and exercise their role as citizens of a community [98]. In line with the digital era and the WHO innovation objective [48], increasing the use of technologies in psychosocial rehabilitation could better respond to health needs. Despite the poor methodological quality of the trials, the implementation of CR interventions with traditional methods showed preliminary evidence for people with BD [47]. The results of our study are consistent with other preliminary studies on the efficacy of the use of traditional CR in the treatment of BD and suggest that it is important to implement randomized clinical trials with larger samples to evaluate the clinical efficacy of CR interventions with fully immersive VR in order to confirm and extend this data. One limitation of this study must be cited: we had some dropouts, and the final analyses were only on completers.
Risk and Benefits
The use of fully immersive VR could be associated with different side effects like dizziness, nausea, headache, eye fatigue, reduced limb control, reduced postural control, reduced sense of presence, and the development of inadequate responses to the real world. However, significant side effects are not expected, as the VR tool has already been used in people with psychosocial disabilities without substantial side effects [99,100]. The benefits of VR in terms of satisfaction and ecological learning and the few side effects satisfy the need to implement an innovative rehabilitation approach in mental health.
6. Conclusions
To date, there is no evidence of the use of fully immersive VR as a CR intervention tool to train all cognitive domains of people with BD, even if cognitive impairment is a core component of the social and personal functioning of this disorder. Implementing a randomized clinical trial with a reproducible method developed by a multidisciplinary team specialized in the specific health needs of BD patients is a pertinent research goal in the field of psychosocial rehabilitation. The results of this study are preliminary and not exhaustive due to the limited sample size. However, the evidence regarding efficacy, the great acceptability and tolerability of the intervention are of interest and suggest the need to conduct studies with more extensive samples that can confirm this data.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12062142/s1. Figures S1–S5. CEREBRUM scenarios.
Author Contributions
Conceptualization, M.G.C. and A.P. (Alessandra Perra); methodology, M.G.C., A.P. (Alessandra Perra), A.P. (Antonio Preti), D.P., A.E.N. and P.K.K.; software, V.D.L., L.D.N. and G.S.; formal analysis, A.P. (Antonio Preti), D.P., A.E.N. and F.S.; investigation R.Z., A.G., A.L. and F.P.; resources M.G.C. and A.P. (Alessandra Perra); data curation, G.C.; writing-original draft preparation, A.P. (Alessandra Perra); writing-review and editing A.P. (Alessandra Perra), M.G.C., V.D.L., R.Z., A.G., A.L., F.P., A.P. (Antonio Preti), T.Z., D.P., L.D.N., A.E.N., P.K.K., G.C., F.S. and G.S.; supervision M.G.C., A.P. (Alessandra Perra) and V.D.L.; project administration, M.G.C.; funding acquisition, M.G.C. and A.P. (Alessandra Perra). All authors have read and agreed to the published version of the manuscript.
Funding
The project is funded by the Fondazione di Sardegna (U351.2020/AI.334.MGB-2020.1581). The funding source had no role in the design of this study (collection, analysis, and interpretation of data), in the writing of the report or in the decision to submit and publish its results.
Institutional Review Board Statement
This protocol was approved by the Local Independent Ethics Committee with the number Prot. PG/2020/21681.
Informed Consent Statement
This study is registered on ClinicalTrials.gov (NCT05070065, September 2021). Even if, in the registered trial, we planned to recruit and randomize 60 subjects who met the inclusion criteria, we stopped the enrollment phase early due to the COVID-19 pandemic, which did not allow us to easily involve all the subjects we prevent in the study protocol. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki [101]. Before inclusion, written informed consent was obtained from all patients (or, alternatively) by their guardians. Comprehensive information about the nature and purpose of the study was provided before participants agreed to participate. Participants were also provided with information about the possibility of interrupting the program at any time. The contact details of the coordinators were made available to participants who wished to have more information on the study. Subjects enrolled in the study were given information about data protection and privacy law. The data of the study was entered into a database to maintain confidentiality in accordance with local laws on Data Protection [102,103]. An operator of the Psychiatry Center associated an alphanumeric code to the name before delivering the database to those involved in statistical analysis. The list of correspondences between individual names and alphanumeric code will remain in the exclusive availability of the guarantor (Psychiatry Center). This procedure will guarantee the complete anonymization of the data.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest, financial or otherwise. Two co-authors (VDL and LDN) are the legal administrators of the societies that have developed CEREBRUM software.
