Mitochondrial Heteroplasmy as a Marker for Premature Coronary Artery Disease: Analysis of the Poly-C Tract of the Control Region Sequence
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. Inclusion Criteria
- -
- Consecutive patients referred to our center for emergency primary angioplasty from 2018 to date.
- -
- STEMI definite diagnosis. MI type 1 is caused by atherothrombotic coronary artery disease (CAD) and is usually precipitated by atherosclerotic plaque. However, in type 2 MI, there is a demand–supply mismatch resulting in myocardial ischemia [30,31]. Atherothrombotic coronary artery disease (CAD) as the cause of the STEMI had to be confirmed by coronary angiogram [32].
- -
- -
- Patients who accepted to participate in this study for investigational purposes.
2.1.2. Exclusion Criteria
- -
- Patients without significant coronary artery disease: individuals with major epicardial coronary arteries angiographically normal or with documented nonsignificant disease (<50% stenosis) by coronary angiogram [32].
- -
- Patients with STEMI due to coronary artery dissection, as the etiopathogenesis differs from atherosclerosis background.
- -
- Patients who died due to the ACS before being able to accept participation in the study.
2.2. Clinical Evaluation
2.3. Control Cohort
2.4. Genetic Testing
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, J.B.; Chinnery, P.F. The dynamics of mitochondrial DNA heteroplasmy: Implications for human health and disease. Nat. Rev. Genet. 2015, 16, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Pagani, I.S.; Kok, C.H.; Saunders, V.A.; Van der Hoek, M.B.; Heatley, S.L.; Schwarer, A.P.; Hahn, C.N.; Hughes, T.P.; White, D.L.; Ross, D.M. A Method for Next-Generation Sequencing of Paired Diagnostic and Remission Samples to Detect Mitochondrial DNA Mutations Associated with Leukemia. J. Mol. Diagn. 2017, 19, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.S.; Alexandrov, L.B.; Gerstung, M.; Martincorena, I.; Nik-Zainal, S.; Ramakrishna, M.; Davies, H.R.; Papaemmanuil, E.; Gundem, G.; Shlien, A.; et al. Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. eLife 2014, 3, e02935. [Google Scholar] [CrossRef] [PubMed]
- Pleasance, E.D.; Stephens, P.J.; O’Meara, S.; McBride, D.J.; Meynert, A.; Jones, D.; Lin, M.-L.; Beare, D.; Lau, K.W.; Greenman, C.; et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010, 463, 184–190. [Google Scholar] [CrossRef]
- van Gisbergen, M.W.; Voets, A.M.; Starmans, M.H.; de Coo, I.F.; Yadak, R.; Hoffmann, R.F.; Boutros, P.C.; Smeets, H.J.; Dubois, L.; Lambin, P. How do changes in the mtDNA and mitochondrial dysfunction influence cancer and cancer therapy? Challenges, opportunities and models. Mutat. Res. Rev. Mutat. Res. 2015, 764, 16–30. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial diseases in man and mouse. Science 1999, 283, 1482–1488. [Google Scholar] [CrossRef]
