Laparoscopic Sleeve Gastrectomy versus Laparoscopic Roux-en-Y Gastric Bypass: An Analysis of Weight Loss Using a Multilevel Mixed-Effects Linear Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Technique
2.3. Data Collection
2.4. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Weight Loss
3.2.1. Two Years %TWL
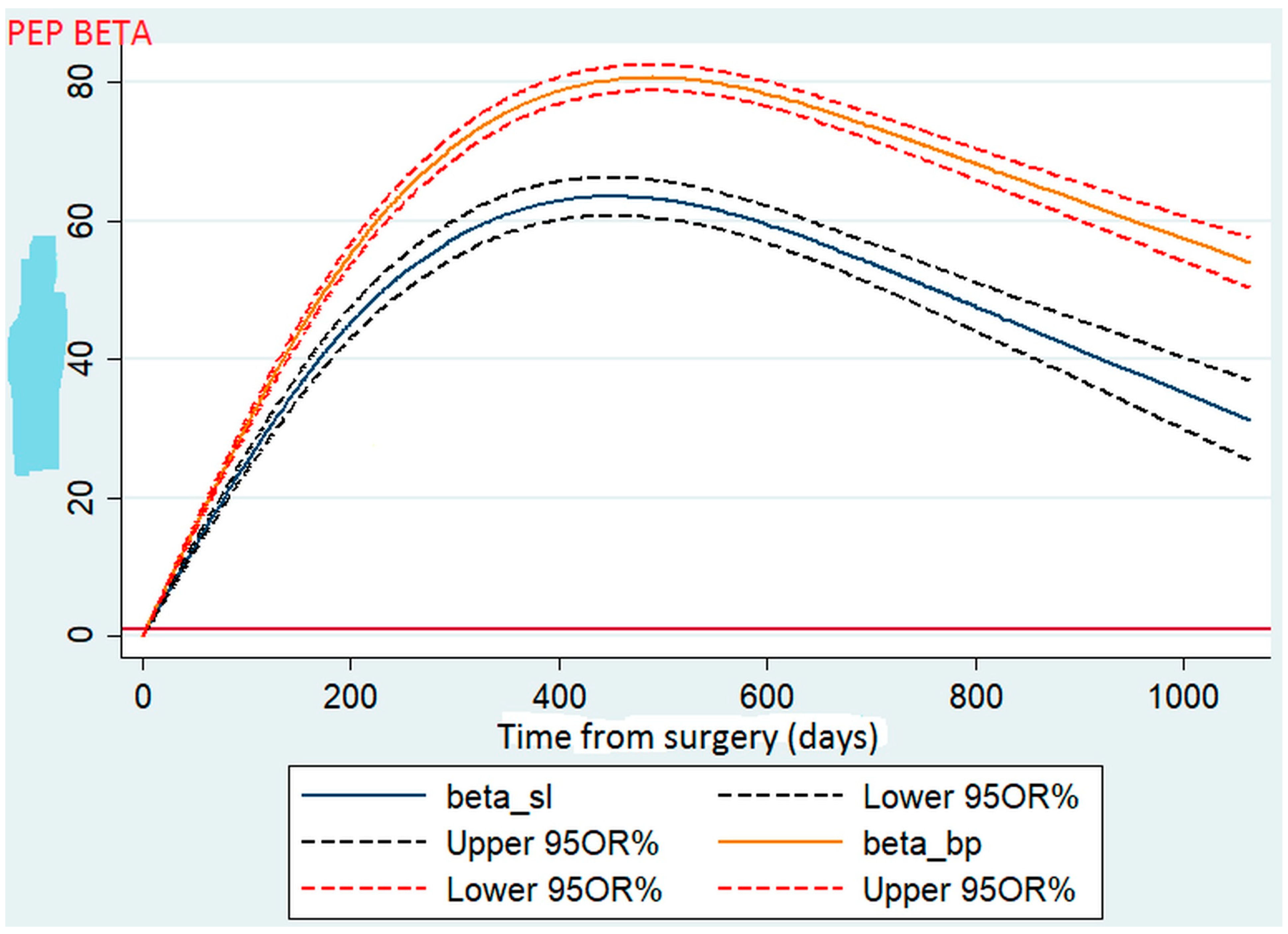
3.2.2. Weight Loss Curve
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, D.D.; Basu, A. Estimating the Medical Care Costs of Obesity in the United States: Systematic Review, Meta-Analysis, and Empirical Analysis. Value Health 2016, 19, 602–613. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Formisano, G.; Buchwald, H.; Scopinaro, N. Bariatric Surgery Worldwide 2013. Obes. Surg. 2015, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric–metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015, 386, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Brethauer, S.A.; Navaneethan, S.D.; Aminian, A.; Pothier, C.E.; Kim, E.S.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—3-Year Outcomes. N. Engl. J. Med. 2014, 370, 2002–2013. [Google Scholar] [CrossRef] [PubMed]
- Melissas, J. IFSO Guidelines for Safety, Quality, and Excellence in Bariatric Surgery. Obes. Surg. 2008, 18, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.; Yumuk, V.; Oppert, J.-M.; Scopinaro, N.; Torres, A.J.; Weiner, R.; Yashkov, Y.; Frühbeck, G. Interdisciplinary European Guidelines on Metabolic and Bariatric Surgery. Obes. Facts 2013, 6, 449–468. [Google Scholar] [CrossRef]
- Maciejewski, M.L.; Arterburn, D.E.; Van Scoyoc, L.; Smith, V.A.; Yancy, W.S., Jr.; Weidenbacher, H.J.; Livingston, E.H.; Olsen, M.K. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg. 2016, 151, 1046–1055. [Google Scholar] [CrossRef]
- Li, J.; Lai, D.; Wu, D. Laparoscopic Roux-en-Y Gastric Bypass Versus Laparoscopic Sleeve Gastrectomy to Treat Morbid Obesity-Related Comorbidities: A Systematic Review and Meta-analysis. Obes. Surg. 2015, 26, 429–442. [Google Scholar] [CrossRef]
- Arterburn, D.; Wellman, R.; Emiliano, A.; Smith, S.R.; Odegaard, A.O.; Murali, S.; Williams, N.; Coleman, K.J.; Courcoulas, A.; Coley, R.Y.; et al. Comparative Effectiveness and Safety of Bariatric Procedures for Weight Loss: A PCORnet cohort study. Ann. Intern. Med. 2018, 169, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Sudan, R.; Maciejewski, M.L.; Wilk, A.R.; Nguyen, N.T.; Ponce, J.; Morton, J.M. Comparative effectiveness of primary bariatric operations in the United States. Surg. Obes. Relat. Dis. 2017, 13, 826–834. [Google Scholar] [CrossRef]
