Low- vs. High-Power Laser for Holmium Laser Enucleation of Prostate
Abstract
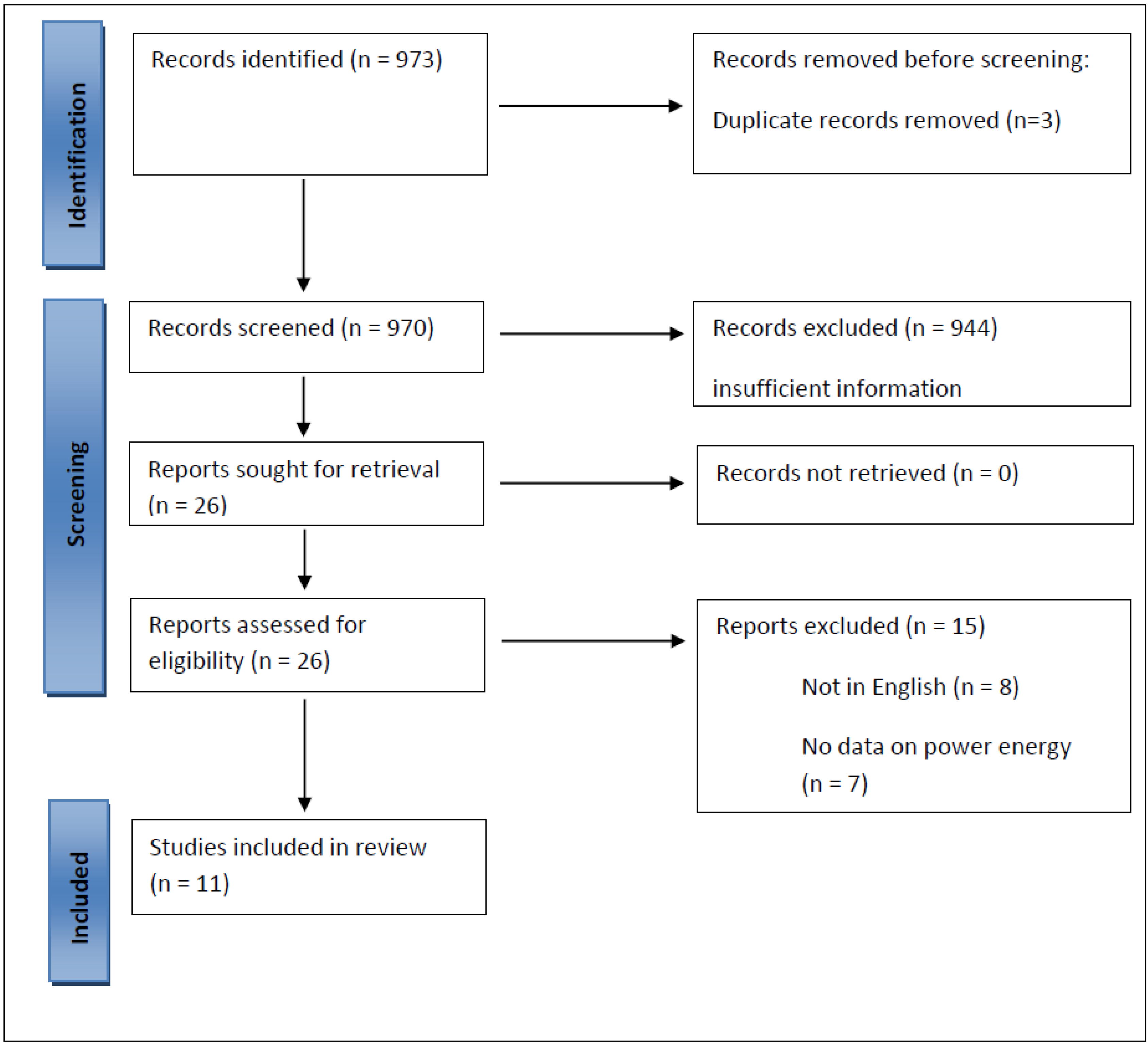
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria
3. Results
3.1. Efficiency and Speed of LP HoLEP
3.2. Functional Outcomes of LP HoLEP
3.3. Postoperative Stress Urinary Incontinence (SIU) and Dysuria after LP HoLEP
3.4. Safety of LP HoLEP
4. Opinion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gratzke, C.; Bachmann, A.; Descazeaud, A.; Drake, M.J.; Madersbacher, S.; Mamoulakis, C.; Oelke, M.; Tikkinen, K.A.; Gravas, S. EAU Guidelines on the Assessment of Non-neurogenic Male Lower Urinary Tract Symptoms including Benign Prostatic Obstruction. Eur. Urol. 2015, 67, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.W.; Gilling, P.J. HoLEP has come of age. World J. Urol. 2015, 33, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Cornu, J.-N.; Ahyai, S.; Bachmann, A.; de la Rosette, J.; Gilling, P.; Gratzke, C.; McVary, K.; Novara, G.; Woo, H.; Madersbacher, S. A Systematic Review and Meta-analysis of Functional Outcomes and Complications Following Transurethral Procedures for Lower Urinary Tract Symptoms Resulting from Benign Prostatic Obstruction: An Update. Eur. Urol. 2015, 67, 1066–1096. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, T.R.W.; Liatsikos, E.N.; Nagele, U.; Traxer, O.; Merseburger, A.S. EAU Guidelines on Laser Technologies. Eur. Urol. 2012, 61, 783–795. [Google Scholar] [CrossRef]
- Suardi, N.; Gallina, A.; Salonia, A.; Briganti, A.; Dehò, F.; Zanni, G.; Abdollah, F.; Naspro, R.; Cestari, A.; Guazzoni, G.; et al. Holmium laser enucleation of the prostate and holmium laser ablation of the prostate: Indications and outcome. Curr. Opin. Urol. 2009, 19, 38–43. [Google Scholar] [CrossRef]
- Gong, Y.-G.; He, D.-L.; Wang, M.-Z.; Li, X.-D.; Zhu, G.-D.; Zheng, Z.-H.; Du, Y.-F.; Chang, L.S.; Nan, X.-Y. Holmium Laser Enucleation of the Prostate: A Modified Enucleation Technique and Initial Results. J. Urol. 2012, 187, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Rassweiler, J.; Roder, M.; Schulze, M.; Muschter, R. Transurethral enucleation of the prostate with the holmium: YAG laser system: How much power is necessary? Urologe A 2008, 47, 441–448. [Google Scholar] [CrossRef]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Gilling, P.; Mason, C.; Reuther, R.; Van Rij, S.; Fraundorfer, M. Use of low-powered HoLEP for the treatment of benign prostatic hyperplasia. BJU Int. 2013, 111 (Suppl. 1), 13–126. [Google Scholar]