- Maassen, J.A.; ’t Hart, L.M.; Janssen, G.M.C.; Reiling, E.; Romijn, J.A.; Lemkes, H.H. Mitochondrial diabetes and its lessons for common Type 2 diabetes. Biochem. Soc. Trans. 2006, 34 Pt 5, 819–823. [Google Scholar] [CrossRef]
- Mueller, E.E.; Eder, W.; Ebner, S.; Schwaiger, E.; Santic, D.; Kreindl, T.; Stanger, O.; Paulweber, B.; Iglseder, B.; Oberkofler, H.; et al. The mitochondrial T16189C polymorphism is associated with coronary artery disease in Middle European populations. PLoS ONE 2011, 6, e16455. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Al-Boudari, O.M.; Mousa, A.; Gonzalez, A.M.; Larruga, J.M.; Cabrera, V.M.; Dzimiri, N. The mitochondrial DNA variant 16189T>C is associated with coronary artery disease and myocardial infarction in Saudi Arabs. Genet. Test. Mol. Biomark. 2010, 14, 43–47. [Google Scholar] [CrossRef]
- 10. Volobueva, A.; Grechko, A.; Yet, S.F.; Sobenin, I.; Orekhov, A. Changes in Mitochondrial Genome Associated with Predisposition to Atherosclerosis and Related Disease. Biomolecules 2019, 9, 377. [Google Scholar] [CrossRef]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Bossola, M.; Manes-Gravina, E.; Landi, F.; Bernabei, R.; Marzetti, E. Circulating Mitochondrial DNA at the Crossroads of Mitochondrial Dysfunction and Inflammation During Aging and Muscle Wasting Disorders. Rejuvenation Res. 2018, 21, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.P.K.; Bennett, M.R. Mitochondrial DNA damage and atherosclerosis. Trends Endocrinol. Metab. 2014, 25, 481–487. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Sazonova, M.A.; Postnov, A.Y.; Salonen, J.T.; Bobryshev, Y.V.; Orekhov, A.N. Association of mitochondrial genetic variation with carotid atherosclerosis. PLoS ONE 2013, 8, e68070. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, M.A.; Sinyov, V.V.; Barinova, V.A.; Ryzhkova, A.I.; Bobryshev, Y.V.; Orekhov, A.N.; Sobenin, I.A. Association of mitochondrial mutations with the age of patients having atherosclerotic lesions. Exp. Mol. Pathol. 2015, 99, 717–719. [Google Scholar] [CrossRef]
- Sazonova, M.A.; Chicheva, M.M.; Zhelankin, A.V.; Sobenin, I.A.; Bobryshev, Y.V.; Orekhov, A.N. Association of mutations in the mitochondrial genome with the subclinical carotid atherosclerosis in women. Exp. Mol. Pathol. 2015, 99, 25–32. [Google Scholar] [CrossRef]
- Sazonova, M.A.; Zhelankin, A.V.; Barinova, V.A.; Sinyov, V.V.; Khasanova, Z.B.; Postnov, A.Y.; Sobenin, I.A.; Bobryshev, Y.V.; Orekhov, A.N. Dataset of mitochondrial genome variants associated with asymptomatic atherosclerosis. Data Brief 2016, 7, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondrial DNA variation in human radiation and disease. Cell 2015, 163, 33–38. [Google Scholar] [CrossRef]