- Peterli, R.; Wölnerhanssen, B.; Peters, T.; Vetter, D.; Kröll, D.; Borbély, Y.; Schultes, B.; Beglinger, C.; Drewe, J.; Schiesser, M.; et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients with Morbid Obesity: The SM-BOSS randomized clinical trial. JAMA 2018, 319, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Salminen, P.; Helmiö, M.; Ovaska, J.; Juuti, A.; Leivonen, M.; Peromaa-Haavisto, P.; Hurme, S.; Soinio, M.; Nuutila, P.; Victorzon, M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients with Morbid Obesity: The SLEEVEPASS randomized clinical trial. JAMA 2018, 319, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Helmiö, M.; Victorzon, M.; Ovaska, J.; Leivonen, M.; Juuti, A.; Jaser, N.; Peromaa, P.; Tolonen, P.; Hurme, S.; Salminen, P. SLEEVEPASS: A randomized prospective multicenter study comparing laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: Preliminary results. Surg. Endosc. 2012, 26, 2521–2526. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Le, Q.A. Effectiveness of bariatric surgical procedures: A systematic review and network meta-analysis of randomized controlled trials. Medicine 2017, 96, e8632. [Google Scholar] [CrossRef]
- Colquitt, J.L.; Pickett, K.; Loveman, E.; Frampton, G.K. Surgery for weight loss in adults. Cochrane Database Syst. Rev. 2014, 2014, CD003641. [Google Scholar] [CrossRef]
- Weinstein, A.L.; Marascalchi, B.J.; Spiegel, M.A.; Saunders, J.K.; Fagerlin, A.; Parikh, M. Patient Preferences and Bariatric Surgery Procedure Selection; the Need for Shared Decision-Making. Obes. Surg. 2014, 24, 1933–1939. [Google Scholar] [CrossRef]
- Laird, N.M.; Ware, J.H. Random-Effects Models for Longitudinal Data. Biometrics 1982, 38, 963. [Google Scholar] [CrossRef]
- Contival, N.; Menahem, B.; Gautier, T.; Le Roux, Y.; Alves, A. Guiding the non-bariatric surgeon through complications of bariatric surgery. J. Visc. Surg. 2018, 155, 27–40. [Google Scholar] [CrossRef]
- Meunier, H.; Le Roux, Y.; Fiant, A.-L.; Marion, Y.; Bion, A.L.; Gautier, T.; Contival, N.; Lubrano, J.; Fobe, F.; Zamparini, M.; et al. Does the Implementation of Enhanced Recovery After Surgery (ERAS) Guidelines Improve Outcomes of Bariatric Surgery? A Propensity Score Analysis in 464 Patients. Obes. Surg. 2019, 29, 2843–2853. [Google Scholar] [CrossRef] [PubMed]
- Vallois, A.; Menahem, B.; Le Roux, Y.; Bion, A.L.; Meunier, H.; Gautier, T.; Contival, N.; Mulliri, A.; Lubrano, J.; Parienti, J.-J.; et al. Revisional Roux-en-Y Gastric Bypass: A Safe Surgical Opportunity? Results of a Case-Matched Study. Obes. Surg. 2018, 29, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Barzin, M.; Khalaj, A.; Motamedi, M.A.; Shapoori, P.; Azizi, F.; Hosseinpanah, F. Safety and effectiveness of sleeve gastrectomy versus gastric bypass: One-year results of Tehran Obesity Treatment Study (TOTS). Gastroenterol. Hepatol. Bed Bench 2016, 9, S62–S69. [Google Scholar] [PubMed]
