Development of National Antimicrobial Intravenous-to-Oral Switch Criteria and Decision Aid
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Protocol and Registration
2.3. Study Question
2.4. Study Design
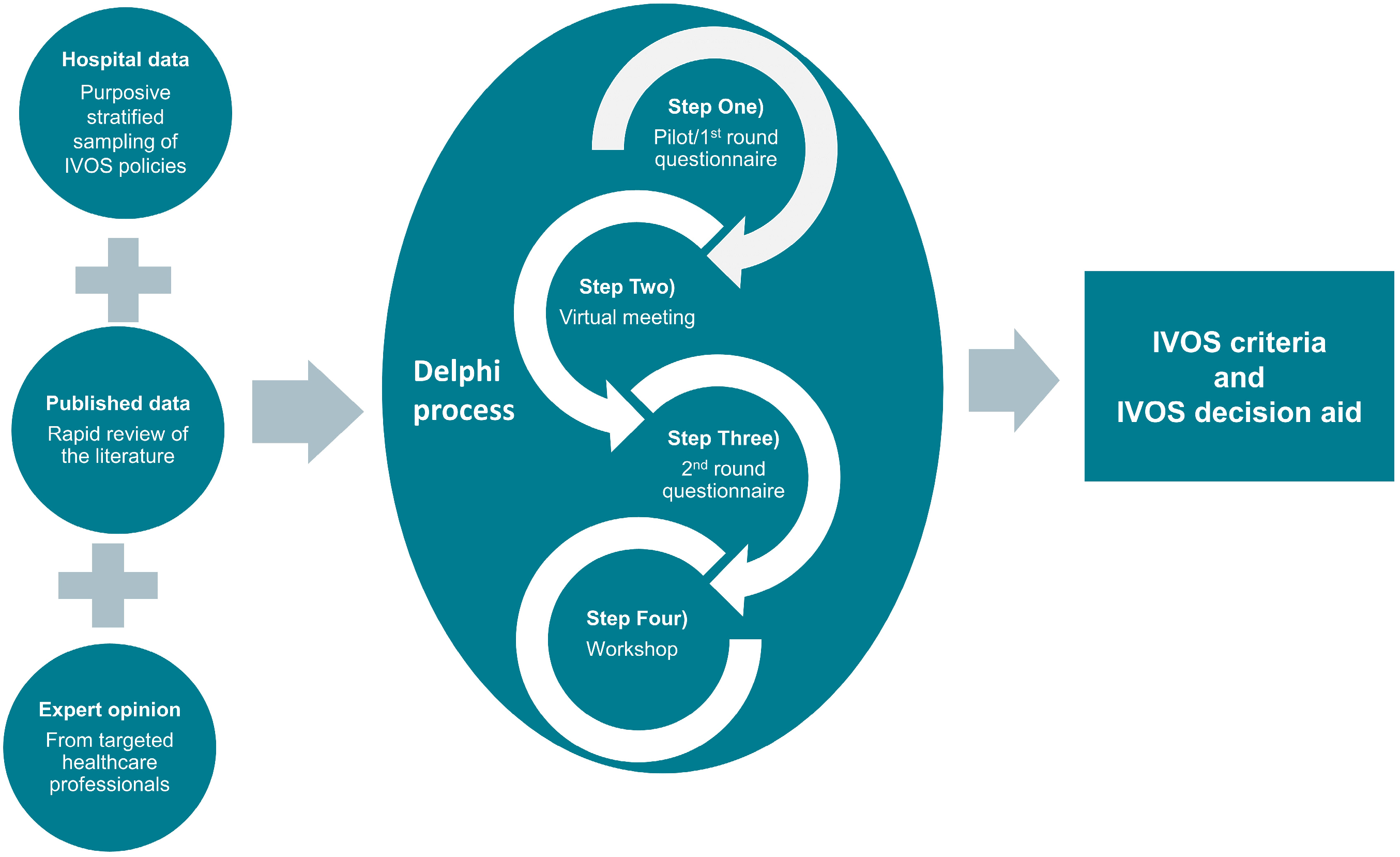
2.5. Delphi Process
2.5.1. (Step One) Pilot/1st Round Questionnaire
- Criteria with a clinical relevance median score of 4 (relevant) or 5 (very relevant) where there was agreement were accepted. Agreement was defined as ≥60% of participants assigning a score of 4 or 5.
- Criteria with a clinical relevance median score of 4 or 5 where there was lack of agreement were marked as uncertain and carried forward for consensus discussion in Step Two) Virtual meeting. Lack of agreement was defined as <60% of participants assigning a score of 4 or 5.
- Criteria with a clinical relevance median score <4 were rejected yet carried forward for consensus discussion in (Step Two) Virtual meeting with the possibility for reinstating.
- Criteria with multiple questionnaire comments suggesting a similar context to support a rephrase were rephrased and carried forward for consensus discussion in (Step Two) Virtual meeting.
2.5.2. (Step Two) Virtual Meeting
2.5.3. (Step Three) 2nd Round Questionnaire
2.5.4. (Step 4) IVOS Decision Aid Workshop
3. Results
3.1. Delphi Process
3.1.1. (Step One) Pilot/1st Round Questionnaire
IVOS Criteria (Step One)
3.1.2. (Step Two) Virtual Meeting
Questionnaire Format
IVOS Criteria (Step Two)
3.1.3. (Step Three) 2nd Round Questionnaire
IVOS Criteria (Step Three)
3.1.4. (Step Four) Workshop
IVOS Criteria (Final Step)
Draft IVOS Decision Aid
3.1.5. Final IVOS Criteria
4. Discussion
4.1. Strengths and Limitations
4.2. Further Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- HM Government. Tackling Antimicrobial Resistance 2019–2024: The UK’s Five-Year National Action Plan. 2019. Available online: https://www.gov.uk/government/publications/uk-5-year-action-plan-for-antimicrobial-resistance-2019-to-2024 (accessed on 20 September 2022).