- Kaneva, K.; Schurr, T.G.; Tatarinova, T.V.; Buckley, J.; Merkurjev, D.; Triska, P.; Liu, X.; Done, J.; Maglinte, D.T.; Deapen, D.; et al. Mitochondrial DNA haplogroup, genetic ancestry, and susceptibility to Ewing sarcoma. Mitochondrion 2022, 67, 6–14. [Google Scholar] [CrossRef]
- van der Walt, J.M.; Dementieva, Y.A.; Martin, E.R.; Scott, W.K.; Nicodemus, K.K.; Kroner, C.C.; Welsh-Bohmer, K.A.; Saunders, A.M.; Roses, A.D.; Small, G.W.; et al. Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neurosci. Lett. 2004, 365, 28–32. [Google Scholar] [CrossRef]
- Van Der Walt, J.M.; Nicodemus, K.K.; Martin, E.R.; Scott, W.K.; Nance, M.A.; Watts, R.L.; Hubble, J.P.; Haines, J.L.; Koller, W.C.; Lyons, K.; et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am. J. Hum. Genet. 2003, 72, 804–811. [Google Scholar] [CrossRef]
- Achilli, A.; Olivieri, A.; Pala, M.; Kashani, B.H.; Carossa, V.; Perego, U.A.; Gandini, F.; Santoro, A.; Battaglia, V.; Grugni, V.; et al. Mitochondrial DNA backgrounds might modulate diabetes complications rather than T2DM as a whole. PLoS ONE 2011, 6, e21029. [Google Scholar] [CrossRef] [PubMed]
- Kenney, M.C.; Chwa, M.; Atilano, S.R.; Falatoonzadeh, P.; Ramirez, C.; Malik, D.; Tarek, M.; Del Carpio, J.C.; Nesburn, A.B.; Boyer, D.S.; et al. Molecular and bioenergetic differences between cells with African versus European inherited mitochondrial DNA haplogroups: Implications for population susceptibility to diseases. Biochim. Biophys. Acta 2014, 1842, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, M.; Wang, J.; Liu, J.; Li, J.; Fang, H.; Lyu, J.; Shen, L. Association between mitochondrial DNA haplogroup variation and coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 960–966. [Google Scholar] [CrossRef]
- Krzywanski, D.M.; Moellering, D.; Westbrook, D.G.; Dunham-Snary, K.; Brown, J.; Bray, A.W.; Feeley, K.P.; Sammy, M.; Smith, M.R.; Schurr, T.G.; et al. Endothelial Cell Bioenergetics and Mitochondrial DNA Damage Differ in Humans Having African or West Eurasian Maternal Ancestry. Circ. Cardiovasc. Genet. 2016, 9, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Kofler, B.; Mueller, E.; Eder, W.; Stanger, O.; Maier, R.; Weger, M.; Haas, A.; Winker, R.; Schmut, O.; Paulweber, B.; et al. Mitochondrial DNA haplogroup T is associated with coronary artery disease and diabetic retinopathy: A case control study. BMC Med. Genet. 2009, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, Y.; Yamada, Y.; Fuku, N.; Matsuo, H.; Segawa, T.; Watanabe, S.; Kato, K.; Yokoi, K.; Yamaguchi, S.; Nozawa, Y.; et al. Mitochondrial haplogroup N9b is protective against myocardial infarction in Japanese males. Hum. Genet. 2007, 120, 827–836. [Google Scholar] [CrossRef]