- Chang, W.W.; Hawkins, D.N.; Brockmeyer, J.R.; Faler, B.J.; Hoppe, S.W.; Prasad, B.M. Factors influencing long-term weight loss after bariatric surgery. Surg. Obes. Relat. Dis. 2019, 15, 456–461. [Google Scholar] [CrossRef]
- Kehagias, I.; Karamanakos, S.N.; Argentou, M.; Kalfarentzos, F. Randomized Clinical Trial of Laparoscopic Roux-en-Y Gastric Bypass Versus Laparoscopic Sleeve Gastrectomy for the Management of Patients with BMI < 50 kg/m2. Obes. Surg. 2011, 21, 1650–1656. [Google Scholar] [CrossRef]
- Ignat, M.; Vix, M.; Imad, I.; D’Urso, A.; Perretta, S.; Marescaux, J.; Mutter, D. Randomized trial of Roux-en-Y gastric bypass versus sleeve gastrectomy in achieving excess weight loss. Br. J. Surg. 2016, 104, 248–256. [Google Scholar] [CrossRef]
- Dogan, K.; Gadiot, R.P.M.; Aarts, E.O.; Betzel, B.; van Laarhoven, C.J.H.M.; Biter, L.U.; Mannaerts, G.H.H.; Aufenacker, T.J.; Janssen, I.M.C.; Berends, F.J. Effectiveness and Safety of Sleeve Gastrectomy, Gastric Bypass, and Adjustable Gastric Banding in Morbidly Obese Patients: A Multicenter, Retrospective, Matched Cohort Study. Obes. Surg. 2014, 25, 1110–1118. [Google Scholar] [CrossRef]
- Shoar, S.; Saber, A.A. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: A systematic review and meta-analysis of comparative studies. Surg. Obes. Relat. Dis. 2016, 13, 170–180. [Google Scholar] [CrossRef]
- Leyba, J.L.; Llopis, S.N.; Aulestia, S.N. Laparoscopic Roux-en-Y Gastric Bypass Versus Laparoscopic Sleeve Gastrectomy for the Treatment of Morbid Obesity. A Prospective Study with 5 Years of Follow-Up. Obes. Surg. 2014, 24, 2094–2098. [Google Scholar] [CrossRef]
- Jammu, G.S.; Sharma, R. A 7-Year Clinical Audit of 1107 Cases Comparing Sleeve Gastrectomy, Roux-En-Y Gastric Bypass, and Mini-Gastric Bypass, to Determine an Effective and Safe Bariatric and Metabolic Procedure. Obes. Surg. 2015, 26, 926–932. [Google Scholar] [CrossRef]
- Jalilvand, A.; Blaszczak, A.; Dewire, J.; Detty, A.; Needleman, B.; Noria, S. Laparoscopic sleeve gastrectomy is an independent predictor of poor follow-up and reaching ≤40% excess body weight loss at 1, 2, and 3 years after bariatric surgery. Surg. Endosc. 2019, 34, 2572–2584. [Google Scholar] [CrossRef] [PubMed]