- Scoffone, C.M.; Ingrosso, M.; Russo, N.; Cracco, C. MP02-10 Low-Power versus High-Power En-Bloc No-Touch Holep: Comparing Feasibility, Safety and Efficacy. J. Urol. 2017, 197 (Suppl. 4), e14. [Google Scholar] [CrossRef]
- Cracco, C.; Ingrosso, M.; Russo, N.; Scoffone, C.M. MP02-11 Postoperative Dysuria after High- and Low-Power en-Bloc No-Touch Holep. J. Urol. 2017, 197 (Suppl. 4), e487–e488. [Google Scholar] [CrossRef]
- Cracco, C.; Cattaneo, G.; Sica, A.; Ndrevataj, D.; Scoffone, C.M. MP32-08 Impact of Adenoma Volume on the Intraoperative Features of 3 Newly Developed Approaches for Holmium Laser Enucleation of the Prostate. J. Urol. 2020, 203 (Suppl. 4), e487–e488. [Google Scholar] [CrossRef]
- Tokatli, Z.; Ferhat, M.; Ibis, M.A.; Sariyildiz, G.T.; Elhan, A.; Sarica, K. Does the power of the laser devices matter for a successful HoLEP procedure? A prospective comparative study. Int. J. Clin. Pract. 2021, 75, e14531. [Google Scholar] [CrossRef] [PubMed]
- Elshal, A.M.; El-Nahas, A.R.; Ghazy, M.; Nabeeh, H.; Laymon, M.; Soltan, M.; Ghobrial, F.K.; El-Kappany, H.A. Low-Power vs. High-Power Holmium Laser Enucleation of the Prostate: Critical Assessment through Randomized Trial. Urology 2018, 121, 58–65. [Google Scholar] [CrossRef]
- Becker, B.; Gross, A.J.; Netsch, C. Safety and efficacy using a low-powered holmium laser for enucleation of the prostate (HoLEP): 12-month results from a prospective low-power HoLEP series. World J. Urol. 2018, 36, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.; Moore, S.L.; Gill, A.; Obi-Njoku, O.; Hughes, S.F.; Saleemi, A.; Ellis, G.; Khan, F.; Shergill, I.S. Safety and efficacy of Holmium laser enucleation of the prostate (HoLEP) in patients with previous transperineal biopsy (TPB): Outcomes from a dual-centre case-control study. BMC Urol. 2019, 19, 97. [Google Scholar] [CrossRef]
- Gazel, E.; Kaya, E.; Yalcın, S.; Okas, T.; Aybal, H.C.; Yılmaz, S.; Tunc, L. The low power effect on holmium laser enucleation of prostate (HoLEP): A comparison between 20 W and 37,5 W energy regarding apical enucleation efficacy and patient safety. Prog. Urol. 2020, 30, 632–638. [Google Scholar] [CrossRef]
- Minagawa, S.; Okada, S.; Morikawa, H. Safety and Effectiveness of Holmium Laser Enucleation of the Prostate Using a Low-power Laser. Urology 2017, 110, 51–55. [Google Scholar] [CrossRef]