References
- Albanna, A.; Choudhry, Z.; Harvey, P.-O.; Fathalli, F.; Cassidy, C.; Sengupta, S.M.; Iyer, S.N.; Rho, A.; Lepage, M.; Malla, A.; et al. TCF4 gene polymorphism and cognitive performance in patients with first episode psychosis. Schizophr. Res. 2014, 152, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, A.L.; Johansson, K.; Lã¸berg, E.-M. Neurobiology of Cognitive Remediation Therapy for Schizophrenia: A Systematic Review. Front. Psychiatry 2014, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, F.-C.; Zhang, L.; Ng, C.H.; Ungvari, G.S.; Li, J.; Xiang, Y.-T. Comparison of cognitive dysfunction between schizophrenia and bipolar disorder patients: A meta-analysis of comparative studies. J. Affect. Disord. 2020, 274, 652–661. [Google Scholar] [CrossRef]
- Goff, D.C.; Hill, M.; Barch, D. The treatment of cognitive impairment in schizophrenia. Pharmacol. Biochem. Behav. 2011, 99, 245–253. [Google Scholar] [CrossRef]
- Solé, B.; Jiménez, E.; Torrent, C.; Reinares, M.; Bonnin, C.; Torres, I.; Varo, C.; Grande, I.; Valls, E.; Salagre, E.; et al. Cognitive Impairment in Bipolar Dis-order: Treatment and Prevention Strategies. Int. J. Neuropsychopharmacol. 2017, 20, 670–680. [Google Scholar] [CrossRef]
- Carta, M.; Angst, J. Screening for bipolar disorders: A public health issue. J. Affect. Disord. 2016, 205, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.G.; Ouali, U.; Perra, A.; Ahmed, A.B.C.; Boe, L.; Aissa, A.; Lorrai, S.; Cossu, G.; Aresti, A.; Preti, A.; et al. Living With Bipolar Disorder in the Time of Covid-19: Biorhythms During the Severe Lockdown in Cagliari, Italy, and the Moderate Lockdown in Tunis, Tunisia. Front. Psychiatry 2021, 12, 634765. [Google Scholar] [CrossRef]
- Merikangas, K.R.; Jin, R.; He, J.-P.; Kessler, R.C.; Lee, S.; Sampson, N.A.; Viana, M.C.; Andrade, L.H.; Hu, C.; Karam, E.G.; et al. Prevalence and Correlates of Bipolar Spectrum Disorder in the World Mental Health Survey Initiative. Arch. Gen. Psychiatry 2011, 68, 241–251. [Google Scholar] [CrossRef]
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef]
- Martinez-Aran, A.; Vieta, E. Cognition as a target in schizophrenia, bipolar disorder and depres-sion. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2015, 25, 151–157. [Google Scholar] [CrossRef]
- Martínez-Arán, A.; Vieta, E.; Colom, F.; Reinares, M.; Benabarre, A.; Gastó, C.; Salamero, M. Cognitive Dysfunctions in Bipolar Disorder: Evidence of Neuropsychological Disturbances. Psychother. Psychosom. 1999, 69, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.; Gonçalves-Pereira, M.; Xavier, M.; Mukaetova-Ladinska, E.B. Affective disorders and risk of developing dementia: Systematic review. Br. J. Psychiatry 2013, 202, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Musat, E.M.; Marlinge, E.; Leroy, M.; Olié, E.; Magnin, E.; Lebert, F.; Gabelle, A.; Bennabi, D.; Blanc, F.; Paquet, C.; et al. Characteristics of Bipolar Patients with Cognitive Impairment of Suspected Neurodegenerative Origin: A Multicenter Cohort. J. Pers. Med. 2021, 11, 1183. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, M.M.; Gerraty, R.T. A meta-analytic investigation of neurocognitive deficits in bipolar illness: Profile and effects of clinical state. Neuropsychology 2009, 23, 551–562. [Google Scholar] [CrossRef]