- Praveenraj, P.; Gomes, R.M.; Kumar, S.; Perumal, S.; Senthilnathan, P.; Parthasarathi, R.; Rajapandian, S.; Palanivelu, C. Comparison of weight loss outcomes 1 year after sleeve gastrectomy and Roux-en-Y gastric bypass in patients aged above 50 years. J. Minimal Access Surg. 2016, 12, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, H.; Cao, Z.; Sun, X.; Zhang, C.; Cai, W.; Liu, R.; Hu, S.; Qin, M. A Randomized Clinical Trial of Laparoscopic Roux-en-Y Gastric Bypass and Sleeve Gastrectomy for the Treatment of Morbid Obesity in China: A 5-Year Outcome. Obes. Surg. 2014, 24, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Grönroos, S.; Helmiö, M.; Juuti, A.; Tiusanen, R.; Hurme, S.; Löyttyniemi, E.; Ovaska, J.; Leivonen, M.; Peromaa-Haavisto, P.; Mäklin, S.; et al. Effect of Laparoscopic Sleeve Gastrectomy vs Roux-en-Y Gastric Bypass on Weight Loss and Quality of Life at 7 Years in Patients with Morbid Obesity: The SLEEVEPASS randomized clinical tria. JAMA Surg. 2021, 156, 137. [Google Scholar] [CrossRef] [PubMed]
- Peterli, R.; Wölnerhanssen, B.; Vetter, D.; Nett, P.; Gass, M.; Borbély, Y.; Peters, T.; Schiesser, M.; Schultes, B.; Beglinger, C.; et al. Laparoscopic Sleeve Gastrectomy Versus Roux-Y-Gastric Bypass for Morbid Obesity—3-Year Outcomes of the Prospective Randomized Swiss Multicenter Bypass or Sleeve Study (SM-BOSS). Ann. Surg. 2017, 265, 466–473. [Google Scholar] [CrossRef]
- Wölnerhanssen, B.K.; Peterli, R.; Hurme, S.; Bueter, M.; Helmiö, M.; Juuti, A.; Meyer-Gerspach, A.C.; Slawik, M.; Peromaa-Haavisto, P.; Nuutila, P.; et al. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: 5-year outcomes of merged data from two randomized clinical trials (SLEEVEPASS and SM-BOSS). Br. J. Surg. 2020, 108, 49–57. [Google Scholar] [CrossRef]
- Ahmed, B.; King, W.C.; Gourash, W.; Belle, S.H.; Hinerman, A.; Pomp, A.; Dakin, G.; Courcoulas, A.P. Long-term weight change and health outcomes for sleeve gastrectomy (SG) and matched Roux-en-Y gastric bypass (RYGB) participants in the Longitudinal Assessment of Bariatric Surgery (LABS) study. Surgery 2018, 164, 774–783. [Google Scholar] [CrossRef]
- van Rijswijk, A.-S.; van Olst, N.; Schats, W.; van der Peet, D.L.; van de Laar, A.W. What Is Weight Loss After Bariatric Surgery Expressed in Percentage Total Weight Loss (%TWL)? A Systematic Review. Obes Surg. Août 2021, 31, 3833–3847. [Google Scholar] [CrossRef]
- Grover, B.T.; Morell, M.C.; Kothari, S.N.; Borgert, A.J.; Kallies, K.J.; Baker, M.T. Defining Weight Loss After Bariatric Surgery: A Call for Standardization. Obes. Surg. 2019, 29, 3493–3499. [Google Scholar] [CrossRef]
- Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence—Oxford Scholarship [Internet]. [cité 19 août 2021]. Available online: https://oxford.universitypressscholarship.com/view/10.1093/acprof:oso/9780195152968.001.0001/acprof-9780195152968 (accessed on 29 December 2022).