- Palacín, M.; Alvarez, V.; Martín, M.; Díaz, M.; Corao, A.I.; Alonso, B.; Díaz-Molina, B.; Lozano, I.; Avanzas, P.; Morís, C.; et al. Mitochondrial DNA and TFAM gene variation in early-onset myocardial infarction: Evidence for an association to haplogroup H. Mitochondrion 2011, 11, 176–181. [Google Scholar] [CrossRef]
- Vilne, B.; Sawant, A.; Rudaka, I. Examining the Association between Mitochondrial Genome Variation and Coronary Artery Disease. Genes 2022, 13, 516. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Cannon, R.O. Chest pain with normal coronary angiograms. N. Engl. J. Med. 1993, 328, 1706–1708. [Google Scholar] [CrossRef]
- Defesche, J.C.; Lansberg, P.J.; Umans-Eckenhausen, M.A.W.; Kastelein, J.J.P. Advanced method for the identification of patients with inherited hypercholesterolemia. Semin. Vasc. Med. 2004, 4, 59–65. [Google Scholar] [CrossRef]
- Williams, R.R.; Hunt, S.C.; Schumacher, M.; Hegele, R.A.; Leppert, M.F.; Ludwig, E.H.; Hopkins, P.N. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am. J. Cardiol. 1993, 72, 171–176. [Google Scholar] [CrossRef]
- Coto, E.; Gómez, J.; Tavira, B.; Tranche, S.; Ortega, F.; Rodríguez, M.I.; Sánchez, E.; Marín, R.; Corao, A.I.; Arenas, J.; et al. A Common APOE Polymorphism Is an Independent Risk Factor for Reduced Glomerular Filtration Rate in the Spanish RENASTUR Cohort. Cardiorenal Med. 2013, 3, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Coto, E.; Tavira, B.; Gómez, J.; Tranche, S.; Corte, C.D. Effect of the FTO rs9930506 Polymorphism on the Main Comorbidities of the Cardiorenal Metabolic Syndrome in an Elderly Spanish Cohort. Cardiorenal Med. 2014, 4, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Coto, D.; Albaiceta, G.M.; Amado-Rodríguez, L.; Clemente, M.G.; Cuesta-Llavona, E.; Gómez, J.; Coto, E. Common mitochondrial haplogroups as modifiers of the onset-age for critical COVID-19. Mitochondrion 2022, 67, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Botto, N.; Berti, S.; Manfredi, S.; Al-Jabri, A.; Federici, C.; Clerico, A.; Ciofini, E.; Biagini, A.; Andreassi, M.G. Detection of mtDNA with 4977 bp deletion in blood cells and atherosclerotic lesions of patients with coronary artery disease. Mutat. Res. 2005, 570, 81–88. [Google Scholar] [CrossRef]
- Sobenin, I.; Sazonova, M.; Ivanova, M.M.; Zhelankin, A.V.; Myasoedova, V.; Postnov, A.; Nurbaev, S.D.; Bobryshev, Y.V.; Orekhov, A. Mutation C3256T of mitochondrial genome in white blood cells: Novel genetic marker of atherosclerosis and coronary heart disease. PLoS ONE 2012, 7, e46573. [Google Scholar] [CrossRef]
- Mitrofanov, K.Y.; Zhelankin, A.V.; Shiganova, G.M.; Sazonova, M.A.; Bobryshev, Y.V.; Postnov, A.Y.; Sobenin, I.A.; Orekhov, A.N. Analysis of mitochondrial DNA heteroplasmic mutations A1555G, C3256T, T3336C, C5178A, G12315A, G13513A, G14459A, G14846A and G15059A in CHD patients with the history of myocardial infarction. Exp. Mol. Pathol. 2016, 100, 87–91. [Google Scholar] [CrossRef]