- Yilmaz, M.; Esser, J.; Kraft, L.; Petzold, R.; Sigle, A.; Gratzke, C.; Suarez-Ibarrola, R.; Miernik, A. Experimental ex-vivo performance study comparing a novel, pulsed thulium solid-state laser, chopped thulium fibre laser, low and high-power holmium:YAG laser for endoscopic enucleation of the prostate. World J. Urol. 2022, 40, 601–606. [Google Scholar] [CrossRef]
- Fraundorfer, M.R.; Gilling, P.J. Holmium:YAG Laser Enucleation of the Prostate Combined with Mechanical Morcellation: Preliminary Results. Eur. Urol. 1998, 33, 69–72. [Google Scholar] [CrossRef]
- Gilling, P.J.; Kennett, K.; Das, A.K.; Thompson, D.; Fraundorfer, M.R. Holmium Laser Enucleation of the Prostate (HoLEP) Combined with Transurethral Tissue Morcellation: An Update on the Early Clinical Experience. J. Endourol. 1998, 12, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Baazeem, A.S.; Elmansy, H.M.; Elhilali, M.M. Holmium Laser Enucleation of the Prostate: Modified Technical Aspects. BJU Int. 2010, 105, 584–585. [Google Scholar] [CrossRef] [PubMed]
- Gilling, P.J.; Aho, T.F.; Frampton, C.M.; King, C.J.; Fraundorfer, M.R. Holmium Laser Enucleation of the Prostate: Results at 6 Years. Eur. Urol. 2008, 53, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, W.; Zattoni, F.; Nigro, F.; Tasca, A. Plasma bubble formation induced by holmium laser: An in vitro study. Urology 2004, 63, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Scoffone, C.M.; Cracco, C.M. High-Power HoLEP: No Thanks! World J. Urol. 2018, 36, 837–838. [Google Scholar] [CrossRef]
- Hardy, L.A.; Kennedy, J.D.; Wilson, C.R.; Irby, P.B.; Fried, N.M. Analysis of thulium fiber laser induced bubble dynamics for ablation of kidney stones. J. Biophotonics 2016, 10, 1240–1249. [Google Scholar] [CrossRef]
- Verdaasdonk, R.; Van Swol, C.F.P.; Grimbergen, M.C.M.; Rem, A.I. Imaging techniques for research and education of thermal and mechanical interactions of lasers with biological and model tissues. J. Biomed. Opt. 2006, 11, 041110. [Google Scholar] [CrossRef]
- Becker, B.; Enikeev, D.; Glybochko, P.; Rapoport, L.; Taratkin, M.; Gross, A.J.; Vinnichenko, V.; Herrmann, T.R.W.; Netsch, C. Effect of optical fiber diameter and laser emission mode (cw vs. pulse) on tissue damage profile using 1.94 µm Tm:fiber lasers in a porcine kidney model. World J. Urol. 2019, 38, 1563–1568. [Google Scholar] [CrossRef]
- Doizi, S.; Germain, T.; Panthier, F.; Compérat, E.; Traxer, O.; Berthe, L. Comparison of Holmium:YAG and Thulium Fiber Lasers on Soft Tissue: An Ex Vivo Study. J. Endourol. 2022, 36, 251–258. [Google Scholar] [CrossRef]
