1. Introduction
Among the complications of cardiopulmonary bypass (CPB) surgery, the incidence of acute kidney injury (AKI) is as high as 47% in children. Of these, 67% of AKI cases occurred in children aged 2 years or below, with a younger age correlated with a higher incidence [
1]. Furthermore, the onset of AKI is a known risk factor for prolonged intensive care unit (ICU) stay and increased mortality [
2,
3].
The standard definition of AKI is increased serum creatinine (sCr) levels or decreased urine output. A decrease in creatinine clearance signals its onset [
3,
4].
To facilitate early and reliable diagnosis, in 2011, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines defined the diagnostic criteria for AKI as decreased urine output for 6 h and increased sCr levels for 48 h. The usefulness of these criteria in a pediatric setting has been reported [
5]. However, since most of these parameters reach their peak values 48 h after AKI onset, a reliable biomarker for the early detection of AKI is needed. Several studies have investigated potential early AKI biomarkers, such as neutrophil gelatinase-associated lipocalin (NGAL), Cystatin C, interleukin-18, renal injury molecule-1 (KIM-1), and others [
6,
7,
8,
9,
10].
Due to the influence of maternal creatinine concentrations, the reference value of sCr in newborns changes daily and gradually increases as infants grow. Furthermore, its increase is slower at the onset of AKI compared to that in adults, and even a slight increase is associated with mortality [
11]. Hence, diagnostic methods that can detect AKI earlier and are more sensitive than the existing methods are desirable in the pediatric setting.
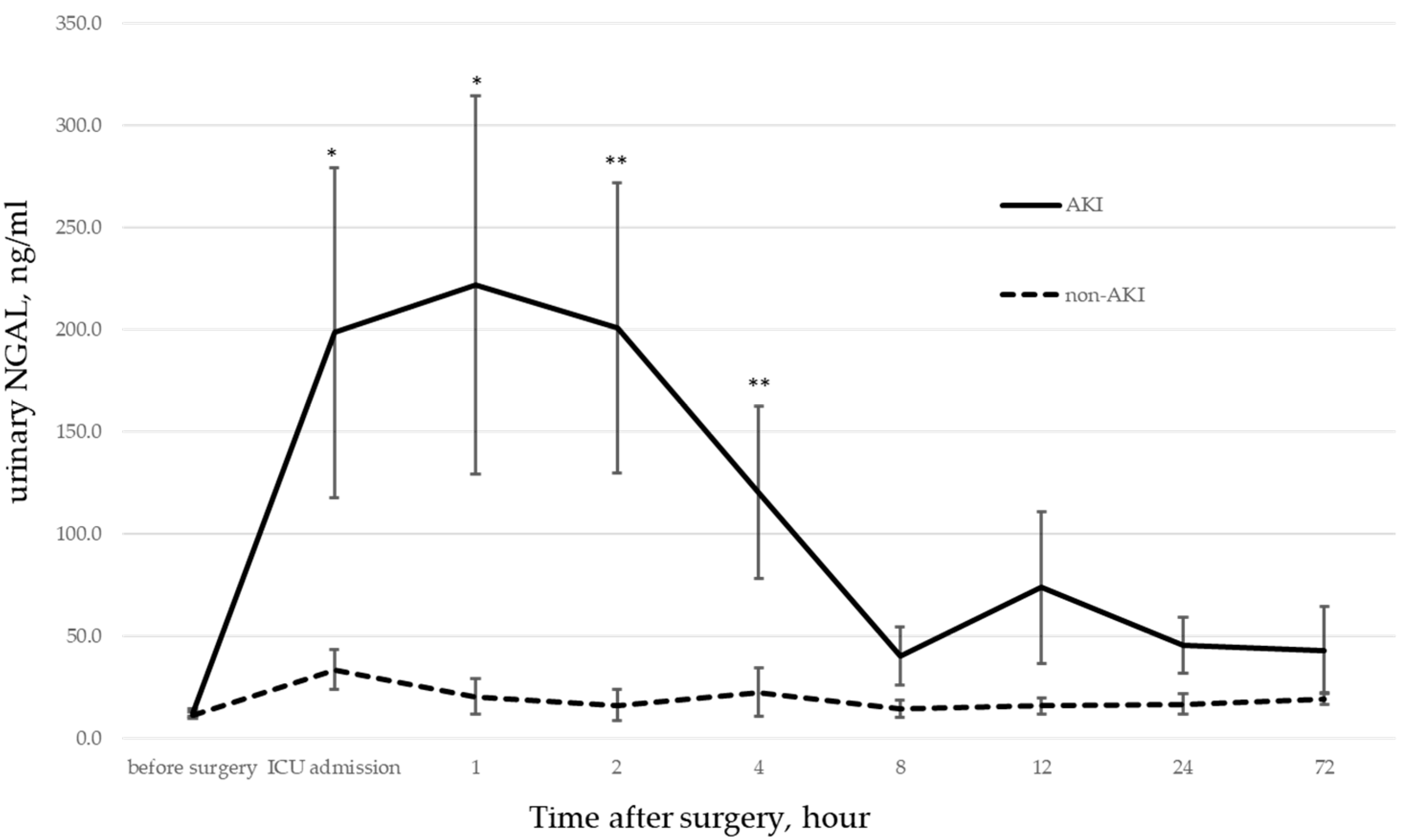
We previously studied the correlation between AKI onset and urinary NGAL levels in pediatric patients undergoing CPB. We found that urinary NGAL levels were significantly elevated immediately after ICU admission in patients who developed postoperative AKI [
12].
Other studies on AKI associated with pediatric cardiac surgery reported that the receiver operating characteristic (ROC) curves of urinary NGAL were 0.998 at 2 h after CPB and 1.000 at 4 h [
13]. However, there are cases where NGAL values did not increase, despite postoperative AKI, or where NGAL values increased, despite the absence of postoperative AKI. These suggest that the values’ predictive performance is not consistent [