- Poulton, J.; Luan, J.; Macaulay, V.; Hennings, S.; Mitchell, J.; Wareham, N.J. Type 2 diabetes is associated with a common mitochondrial variant: Evidence from a population-based case-control study. Hum. Mol. Genet. 2002, 11, 1581–1583. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Chan, J.; Chuang, L.-M.; Suzuki, S.; Araki, E.; Nanjo, K.; Ji, L.; Ng, M.; Nishi, M.; Furuta, H.; et al. A mitochondrial DNA variant at position 16189 is associated with type 2 diabetes mellitus in Asians. Diabetologia 2008, 51, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Umbria, M.; Ramos, A.; Aluja, M.P.; Santos, C. The role of control region mitochondrial DNA mutations in cardiovascular disease: Stroke and myocardial infarction. Sci. Rep. 2020, 10, 2766. [Google Scholar] [CrossRef]
- Takamatsu, C.; Umeda, S.; Ohsato, T.; Ohno, T.; Abe, Y.; Fukuoh, A.; Shinagawa, H.; Hamasaki, N.; Kang, D. Regulation of mitochondrial D-loops by transcription factor A and single-stranded DNA-binding protein. EMBO Rep. 2002, 3, 451–456. [Google Scholar] [CrossRef]
- Fish, J.; Raule, N.; Attardi, G. Discovery of a major D-loop replication origin reveals two modes of human mtDNA synthesis. Science 2004, 306, 2098–2101. [Google Scholar] [CrossRef] [PubMed]
- Hefti, E.; Blanco, J.G. Mitochondrial DNA heteroplasmy in cardiac tissue from individuals with and without coronary artery disease. Mitochondrial DNA Part DNA Mapp. Seq. Anal. 2018, 29, 587–593. [Google Scholar] [CrossRef]
- Wei, R.; Ni, Y.; Bazeley, P.; Grandhi, S.; Wang, J.; Li, S.T.; Hazen, S.L.; Tang, W.H.W.; LaFramboise, T. Mitochondrial DNA Content Is Linked to Cardiovascular Disease Patient Phenotypes. J. Am. Heart Assoc. 2021, 10, e018776. [Google Scholar] [CrossRef]
| Total (n = 452) | Cases (n = 188) | Control (n = 271) | p * | |
|---|---|---|---|---|
| Gender (men) | 69.47% (314) | 83.51% (157) | 59.47% (157) | <0.0001 |
| Cardiovascular risk factors | ||||
| Previous/current smoker | 46.02% (208) | 80.85% (152) | 21.21% (56) | <0.0001 |
| High blood pressure | 23.67% (107) | 27.13% (51) | 21.21% (56) | 0.1448 |
| Diabetes mellitus | 12.17% (55) | 9.57% (18) | 14.02% (37) | 0.1546 |
| Dyslipidemia | 32.74% (148) | 38.83% (73) | 28.41% (75) | 0.02 |
| Family history of premature coronary artery disease | 48 (25.5%) | unavailable | ||
| Mitochondrial analysis | ||||
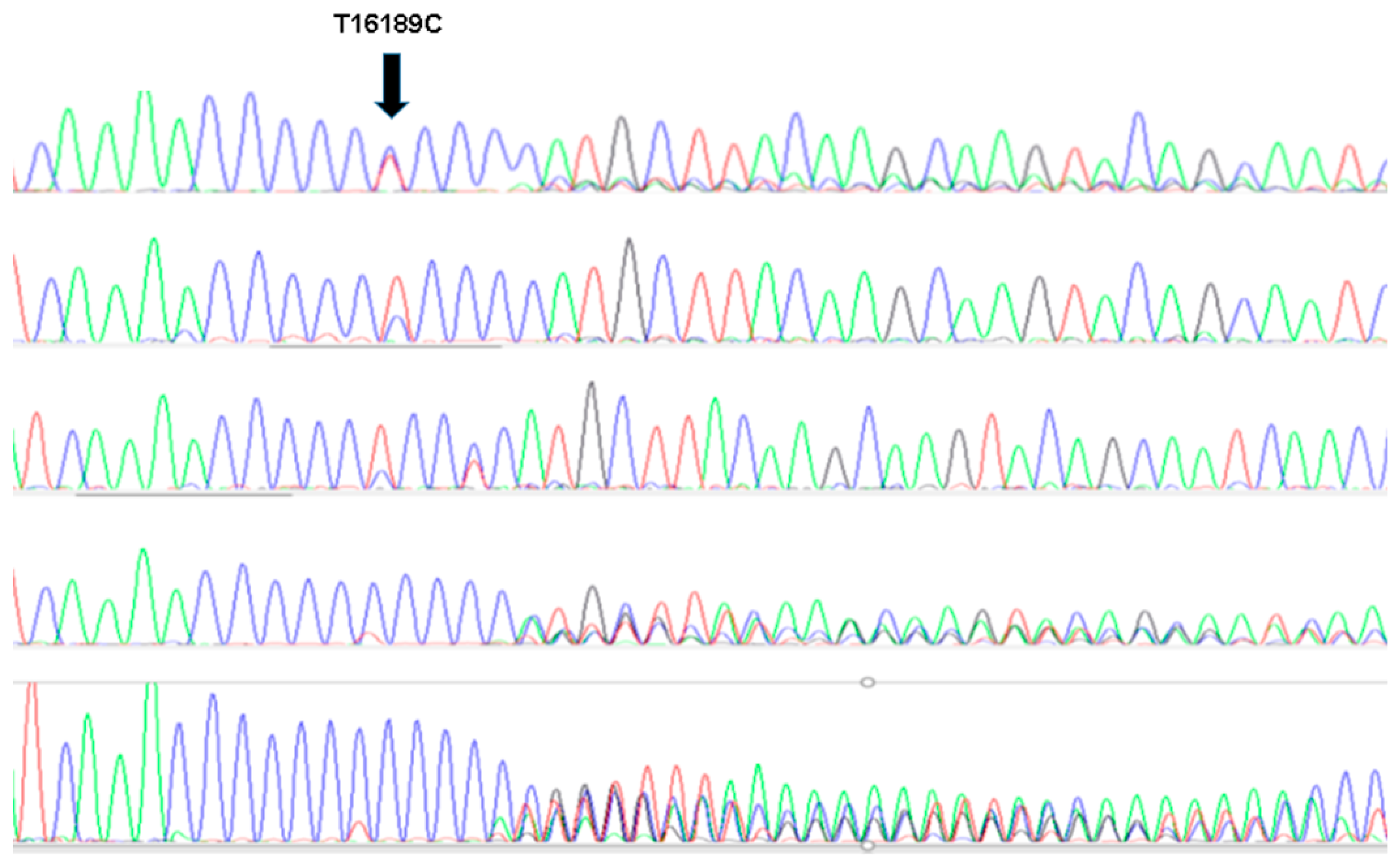
| 16,189 Heteroplasmy | 10.18% (46) | 14.89% (28) | 8% (22) | 0.0051 |
| 16223T | 7.08% (32) | 13.30% (25) | 4% (12) | <0.001 |