- Torres, I.J.; DeFreitas, V.G.; DeFreitas, C.M.; Kauer-Sant’Anna, M.; Bond, D.J.; Honer, W.G.; Lam, R.W.; Yatham, L.N. Neurocognitive Functioning in Patients With Bipolar I Disorder Recently Recovered From a First Manic Episode. J. Clin. Psychiatry 2010, 71, 1234–1242. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Bortolato, B.; Miskowiak, K.; Vieta, E.; Köhler, C. Cognitive dysfunction in bipolar disorder and schizophrenia: A systematic review of meta-analyses. Neuropsychiatr. Dis. Treat. 2015, 11, 3111–3125. [Google Scholar] [CrossRef] [PubMed]
- Douglas, K.M.; Gallagher, P.; Robinson, L.J.; Carter, J.D.; McIntosh, V.V.; Frampton, C.M.; Watson, S.; Young, A.H.; Ferrier, I.N.; Porter, R.J. Prevalence of cognitive impairment in major depression and bipolar disor-der. Bipolar Dis. 2018, 20, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Brissos, S.; Dias, V.V.; Kapczinski, F. Cognitive performance and quality of life in bipolar disor-der. Canadian journal of psychiatry. Revue Can. Psychiatr. 2008, 53, 517–524. [Google Scholar] [CrossRef]
- American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am. J. Psychiatry 2002, 159, 1–50. [Google Scholar]
- Goodwin, G.; Haddad, P.; Ferrier, I.; Aronson, J.; Barnes, T.; Cipriani, A.; Coghill, D.R.; Fazel, S.; Geddes, J.; Grunze, H.; et al. Evidence-based guidelines for treating bipolar disorder: Revised third edition recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2016, 30, 495–553. [Google Scholar] [CrossRef]
- Vieta, E. The influence of medications on neurocognition in bipolar disorder. Acta Psychiatr. Scand. 2009, 120, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Steardo, L.; de Filippis, R.; Carbone, E.A.; Segura-Garcia, C.; Verkhratsky, A.; De Fazio, P. Sleep Disturbance in Bipolar Disorder: Neuroglia and Circadian Rhythms. Front. Psychiatry 2019, 10, 501. [Google Scholar] [CrossRef]
- Cullen, C.; Kappelmann, N.; Umer, M.; Abdolizadeh, A.; Husain, M.O.; Bonato, S.; Sharma, G.; Xue, S.; Ortiz, A.; Kloiber, S.M.; et al. Efficacy and acceptability of pharmacotherapy for comorbid anxiety symptoms in bipolar disorder: A systematic review and meta-analysis. Bipolar Disord. 2021, 23, 754–766. [Google Scholar] [CrossRef]
- Albert, U.; Rosso, G.; Maina, G.; Bogetto, F. Impact of anxiety disorder comorbidity on quality of life in euthymic bipolar disorder patients: Differences between bipolar I and II subtypes. J. Affect. Disord. 2008, 105, 297–303. [Google Scholar] [CrossRef]
- Assembly, U.G. Convention on the Rights of Persons with Disabilities. GA Res. 2006, 61, 106. [Google Scholar]
- Vita, A.; Barlati, S.; Ceraso, A.; Nibbio, G.; Ariu, C.; Deste, G.; Wykes, T. Effectiveness, Core Elements, and Moderators of Response of Cognitive Remediation for Schizophrenia: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Psychiatry 2021, 78, 848–858. [Google Scholar] [CrossRef]
- Thérond, A.; Pezzoli, P.; Abbas, M.; Howard, A.; Bowie, C.R.; Guimond, S. The Efficacy of Cognitive Remediation in Depression: A Systematic Literature Review and Meta-Analysis. J. Affect. Disord. 2021, 284, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Stubbs, B.; Rosenbaum, S.; Vancampfort, D.; Malchow, B.; Schuch, F.; Elliott, R.; Nuechterlein, K.H.; Yung, A.R. Aerobic Exercise Improves Cognitive Functioning in People With Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2016, 43, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Bottai, T.; Biloa-Tang, M.; Christophe, S.; Dupuy, C.; Jacquesy, L.; Kochman, F.; Meynard, J.-A.; Papeta, D.; Rahioui, H.; Adida, M.; et al. Thérapie interpersonnelle et aménagement des rythmes sociaux (TIPARS): Du concept anglo-saxon à l’expérience française. L’Encephale 2010, 36, S206–S217. [Google Scholar] [CrossRef]