14]. Similar results were observed in our study [
12].
A previous study found that urinary NGAL was elevated at ICU admission in patients who developed AKI during the perioperative period. This suggests that many events that cause renal dysfunction might have occurred intraoperatively. Hence, it is necessary to evaluate intraoperative kidney perfusion for early AKI diagnosis. Therefore, we decided to use intraoperative near-infrared spectroscopy (NIRS) monitoring to evaluate blood flow to the kidney and measure renal oxygen saturation. NIRS is a non-invasive, continuous, and real-time method for measuring regional saturation of oxygen (rSO
2) around the target organs. rSO
2 captures the shift in the balance of oxygen supply and demand, reflecting tissue perfusion and metabolic status. NIRS can calculate the two-dimensional parameters of time elapsed below a set threshold and the value of the decline as the area under the curve (AUC). This allows the degree of desaturation to be quantified in terms of cumulative saturation (% min). The threshold for cumulative saturation can be set to the level of decline from the baseline or the level of decline at a set rate of decrease. In addition, since rSO
2 provides real-time information about tissue perfusion—and the near-infrared light emitted by the sensors can reach several centimeters deep—we planned to examine its use in monitoring the perirenal region in children [
15,
16].
In this study, we examined the use of urinary NGAL and renal NIRS as indicators of AKI during the perioperative period of pediatric patients who underwent cardiac surgery under CPB.
2. Materials and Methods
2.1. Patient Population
This prospective observational study was approved (approval number 17-304) by the Center for Clinical Research and Clinical Trials of Juntendo University Hospital, which approves the clinical trial registry, and complies with the 1975 Declaration of Helsinki (revised 1983).
The study period ranged from June 2018 to January 2020, and all pediatric patients aged under 204 months with congenital heart disease undergoing cardiac surgery with CPB were eligible. Patients with written consent from their legal guardians were enrolled in the study. The exclusion criteria included cases requiring total circulatory arrest; beating-heart surgery; and before-surgery renal dysfunction, such as congenital renal disease.