- Khoder, W.Y.; Zilinberg, K.; Waidelich, R.; Stief, C.G.; Becker, A.J.; Pangratz, T.; Hennig, G.; Sroka, R. Ex vivo comparison of the tissue effects of six laser wavelengths for potential use in laser supported partial nephrectomy. J. Biomed. Opt. 2012, 17, 068005. [Google Scholar] [CrossRef]
- Ortner, G.; Rice, P.; Nagele, U.; Herrmann, T.R.W.; Somani, B.K.; Tokas, T. Tissue thermal effect during lithotripsy and tissue ablation in endourology: A systematic review of experimental studies comparing Holmium and Thulium lasers. World J. Urol. 2023, 41, 1–12. [Google Scholar] [CrossRef]
- Enikeev, D.; Glybochko, P.; Rapoport, L.; Okhunov, Z.; O’Leary, M.; Potoldykova, N.; Sukhanov, R.; Enikeev, M.; Laukhtina, E.; Taratkin, M. Impact of endoscopic enucleation of the prostate with thulium fiber laser on the erectile function. BMC Urol. 2018, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Chiang, P.-H.; Lee, W.-C.; Chuang, Y.-C.; Kang, C.-H.; Hsu, C.-C.; Chen, Y.-T.; Cheng, Y.-T. High-intensity diode laser in combination with bipolar transurethral resection of the prostate: A new strategy for the treatment of large prostates (>80 mL). Lasers Surg. Med. 2012, 44, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Wisenbaugh, E.S.; Nunez-Nateras, R.; Mmeje, C.O.; Warner, J.N.; Humphreys, M.R. Does prostate morphology affect outcomes after holmium laser enucleation? Urology 2013, 81, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Hurle, R.; Vavassori, I.; Piccinelli, A.; Manzetti, A.; Valenti, S.; Vismara, A. Holmium laser enucleation of the prostate combined with mechanical morcellation in 155 patients with benign prostatic hyperplasia. Urology 2002, 60, 449–453. [Google Scholar] [CrossRef]
- Naspro, R.; Suardi, N.; Salonia, A.; Scattoni, V.; Guazzoni, G.; Colombo, R.; Cestari, A.; Briganti, A.; Mazzoccoli, B.; Rigatti, P.; et al. Holmium Laser Enucleation of the Prostate versus Open Prostatectomy for Prostates >70g: 24-Month Follow-up. Eur. Urol. 2006, 50, 563–568. [Google Scholar] [CrossRef]
- Tan, A.H.; Gilling, P.; Kennett, K.; Frampton, C.; Westenberg, A.; Fraundorfer, M. A Randomized Trial Comparing Holmium Laser Enucleation of the Prostate with Transurethral Resection of the Prostate for the Treatment of Bladder Outlet Obstruction Secondary to Benign Prostatic Hyperplasia in Large Glands (40 to 200 Grams). J. Urol. 2003, 170 Pt 1, 1270–1274. [Google Scholar] [CrossRef]