- Hu, Z.; Sun, J.; Li, R.; Wang, Z.; Ding, H.; Zhu, T.; Wang, G. A Comprehensive Comparison of LRYGB and LSG in Obese Patients Including the Effects on QoL, Comorbidities, Weight Loss, and Complications: A Systematic Review and Meta-Analysis. Obes. Surg. 2019, 30, 819–827. [Google Scholar] [CrossRef]
- Han, Y.; Jia, Y.; Wang, H.; Cao, L.; Zhao, Y. Comparative analysis of weight loss and resolution of comorbidities between laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass: A systematic review and meta-analysis based on 18 studies. Int. J. Surg. 2020, 76, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Toolabi, K.; Sarkardeh, M.; Vasigh, M.; Golzarand, M.; Vezvaei, P.; Kooshki, J. Comparison of Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Sleeve Gastrectomy on Weight Loss, Weight Regain, and Remission of Comorbidities: A 5 Years of Follow-up Study. Obes. Surg. 2019, 30, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Auge, M.; Dejardin, O.; Menahem, B.; Bion, A.L.; Savey, V.; Launoy, G.; Bouvier, V.; Alves, A. Analysis of the Lack of Follow-Up of Bariatric Surgery Patients: Experience of a Reference Center. J. Clin. Med. 2022, 11, 6310. [Google Scholar] [CrossRef] [PubMed]
- Auge, M.; Menahem, B.; Savey, V.; Bion, A.L.; Alves, A. Long-term complications after gastric bypass and sleeve gastrectomy: What information to give to patients and practitioners, and why? J. Visc. Surg. 2022, 159, 298–308. [Google Scholar] [CrossRef] [PubMed]
| LRYGB | LSG | ||||
|---|---|---|---|---|---|
| (n = 266) | (n = 169) | ||||
| Variables | p Values | ||||
| Sex | <0.001 | ||||
| Female | 227 | 85.3% | 117 | 69.2% | |
| Male | 39 | 14.7% | 52 | 30.8% | |
| Age | 0.382 | ||||
| Continuous (years), mean ± SD | 266 | 43.4 ± 0.7 | 169 | 42.5 ± 0.9 | |
| Preoperative bodyweight | <0.001 | ||||
| Continuous (kg), mean ± SD | 266 | 110.1 ± 0.9 | 169 | 123.6 ± 1.8 | |
| Preoperative excess weight | <0.001 | ||||
| Continuous (kg) mean ± SD | 266 | 45.3 ± 0.7 | 169 | 56.1 ± 1.6 | |
| Preoperative BMI | <0.001 | ||||
| Continuous (kg/m2) mean ± SD | 266 | 40.5 ± 0.2 | 169 | 44.0 ± 0.6 | |
| ASA score | 0.01 | ||||
| 2 | 222 | 83.4% | 116 | 68.7% | |
| 3 | 44 | 16.6% | 53 | 31.3 | |
| Former smokers | 0.534 | ||||
| Yes | 42 | 15.8% | 23 | 13.6% | |
| No | 224 | 84.2% | 146 | 86.4% | |
| Diabetes | 0.521 | ||||
| Yes | 67 | 25.2% | 38 | 22.5% | |
| No | 199 | 74.8% | 131 | 77.5% | |
| Hypertension | 0.447 | ||||
| Yes | 82 | 30.8% | 58 | 34.3% | |
| No | 184 | 69.2% | 111 | 65.7% | |
| Dyslipidaemia | 0.485 | ||||
| Yes | 69 | 25.9% | 49 | 29.0% | |
| No | 197 | 74.1% | 120 | 71.0% | |
| Sleep apnoea | 0.184 | ||||
| Yes | 118 | 44.4% | 86 | 50.9% | |
| No | 148 | 55.6% | 83 | 49.1% | |
| Length of hospital stay (days) | 266 | 3.4 ± 0.1 | 169 | 3.0 ± 0.1 | 0.026 |
| (n = 435) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Percentage | Univariable Analysis | Multivariable Analysis * | |||||||
| Variables | β ** | 95% CI | p Values | β ** | 95% CI | p Values | ||||