- Sancassiani, F.; Lorrai, S.; Cossu, G.; Cocco, A.; Trincas, G.; Floris, F.; Mellino, G.; Machado, S.; Nardi, A.E.; Fabrici, E.P.; et al. The Effects of “VelaMente?!” Project on Social Functioning of People With Severe Psychosocial Disabilities. Clin. Pract. Epidemiol. Ment. Health 2017, 13, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.G.; Cossu, G.; Pintus, E.; Zaccheddu, R.; Callia, O.; Conti, G.; Pintus, M.; Gonzalez, C.I.A.; Massidda, M.V.; Mura, G.; et al. Moderate Exercise Improves Cognitive Function in Healthy Elderly People: Results of a Randomized Controlled Trial. Clin. Pract. Epidemiol. Ment. Health 2021, 17, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.G.; Cossu, G.; Pintus, E.; Zoccheddu, R.; Callia, O.; Conti, G.; Pintus, M.; Gonzalez, C.I.A.; Massidda, M.V.; Mura, G.; et al. Active elderly and health—Can moderate exercise improve health and wellbeing in older adults? Protocol for a randomized controlled trial. Trials 2021, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Angevaren, M.; Rusted, J.; Tabet, N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2015, 4, CD005381. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Gonzalez, C.I.A.; Minerba, L.; Demontis, R.; Pau, M.; Velluzzi, F.; Ferreli, C.; Atzori, L.; Machado, S.; Fortin, D.; et al. Exercise Improves Long-Term Social and Behavioral Rhythms in Older Adults: Did it Play a Role during the COVID-19 Lockdown? J. Public Health Res. 2022, 11, 2432. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.G.; Fornaro, M.; Minerba, L.; Pau, M.; Velluzzi, F.; Atzori, L.; Gonzalez, C.I.A.; Romano, F.; Littera, R.; Chessa, L.; et al. Previous Functional Social and Behavioral Rhythms Affect Resilience to Covid-19-Related Stress among Old Adults. J. Public Health Res. 2022, 11, 2768. [Google Scholar] [CrossRef] [PubMed]
- Wykes, T.; Spaulding, W.D. Thinking About the Future Cognitive Remediation Therapy--What Works and Could We Do Better? Schizophr. Bull. 2011, 37, S80–S90. [Google Scholar] [CrossRef] [PubMed]
- Elgamal, S.; McKINNON, M.C.; Ramakrishnan, K.; Joffe, R.T.; MacQUEEN, G. Successful computer-assisted cognitive remediation therapy in patients with unipolar depression: A proof of principle study. Psychol. Med. 2007, 37, 1229–1238. [Google Scholar] [CrossRef]
- Bellani, M.; Biagianti, B.; Zovetti, N.; Rossetti, M.G.; Bressi, C.; Perlini, C.; Brambilla, P. The effects of cognitive remediation on cognitive abilities and real-world functioning among people with bipolar disorder: A systematic review: Special Section on “Translational and Neuroscience Studies in Affective Disorders”. Section Editor, Maria Nobile MD, PhD. This Section of JAD focuses on the relevance of translational and neuroscience studies in providing a better understanding of the neural basis of affective disorders. The main aim is to briefly summaries relevant research findings in clinical neuroscience with particular regards to specific innovative topics in mood and anxiety disorders. J. Affect. Dis. 2019, 257, 691–697. [Google Scholar]
- Choi, J.; Medalia, A. Factors Associated With a Positive Response to Cognitive Remediation in a Community Psychiatric Sample. Psychiatr. Serv. 2005, 56, 602–604. [Google Scholar] [CrossRef]
- Tchanturia, K.; Davies, H.; Campbell, I.C. Cognitive remediation therapy for patients with anorexia nervosa: Preliminary findings. Ann. Gen. Psychiatry 2007, 6, 14. [Google Scholar] [CrossRef]
- Gold, A.K.; Montana, R.E.; Sylvia, L.G.; Nierenberg, A.A.; Deckersbach, T. Cognitive Remediation and Bias Modification Strategies in Mood and Anxiety Disorders. Curr. Behav. Neurosci. Rep. 2016, 3, 340–349. [Google Scholar] [CrossRef]