- Wei, Y.; Ke, Z.; Xu, N.; Xue, X. Complications of anatomical endoscopic enucleation of the prostate. Andrologia 2020, 52, e13557. [Google Scholar] [CrossRef]
- Ahyai, S.A.; Gilling, P.; Kaplan, S.A.; Kuntz, R.M.; Madersbacher, S.; Montorsi, F.; Speakman, M.J.; Stief, C.G. Meta-analysis of Functional Outcomes and Complications Following Transurethral Procedures for Lower Urinary Tract Symptoms Resulting from Benign Prostatic Enlargement. Eur. Urol. 2010, 58, 384–397. [Google Scholar] [CrossRef]
- Gilling, P.J.; Wilson, L.C.; King, C.J.; Westenberg, A.M.; Frampton, C.M.; Fraundorfer, M.R. Long-term results of a randomized trial comparing holmium laser enucleation of the prostate and transurethral resection of the prostate: Results at 7 years. BJU Int. 2012, 109, 408–411. [Google Scholar] [CrossRef]
- Kuntz, R.M.; Lehrich, K.; Ahyai, S.A. Holmium Laser Enucleation of the Prostate versus Open Prostatectomy for Prostates Greater than 100 Grams: 5-Year Follow-Up Results of a Randomised Clinical Trial. Eur. Urol. 2008, 53, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Elzayat, E.; Habib, E.; Elhilali, M. Holmium laser enucleation of the prostate (HoLEP): A size independent new gold standard. Urology 2005, 66 (Suppl. 5), 108–113. [Google Scholar] [CrossRef] [PubMed]
| Author | Design | Laser | Pts (N) | Laser Power | Type of Enucleation | Mean Age ± SD (Years) | Mean PV ± SD (mL) | Mean Adenoma Weight (gr) | Median Time to Catheter Removal (Days) (Range) | Median Hospital Stay (Days) |
|---|---|---|---|---|---|---|---|---|---|---|
| Becker 2018 [15] | Prospective, not randomized, full text | LP HoLEP | 54 | 2.2 J, 18 Hz~39.6 W | Modified technique | 72.5 | 74.5 | 46 | 2 | 2 |
| Bell 2019 [16] | Retrospective, full text | LP HoLEP (with previous TBP) LP HoLEP (biopsy-naïve) | 24 85 | 50 W 50 W | 3-lobe 3-lobe | 66.8 ± 8.2 71.8 ± 8.7 | 76.1 ± 35.0 69.3 ± 31.8 | NR NR | NR NR | 1.3 3.0 |
| Gazel 2020 [17] | Retrospective, full text | LP HoLEP LP HoLEP | 80 80 | 1 J, 20 Hz~20 W 1.5 J, 25 Hz~37.5 W | 3-lobe 3-lobe | 63 ± 7.97 62 ± 7.07 | 79 ± 35.71 68.5 ± 47.37 | NR NR | 42 ± 27.74 (hours) 27 ± 14.38 (hours) | 28 ± 6.06 (hours) 33 ± 8.03 (hours) |
| Minagawa 2017 [18] | Retrospective, full text | HP HoLEP | 74 | 1.5 J, 20 Hz~30 W | En-bloc | 75.4 ± 7.1 | 94.5 ± 61.0 | 51.8 | 2.6 | 5.3 |