| Sex | ||||||||||
| Female | 1240 | 77.5% | Ref | <0.001 | Ref | 0.325 | ||||
| Male | 359 | 22.5% | −5.46 | −7.49 | −3.42 | −0.89 | −2.65 | 0.88 | ||
| Surgery age | ||||||||||
| Continuous | 1599 | 100% | −0.29 | −0.36 | −0.22 | <0.001 | −0.15 | −0.21 | −0.09 | <0.001 |
| Surgery BMI | ||||||||||
| Continuous | 1599 | 100% | −1.3 | −1.55 | −1.05 | <0.001 | −1.17 | −1.35 | −0.98 | <0.001 |
| Type of surgery | ||||||||||
| LRYGB | 1069 | 66.8% | Ref | <0.001 | Ref | <0.001 | ||||
| LSG | 530 | 33.2% | −10.80 | −12.56 | −9.03 | −6.67 | −8.33 | −5.00 | ||
| Year of surgery | ||||||||||
| Continuous | 1599 | 100% | −0.51 | −0.81 | −0.21 | <0.001 | 0.48 | 0.21 | 0.74 | <0.001 |
| Time since surgery | ||||||||||
| Continuous | 1599 | 100% | 0.78 | 0.75 | 0.81 | <0.001 | 0.78 | 0.75 | 0.82 | <0.001 |
| Former smokers | ||||||||||
| No | 1368 | 85.5% | Ref | 0.017 | Ref | 0.003 | ||||
| Yes | 231 | 14.5% | 2.97 | 0.52 | 5.41 | 3.14 | 1.06 | 5.20 | ||
| Diabetes | ||||||||||
| No | 1197 | 74.9% | Ref | <0.001 | Ref | 0.003 | ||||
| Yes | 409 | 25.1% | −4.81 | −6.73 | −2.90 | −2.63 | −4.37 | −0.88 | ||
| Hypertension | ||||||||||
| No | 1032 | 64.5% | Ref | <0.001 | ||||||
| Yes | 567 | 35.5% | −6.15 | −7.88 | −4.41 | |||||
| Dyslipidaemia | ||||||||||
| No | 1268 | 79.3% | Ref | <0.001 | ||||||
| Yes | 331 | 20.7% | −4.51 | −6.61 | −2.40 | |||||
| Sleep apnoea | ||||||||||
| No | 999 | 62.5% | Ref | <0.001 | ||||||
| Yes | 600 | 37.5% | −6.59 | −8.31 | −4.87 | |||||
| Individual variance | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pouchucq, C.; Dejardin, O.; Bouvier, V.; Lee Bion, A.; Savey, V.; Launoy, G.; Menahem, B.; Alves, A. Laparoscopic Sleeve Gastrectomy versus Laparoscopic Roux-en-Y Gastric Bypass: An Analysis of Weight Loss Using a Multilevel Mixed-Effects Linear Model. J. Clin. Med. 2023, 12, 2132. https://doi.org/10.3390/jcm12062132
Pouchucq C, Dejardin O, Bouvier V, Lee Bion A, Savey V, Launoy G, Menahem B, Alves A. Laparoscopic Sleeve Gastrectomy versus Laparoscopic Roux-en-Y Gastric Bypass: An Analysis of Weight Loss Using a Multilevel Mixed-Effects Linear Model. Journal of Clinical Medicine. 2023; 12(6):2132. https://doi.org/10.3390/jcm12062132
Chicago/Turabian StylePouchucq, Camille, Olivier Dejardin, Véronique Bouvier, Adrien Lee Bion, Véronique Savey, Guy Launoy, Benjamin Menahem, and Arnaud Alves. 2023. "Laparoscopic Sleeve Gastrectomy versus Laparoscopic Roux-en-Y Gastric Bypass: An Analysis of Weight Loss Using a Multilevel Mixed-Effects Linear Model" Journal of Clinical Medicine 12, no. 6: 2132. https://doi.org/10.3390/jcm12062132
APA StylePouchucq, C., Dejardin, O., Bouvier, V., Lee Bion, A., Savey, V., Launoy, G., Menahem, B., & Alves, A. (2023). Laparoscopic Sleeve Gastrectomy versus Laparoscopic Roux-en-Y Gastric Bypass: An Analysis of Weight Loss Using a Multilevel Mixed-Effects Linear Model. Journal of Clinical Medicine, 12(6), 2132. https://doi.org/10.3390/jcm12062132