- Vita, A.; Deste, G.; Barlati, S.; Poli, R.; Cacciani, P.; De Peri, L.; Sacchetti, E. Feasibility and effectiveness of cognitive remediation in the treatment of borderline personality disorder. Neuropsychol. Rehabilitation 2016, 28, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Preti, A.; Edwards, C.; Dow, T.; Wykes, T. Cognitive remediation for negative symptoms of schizophrenia: A network meta-analysis. Clin. Psychol. Rev. 2017, 52, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Thapa, N.; Park, H.J.; Yang, J.-G.; Son, H.; Jang, M.; Lee, J.; Kang, S.W.; Park, K.W.; Park, H. The Effect of a Virtual Reality-Based Intervention Program on Cognition in Older Adults with Mild Cognitive Impairment: A Randomized Control Trial. J. Clin. Med. 2020, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Velligan, D.I.; Kern, R.S.; Gold, J.M. Cognitive Rehabilitation for Schizophrenia and the Putative Role of Motivation and Expectancies. Schizophr. Bull. 2005, 32, 474–485. [Google Scholar] [CrossRef]
- Ventura, J.; Subotnik, K.L.; Gretchen-Doorly, D.; Casaus, L.; Boucher, M.; Medalia, A.; Bell, M.D.; Hellemann, G.S.; Nuechterlein, K.H. Cognitive remediation can improve negative symptoms and social functioning in first-episode schizophrenia: A randomized controlled trial. Schizophr. Res. 2017, 203, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tsapekos, D.; Seccomandi, B.; Mantingh, T.; Cella, M.; Wykes, T.; Young, A. Cognitive enhancement interventions for people with bipolar disorder: A systematic review of methodological quality, treatment approaches, and outcomes. Bipolar Disord. 2019, 22, 216–230. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. In Proceedings of the European Advisory Committee on Health Research: Fourth MeetingL World Health Organization, Copenhagen, Denmark, 10–11 December 2013; Regional Office for Europe: Geneva, Switzerland, 2014. [Google Scholar]
- Freeman, D.; Reeve, S.; Robinson, A.; Ehlers, A.; Clark, D.; Spanlang, B.; Slater, M. Virtual reality in the assessment, understanding, and treatment of mental health disorders. Psychol. Med. 2017, 47, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Kim, D.J.; Lee, U.; Na, E.J.; Jeon, H.J. A Literature Overview of Virtual Reality (VR) in Treatment of Psychiatric Disorders: Recent Advances and Limitations. Front. Psychiatry 2019, 10, 505. [Google Scholar] [CrossRef]
- Costa, M.; Vieira, L.P.; Barbosa, E.O.; Mendes Oliveira, L.; Maillot, P.; Otero Vaghetti, C.A.; Giovani Carta, M.; Machado, S.; Gatica-Rojas, V.; Monteiro-Junior, R.S. Virtual Reality-Based Exercise with Exergames as Medicine in Different Contexts: A Short Review. Clin. Pract. Epidemiol. Ment. Health CP EMH 2019, 15, 74. [Google Scholar] [CrossRef]
- Lima, J.L.; Axt, G.; Teixeira, D.S.; Monteiro, D.; Cid, L.; Yamamoto, T.; Murillo-Rodriguez, E.; Machado, S. Exergames for Children and Adolescents with Autism Spectrum Disorder: An Overview. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.H.; König, A.; Amieva, H.; Andrieu, S.; Bremond, F.; Bullock, R.; Manera, V. Recommendations for the use of serious games in people with Alzheimer’s disease, related disorders and frailty Front. Aging Neurosci. 2014, 6, 54. [Google Scholar] [CrossRef]