| Tokatli 2021 [13] | Prospective, randomized, full text | MP HoLEP MP HoLEP | 60 60 | 2.2 J, 18 Hz~39.6 W 1.2 J, 35 Hz~42 W | 2-lobe and 3-lobe 2-lobe and 3-lobe | 66.5 67 | 95 91 | 44 40 | 2 2 | 3 3 |
| Author | Design | Laser | Pts (N) | Laser Power | Type of Enucleation | Mean Age ± SD (Years) | Mean PV ± SD (mL) | Mean Adenoma Weight (gr) | Median Time to Catheter Removal (Days) (Range) | Median Hospital Stay (Days) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gilling 2013 [9] | Prospective, meeting abstract | LP HoLEP HP HoLEP | 20 20 | 50 W 100 W | NR NR | 67.4 ± 11.2 68.9 ± 2.0 | NR N | 22.4 21.7 | 17.5 (hours) 25.1 (hours) | 26.6 (hours) 32.5 (hours) |
| Scoffone 2017 [10] | Prospective, meeting abstract | LP HoLEP HP HoLEP | 102 214 | 2.2 J, 18 Hz~40 W 2 J, 50 Hz~100 W | En-bloc En-bloc | 67.7 ± 8 69.4 ± 7.5 | NR NR | 46 55 | NR NR | NR NR |
| Cracco 2017 [11] | Prospective, meeting abstract | LP HoLEP HP HoLEP | 102 214 | 2.2 J, 18 Hz~40 W 52 J, 50 Hz~100 W | En-bloc En-bloc | 67.7 ± 8 69.4 ± 7.5 | NR NR | 46 55 | NR NR | NR NR |
| Elshal 2018 [14] | Prospective, randomized controlled trial, full text | LP HoLEP HP HoLEP | 61 60 | 2 J, 25 Hz~50 W 2 J, 50 Hz~100 W | 2-lobe and 3-lobe 2-lobe and 3-lobe | 66.4 ± 7 67.0 ± 7 | 137.6 ± 58 137.6 ± 58 | 75.5 77 | 1 (1–5) 1 (1–5) | 1 (1–3) 1 (1–5) |
| Cracco 2020 [12] | Retrospective, meeting abstract | LP HoLEP HP HoLEP | 326 212 | 40 W 100 W | Partial and total en-bloc Partial en-bloc | NR NR | NR NR | 48,9 53.3 | NR NR | NR NR |
| Yilmaz 2022 [19] | Ex vivo, porcine belly | LP HoLEP HP HoLEP | NR NR | 3.5 J, 10 Hz~35 W 4.5 J, 22.2 Hz~100 W | NR NR | NR NR | NR NR | NR NR | NR NR | NR NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkolezakis, V.; Somani, B.K.; Tokas, T. Low- vs. High-Power Laser for Holmium Laser Enucleation of Prostate. J. Clin. Med. 2023, 12, 2084. https://doi.org/10.3390/jcm12052084
Gkolezakis V, Somani BK, Tokas T. Low- vs. High-Power Laser for Holmium Laser Enucleation of Prostate. Journal of Clinical Medicine. 2023; 12(5):2084. https://doi.org/10.3390/jcm12052084
Chicago/Turabian StyleGkolezakis, Vasileios, Bhaskar Kumar Somani, and Theodoros Tokas. 2023. "Low- vs. High-Power Laser for Holmium Laser Enucleation of Prostate" Journal of Clinical Medicine 12, no. 5: 2084. https://doi.org/10.3390/jcm12052084
APA StyleGkolezakis, V., Somani, B. K., & Tokas, T. (2023). Low- vs. High-Power Laser for Holmium Laser Enucleation of Prostate. Journal of Clinical Medicine, 12(5), 2084. https://doi.org/10.3390/jcm12052084