Based on the 2016 Japanese Clinical Practice Guideline for AKI [
17], which uses the KDIGO diagnostic criteria, AKI was classified according to sCr values, with those with stage 1 or higher considered in the AKI group [
18].
2.2. Data Collection
Demographic and clinical data included patient background, duration of surgery, duration of CPB, duration of aortic cross-clamp, duration of postoperative ventilator use, PaO2/FiO2 (P/F) ratio before and after surgery, use of nitric oxide (NO), and serum lactate levels. sCr was evaluated before surgery and at 12 h and 36 h after surgery.
Disease and surgical factors were evaluated using the Risk Adjustment in Congenital Heart Surgery System score (RACHS-1 score), which evaluates surgical outcomes in cases of complex congenital heart disease [
19].
2.3. CPB
The CPB priming volume was calculated according to institutional protocols, with the target flow rate based on a perfusion index of 2.6 L/min/m2. Body temperature was maintained at mild to moderate hypothermic levels. Blood was transfused to correct low hematocrit values (lower limit of 18%) whenever feasible. In all cases, modified ultrafiltration was performed immediately after CPB discontinuation.
CPB data collected from the automatic recording device ORSYS® (Philips Co., Ltd., Amsterdam, The Netherlands) included dilution rate, perfusion index (PI = Perfusion flow/body surface area), hemoglobin level (HGB), hematocrit, and oxygen supply per body surface area (DO2i = 10 × pump flow (L/minute/m2) × HGB (mg/dL) × 1.34 × HGB saturation (%) + 0.003 × O2 tension (mmHg)).
2.4. Perioperative Management
Perioperative management was performed by an anesthesiologist, while postoperative management was handled by a cardiovascular surgeon. Hemodynamic management included the use of dopamine, dobutamine, noradrenaline, nitroglycerin, and other drugs. Ventilation management was mainly accomplished using pressure control ventilation with NO therapy as appropriate. Due to difficulties in monitoring urine output because of leakage caused by a catheter size mismatch and an irregular administration of diuretics, urine output was not included as an indicator.
2.5. Urinary NGAL
Urine collection was performed before surgery, at ICU admission (0 h), and at 1, 2, 4, 8, 12, 24, and 72 h. Urine samples were centrifuged at 500× g of relative centrifugal force for 5 min and frozen at −80 °C. Urinary NGAL measurements were performed using a chemiluminescence immunoassay with the immunoassay analyzer ARCHITECT i200SR® (Abbott Japan Co., Ltd., Tokyo, Japan). Absolute values for urinary NGAL were used, without corrections for urinary creatinine or other parameters.
2.6. NIRS Monitoring
The INVOS 5100C (Medtronic, Minneapolis, MN, USA) was used for NIRS monitoring. Sensors were placed on the forehead and on the right perirenal area, with the renal sensor affixed to the ribs and midline of the pelvis (T10-L2), lateral to the spine, as reported by Ruf et al. [
20]. NIRS data were collected every 2 s, from the time of entry into the operating room to the time of exit.
2.7. NIRS Monitoring Analysis
Analyses were performed using Medtronic’s INVOS software. The decrease in rSO2 from baseline (at the beginning of the surgery) was compared to its lowest value during surgery. Changes in rSO2 while on CPB were examined by comparing the mean, nadir, and rate of decrease from CPB initiation to the lowest rSO2 value. The decrease in rSO2 values during CPB was quantified and calculated to determine the cumulative saturation (defined as the renal rSO2 score). When the decrease in renal rSO2 scores from CPB initiation exceeded 20% and 25%, the amount of decline was integrated and calculated as the AUC (% min). The rSO2 score is {(baseline rSO2 − current rSO2 (%)) × time (min)}. The numerator of this score was changed from seconds to minutes to better accommodate longer time measurements.
2.8. Statistical Analysis
For statistical analysis, nonparametric tests, logistic regression analysis, and multiple regression analysis were performed using JMP® 16.0.0 (SAS Institute Inc., Cary, NC, USA). The ROC curve was used to evaluate independent factors. The AUC was calculated, and the optimal cutoff value for discrimination of AKI onset was determined using the Youden index (defined as sensitivity + specificity − 1).