- Manera, V.; Chapoulie, E.; Bourgeois, J.; Guerchouche, R.; David, R.; Ondrej, J.; Drettakis, G.; Robert, P. A Feasibility Study with Image-Based Rendered Virtual Reality in Patients with Mild Cognitive Impairment and Dementia. PLoS ONE 2016, 11, e0151487. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, W.; Chen, C.; Liu, C.; Yang, J.; Zhang, Y. A Review of the Application of Virtual Reality Technology in the Diagnosis and Treatment of Cognitive Impairment. Front. Aging Neurosci. 2019, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.B.; Emmelkamp, P.M. Virtual reality exposure therapy for anxiety disorders: A meta-analysis. J. Anxiety Disord. 2008, 22, 561–569. [Google Scholar] [CrossRef]
- Maples-Keller, J.L.; Bunnell, B.E.; Kim, S.-J.B.; Rothbaum, B.O. The Use of Virtual Reality Technology in the Treatment of Anxiety and Other Psychiatric Disorders. Harv. Rev. Psychiatry 2017, 25, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Norr, A.M.; Smolenski, D.J.; Reger, G.M. Effects of prolonged exposure and virtual reality exposure on suicidal ideation in active duty soldiers: An examination of potential mechanisms. J. Psychiatr. Res. 2018, 103, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Lasaponara, S.; Marson, F.; Doricchi, F.; Cavallo, M. A Scoping Review of Cognitive Training in Neurodegenerative Diseases via Computerized and Virtual Reality Tools: What We Know So Far. Brain Sci. 2021, 11, 528. [Google Scholar] [CrossRef]
- García-Betances, R.I.; Arredondo Waldmeyer, M.T.; Fico, G.; Cabrera-Umpierrez, M.F. A Succinct Overview of Virtual Reality Technology Use in Alzheimer’s Disease. Front. Aging Neurosci. 2015, 7, 80. [Google Scholar] [CrossRef]
- Zakzanis, K.K.; Quintin, G.; Graham, S.J.; Mraz, R. Age and dementia related differences in spatial navigation within an immersive virtual environment. Med. Sci. Monit. 2009, 15, CR140–CR150. [Google Scholar]
- Cotelli, M.; Manenti, R.; Brambilla, M.; Gobbi, E.; Ferrari, C.; Binetti, G.; Cappa, S.F. Cognitive telereha-bilitation in mild cognitive impairment, Alzheimer’s disease and frontotemporal dementia: A systematic re-view. J. Telemed. Telecare 2019, 25, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Perra, A.; De Lorenzo, V.; Zaccheddu, R.; Locci, A.; Piludu, F.; Preti, A.; Di Natale, L.; Galetti, A.; Nardi, A.E.; Cossu, G.; et al. Cognitive Remediation Virtual Reality Tool a Recovery-Oriented Project for People with Bipolar Disorder: Protocol of a Feasibility Randomized Clinical Trial. Clin. Pract. Epidemiol. Ment. Health 2022, 18, e174501792208220. [Google Scholar] [CrossRef]
- Jahn, F.S.; Skovbye, M.; Obenhausen, K.; Jespersen, A.E.; Miskowiak, K.W. Cognitive training with fully immersive virtual reality in patients with neurological and psychiatric disorders: A systematic review of randomized controlled trials. Psychiatry Res. 2021, 300, 113928. [Google Scholar] [CrossRef]
- Riva, G.; Mancuso, V.; Cavedoni, S.; Stramba-Badiale, C. Virtual reality in neurorehabilitation: A review of its effects on multiple cognitive domains. Expert Rev. Med. Devices 2020, 17, 1035–1061. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; McDonough, D.J.; Gao, Z. The Effectiveness of Virtual Reality Exercise on Individual’s Physiological, Psychological and Rehabilitative Outcomes: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4133. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster GA PAFS Consensus Group. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.; et al. A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. BMJ 2021, 374, n2061. [Google Scholar] [CrossRef]
- Shaigetz, V.G.; Proulx, C.; Cabral, A.; Choudhury, N.; Hewko, M.; Kohlenberg, E.; Segado, M.; Smith, M.S.D.; Debergue, P. An Immersive and Interactive Platform for Cognitive Assessment and Rehabilitation (bWell): Design and Iterative Development Process. JMIR Rehabilitation Assist. Technol. 2021, 8, e26629. [Google Scholar] [CrossRef]