| 7028C Haplogroup H | 45.13% (204) | 48.94% (92) | 42% (112) | 0.1703 |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Cases (n =157) | Controls (n = 157) | p * | Cases (n = 31) | Controls (n = 107) | p * | |
| Cardiovascular Risk Factors | ||||||
| Previous/current smoker | 78.34% (123) | 24.84% (39) | <0.001 | 93.55% (29) | 15.89% (17) | <0.001 |
| High blood pressure | 28.03% (44) | 16.56% (26) | 0.015 | 22.58% (7) | 28.04% (30) | 0.546 |
| Diabetes mellitus | 9.55% (15) | 11.46% (18) | 0.581 | 9.68% (3) | 17.76% (19) | 0.405 |
| Dyslipidemia | 38.85% (61) | 26.11% (41) | 0.016 | 38.71% (12) | 31.78% (34) | 0.471 |
| Mitochondrial Analysis | ||||||
| 16,189 Heteroplasmy | 15.92% (25) | 5.73% (9) | 0.004 | 9.68% (3) | 8.41% (9) | 0.732 |
| C16223T mtDNA | 15.29% (24) | 0% (0) | <0.001 | 3.23% (1) | 6.54% (7) | 0.487 |
| 7028CHaplogroup H | 47.77% (75) | 43.31% (68) | 0.428 | 54.84% (17) | 41.12% (44) | 0.176 |
| Odds Ratio | IC 95% | p Value | |
|---|---|---|---|
| 1 Factor | 0.81 | 0.16–4.08 | 0.798 |
| 2 Factor | 0.94 | 0.19–4.49 | 0.938 |
| 3 Factor | 1.17 | 0.20–6.02 | 0.906 |
| 4 Factor | 5.17 | 0.94–28.35 | 0.059 |
| 5 Factor | 15.5 | 1.36–175.38 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorca, R.; Aparicio, A.; Gómez, J.; Álvarez-Velasco, R.; Pascual, I.; Avanzas, P.; González-Urbistondo, F.; Alen, A.; Vázquez-Coto, D.; González-Fernández, M.; et al. Mitochondrial Heteroplasmy as a Marker for Premature Coronary Artery Disease: Analysis of the Poly-C Tract of the Control Region Sequence. J. Clin. Med. 2023, 12, 2133. https://doi.org/10.3390/jcm12062133
Lorca R, Aparicio A, Gómez J, Álvarez-Velasco R, Pascual I, Avanzas P, González-Urbistondo F, Alen A, Vázquez-Coto D, González-Fernández M, et al. Mitochondrial Heteroplasmy as a Marker for Premature Coronary Artery Disease: Analysis of the Poly-C Tract of the Control Region Sequence. Journal of Clinical Medicine. 2023; 12(6):2133. https://doi.org/10.3390/jcm12062133
Chicago/Turabian StyleLorca, Rebeca, Andrea Aparicio, Juan Gómez, Rut Álvarez-Velasco, Isaac Pascual, Pablo Avanzas, Francisco González-Urbistondo, Alberto Alen, Daniel Vázquez-Coto, Mar González-Fernández, and et al. 2023. "Mitochondrial Heteroplasmy as a Marker for Premature Coronary Artery Disease: Analysis of the Poly-C Tract of the Control Region Sequence" Journal of Clinical Medicine 12, no. 6: 2133. https://doi.org/10.3390/jcm12062133
APA StyleLorca, R., Aparicio, A., Gómez, J., Álvarez-Velasco, R., Pascual, I., Avanzas, P., González-Urbistondo, F., Alen, A., Vázquez-Coto, D., González-Fernández, M., García-Lago, C., Cuesta-Llavona, E., Morís, C., & Coto, E. (2023). Mitochondrial Heteroplasmy as a Marker for Premature Coronary Artery Disease: Analysis of the Poly-C Tract of the Control Region Sequence. Journal of Clinical Medicine, 12(6), 2133. https://doi.org/10.3390/jcm12062133