4. Discussion
This study analyzed trends in urinary NGAL and renal rSO2 scores in patients younger than 204 months who developed postoperative AKI after cardiac surgery.
The RACHS-1 score showed that the AKI group included many patients with cyanotic heart disease who required more challenging surgery due to their younger age and complex cardiac malformations.
Previous studies reported that urinary NGAL is more sensitive than other biomarkers for detecting AKI [
13,
14,
21]. Compared to our previous study involving 64 patients, perioperative urinary NGAL showed more sensitivity in predicting AKI in this study since the number of patients is higher [
12]. Significant differences in urinary NGAL during ICU admissions suggest that factors that caused the elevated NGAL levels were present during the intraoperative period.
NIRS monitoring showed lower cerebral and renal rSO2 values in the AKI group than in the non-AKI group before the start of surgery. This is probably because the AKI group included more patients whose oxygen levels were already low, requiring respiratory support.
Under CPB conditions, patients are exposed to a non-physiological environment. In such environments, cerebral blood flow is significantly maintained by cerebrovascular autoregulation [
22,
23,
24]. Although the kidneys are believed to exhibit regional autoregulation [
25], the main cause of the decreased renal rSO
2 was the decreased blood flow to the kidneys and other organs. This notion is based on the understanding that the kidneys are also considered to exhibit local autoregulatory functions [
25], and the main cause of reduced kidney rSO
2 is reduced blood flow to the organ due to blood shunting to the brain, which is sensitive to reduced blood flow.
NIRS monitoring is reported to produce artifacts and noise in the data, which can reduce its sensitivity and specificity [
26,
27,
28]. There is also a lack of normative data and standardized definitions for renal ischemia, especially in the pediatric setting [
29]. However, NIRS is non-invasive and provides continuous, real-time information about local tissue oxygen delivery and consumption in neonates [
15,
16]. Previous studies reported a significant correlation between changes in renal NIRS and the onset of AKI following CPB in critically ill children [
20,
22,
30,
31,
32,
33,
34]. Furthermore, studies correlating renal blood flow with renal rSO
2 also support this association [
35].
NIRS is often used to monitor and detect changes, although it has no defined reference values. However, concerning patients with low values before surgery and the effects of artifacts, monitoring only absolute values or changes in values is inadequate. This is especially true in the CPB setting, where changes in hemodynamics, the partial pressure of oxygen, and HGB can be significant. The renal rSO2 score, which reflects cumulative changes in oxygen desaturation, should also be monitored to determine the baseline and the limit of acceptable desaturation levels.
Based on the results of this study, in addition to the conventional method of using only DO2i as an indicator of organ perfusion, we believe that monitoring the renal rSO2 score and limiting its decline might be useful in preventing AKI development. However, this study did not investigate the reasons for rSO2 decrease. In the future, we intend to further the research by examining factors, such as perfusion volume, blood pressure, partial pressure of oxygen, partial pressure of carbon dioxide, HGB, and body temperature, that might cause rSO2 decrease to determine methods that can prevent the onset of renal dysfunction.
This study had some limitations related to patient selection. Because this study included consecutive patients who had undergone surgery, we did not classify the patients by age in months as we did for neonates and infants. Moreover, we did not classify patients according to disease or disease severity, such as the presence or absence of cyanotic heart disease. In addition, cases in which it was impossible to collect urine were excluded. These included cases of leakage from urinary catheters, cases in which urinary drainage could only be seen in the urine bag tube, and cases in which the patient had to be switched to “nappies” early in the ICU. For these reasons, subgroup analysis and stratification were also deemed difficult due to the small number of valid cases. Additionally, hemolysis was not considered since no test was performed to assess it. Lastly, the renal rSO2 sensor is placed on the ‘perirenal’ area. It may not reliably reflect actual renal saturation, depending on body size, given the reach of near-infrared light. However, we also believe that it reflects abdominal blood flow, even if it does not directly reflect the renal. It may reflect the ischemic state of the major abdominal organs.