- Saxena, S.; Funk, M.; Chisholm, D. World Health Assembly adopts Comprehensive Mental Health Action Plan 2013–2020. Lancet 2013, 381, 1970–1971. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol. Sci. 2002, 22, 443–447. [Google Scholar] [CrossRef]
- Spinnler, H.; Tognoni, G. Standardizzazione e taratura italiana di test neuropsicologici. Ital. J. Neurol. Sci. 1987, 8, 21–120. [Google Scholar]
- Caltagirone, C.; Gainotti, G.; Carlesimo, G.A.; Parnetti, L. Batteria per la valutazione del deterioramento mentale: I/II. Descrizione di uno strumento di diagnosi neuropsicologica. Arch. Psicol. Neurol. Psichiatria 1995, 14, 109–138. [Google Scholar]
- Carlesimo, G.A.; Buccione, I.; Fadda, L.; Graceffa, A.; Mauri, M.; Lorusso, S.; Caltagirone, C. Standardizzazione di due test di memoria per uso clinico: Breve Racconto e Figura di Rey. Nuova Riv. Neurol. 2002, 12, 1–13. [Google Scholar]
- Orsini, A.; Grossi, D.; Capitani, E.; Laiacona, M.; Papagno, C.; Vallar, G. Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Neurol. Sci. 1987, 8, 537–548. [Google Scholar] [CrossRef]
- Bisiacchi, P.S.; Mapelli, D.; Mondini, S.; Vestri, A. Esame Neuropsicologico Breve, una Batteria di Test per lo Screening Neuropsicologico; Raffaello Cortina Editore: Milano, Italy, 2003. [Google Scholar]
- Giovagnoli, A.R.; Del Pesce, M.; Mascheroni, S.; Simoncelli, M.; Laiacona, M.; Capitani, E. Trail making test: Normative values from 287 normal adult controls. Neurol. Sci. 1996, 17, 305–309. [Google Scholar] [CrossRef]
- Novelli, G.; Papagno, C.; Capitani, E.; Laiacona, M. Tre test clinici di ricerca e produzione lessicale. Taratura su sogetti normali. Arch. Psicol. Neurol. Psichiatria 1986, 47, 477–506. [Google Scholar]
- Amodio, P.; Wenin, H.; Del Piccolo, F.; Mapelli, D.; Montagnese, S.; Pellegrini, A.; Umiltà, C. Variability of trail making test, symbol digit test and line trait test in normal people. A normative study taking into account age-dependent decline and sociobiological variables. Aging Clin. Exp. Res. 2002, 14, 117–131. [Google Scholar] [CrossRef]
- Amodio, P.; Campagna, F.; Olianas, S.; Iannizzi, P.; Mapelli, D.; Penzo, M.; Angeli, P.; Gatta, A. Detection of minimal hepatic encephalopathy: Normalization and optimization of the Psychometric Hepatic Encephalopathy Score. A neuropsychological and quantified EEG study. J. Hepatol. 2008, 49, 346–353. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Una versione abbreviata del test di Stroop: Dati normativi nella popolazione italiana. Nuova Riv. Neurol. 2002, 12, 111–115. [Google Scholar]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B.F.A.B. The FAB: A frontal assessment battery at bed-side. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef]
- Scarpina, F.; D’Aniello, G.E.; Mauro, A.; Castelnuovo, G.; MacPherson, S.E. How many segments are there in an orange: Normative data for the new Cognitive Estimation Task in an Italian population. Neurol. Sci. 2015, 36, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, S.; MacPherson, S.E.; Phillips, L.; Sacco, L.; Spinnler, H. How many camels are there in Italy? Cognitive estimates standardised on the Italian population. Neurol. Sci. 2003, 24, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Bagby, R.M.; Parker, J.D.A.; Taylor, G.J. The twenty-item Toronto Alexithymia scale—I. Item selection and cross-validation of the factor structure. J. Psychosom. Res. 1994, 38, 23–32. [Google Scholar] [CrossRef]
- Zung, W.W. A rating instrument for anxiety disorders. Psychosom. J. Consult. Liaison Psychiatry 1971, 12, 371–379. [Google Scholar] [CrossRef]
- Rizzo, R.; Piccinelli, M.; Mazzi, M.A.; Bellantuono, C.; Tansella, M. The Personal Health Questionnaire: A new screening instrument for detection of ICD-10 depressive disorders in primary care. Psychol. Med. 2000, 30, 831–840. [Google Scholar] [CrossRef]
- Wing, J.K.; Curtis, R.; Beevor, A. Health of the Nation Outcome Scales; Royal College of Psychiatrists: London, UK, 1996. [Google Scholar]
- Moro, M.F.; Carta, M.G.; Pintus, M.; Pintus, E.; Melis, R.; Kapczinski, F.; Colom, F. Validation of the Italian Version of the Biological Rhythms Interview of Assessment in Neuropsychiatry (BRIAN): Some Considerations on its Screening Usefulness. Clin. Pract. Epidemiol. Ment. Health 2014, 10, 48–52. [Google Scholar] [CrossRef]
- Ott, C.V.; Macoveanu, J.; Bowie, C.R.; Fisher, P.M.; Knudsen, G.M.; Kessing, L.V.; Miskowiak, K.W. Change in prefrontal activity and executive functions after action-based cognitive remediation in bipolar disorder: A randomized controlled trial. Neuropsychopharmacology 2020, 46, 1113–1121. [Google Scholar] [CrossRef]
- Abdulatif, M.; Mukhtar, A.; Obayah, G. Pitfalls in reporting sample size calculation in randomized controlled trials published in leading anaesthesia journals: A systematic review. Br. J. Anaesth. 2015, 115, 699–707. [Google Scholar] [CrossRef]
- Wright, I.; Mughal, F.; Bowers, G.; Meiser-Stedman, R. Dropout from randomised controlled trials of psychological treatments for depression in children and youth: A systematic review and meta-analyses. J. Affect. Disord. 2020, 281, 880–890. [Google Scholar] [CrossRef]
- Amico, K.R. Percent Total Attrition: A Poor Metric for Study Rigor in Hosted Intervention Designs. Am. J. Public Health 2009, 99, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, V.; Fallowfield, L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br. J. Cancer 2000, 82, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.G.; Perra, A.; Atzeni, M.; D’Oca, S.; Moro, M.F.; Kurotschka, P.K.; Moro, D.; Sancassiani, F.; Minerba, L.; Brasesco, M.V.; et al. An evolutionary approach to mania studying Sardinian immigrants to Argentina. Rev. Bras. Psiquiatr. 2017, 39, 147–153. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Comprehensive Mental Health Action Plan 2013–2030; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Garcia-Palacios, A.; Hoffman, H.; Carlin, A.; Furness, T.; Botella, C. Virtual reality in the treatment of spider phobia: A controlled study. Behav. Res. Ther. 2002, 40, 983–993. [Google Scholar] [CrossRef]
- Klinger, E.; Bouchard, S.; Légeron, P.; Roy, S.; Lauer, F.; Chemin, I.; Nugues, P. Virtual Reality Therapy Versus Cognitive Behavior Therapy for Social Phobia: A Preliminary Controlled Study. CyberPsychol. Behav. 2005, 8, 76–88. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale della Repubblica Italiana. Decreto Legislativo 30 Giugno; Gazzetta Ufficiale della Repubblica Italiana: Roma, Italy, 2003; p. 196. [Google Scholar]
- European Union. Regolamento (UE) 2016/679 del Parlamento Europeo e del Consiglio; del 27 Aprile 2016, Relativo Alla Protezione Delle Persone Fisiche Con Riguardo al Trattamento Dei Dati Personali, Nonché Alla Libera Circolazione di Tali Dati e Che Abroga la Direttiva 95/46/CE (regolamento generale sulla protezione dei dati); European Union Regulation: Bruxelles, Belgium, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).