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs. 2019. Available online: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf (accessed on 20 September 2022).
- Matuluko, A.; MacDonald, J.; Ness, V.; Currie, K. Interventions to improve the review of antibiotic therapy in acute care hospitals: A systematic review and narrative synthesis. JAC Antimicrob. Resist. 2020, 2, dlaa065. [Google Scholar] [CrossRef]
- World Health Organization. Library of AMR National Action Plans. 2022. Available online: https://www.who.int/teams/surveillance-prevention-control-AMR/national-action-plan-monitoring-evaluation/library-of-national-action-plans (accessed on 27 October 2022).
- Government of Canada. Federal Action Plan on Antimicrobial Resistance and Use in Canada: Building on the Federal Framework for Action; Public Health Agency of Canada: Toronto, ON, Canada, 2015. Available online: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/federal-action-plan-antimicrobial-resistance-canada.html (accessed on 20 September 2022).
- Government of Canada. Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action. 2017. Available online: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/canada_tackling-antimicrobial-resistance-use-pan-canadian-framework-action.pdf?sfvrsn=7b8000b3_1&download=true (accessed on 20 September 2022).
- Australian Government. Australia’s National Antimicrobial Resistance Strategy 2020 & Beyond; Department of Health and Department of Agriculture. 2019. Available online: https://www.who.int/publications/m/item/australia-national-antimicrobial-resistance-strategy-2020-and-beyond (accessed on 20 September 2022).
- The Government of Japan. National Action Plan on Antimicrobial Resistance (AMR) 2016–2020. 2016. Available online: https://www.who.int/publications/m/item/japan-national-action-plan-on-antimicrobial-resistance-(amr) (accessed on 20 September 2022).
- Davey, P.; Brown, E.; Charani, E.; Fenelon, L.; Gould, I.M.; Holmes, A.; Ramsay, C.R.; Wiffen, P.J.; Wilcox, M. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2013, CD003543. [Google Scholar] [CrossRef]
- McLaughlin, C.M.; Bodasing, N.; Boyter, A.C.; Fenelon, C.; Fox, J.; Seaton, R. Pharmacy-implemented guidelines on switching from intravenous to oral antibiotics: An intervention study. QJM Mon. J. Assoc. Physicians 2005, 98, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Senn, L.; Burnand, B.; Francioli, P.; Zanetti, G. Improving appropriateness of antibiotic therapy: Randomized trial of an intervention to foster reassessment of prescription after 3 days. J. Antimicrob. Chemother. 2004, 53, 1062–1067. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Mai-Phan, T.A.; Tran, M.H.; Pham, H.T. The effect of early switching from intravenous to oral antibiotic therapy: A randomized controlled trial. J. Pharm. Pharmacogn. Res. 2021, 9, 695–703. [Google Scholar] [CrossRef]
- Schuts, E.C.; Hulscher, M.; Mouton, J.W.; Verduin, C.M.; Stuart, J.W.T.C.; Overdiek, H.W.P.M.; van der Linden, P.D.; Natsch, S.; Hertogh, C.M.P.M.; Wolfs, T.F.W.; et al. Current evidence on hospital antimicrobial stewardship objectives: A systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 847–856. [Google Scholar] [CrossRef]
- Tennison, I.; Roschnik, S.; Ashby, B.; Boyd, R.; Hamilton, I.; Oreszczyn, T.; Owen, A.; Romanello, M.; Ruyssevelt, P.; Sherman, J.D.; et al. Health care’s response to climate change: A carbon footprint assessment of the NHS in England. Lancet Planet. Health 2021, 5, e84–e92. [Google Scholar] [CrossRef] [PubMed]
- Cyriac, J.M.; James, E. Switch over from intravenous to oral therapy: A concise overview. J. Pharmacol. Pharmacother. 2014, 5, 83–87. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.; Avent, M. Oral or intravenous antibiotics? Aust. Prescr. 2020, 43, 45–48. [Google Scholar] [CrossRef]
- Mandell, L.A.; Bergeron, M.G.; Gribble, M.J.; Jewesson, P.J.; Low, D.E.; Marrie, T.J.; Nicolle, L.E. Sequential antibiotic therapy: Effective cost management and patient care. Can. J. Infect. Dis. 1995, 6, 306–315. [Google Scholar] [CrossRef]
- Wongkamhla, T.; Khan-Asa, B.; Tongsai, S.; Angkasekwinai, N. Infectious Disease Team Review Using Antibiotic Switch and Discharge Criteria Shortens the Duration of Intravenous Antibiotic: A Single-Center Cluster-Randomized Controlled Trial in Thailand. Open Forum Infect. Dis. 2020, 7, ofaa539. [Google Scholar] [CrossRef]
- Doncaster & Bassetlaw Teaching Hospitals NHS Foundation Trust. IV to Oral Switch and 5 Day Stop Policy. 2020. Available online: https://oesn11hpbml2xaq003wx02ib-wpengine.netdna-ssl.com/wp-content/uploads/2020/09/IV-to-oral-switch-policy-_final2020.pdf (accessed on 20 September 2022).
- Harvey, E.J.; McLeod, M.; De Brun, C.; Ashiru-Oredope, D. Criteria to Achieve Safe Antimicrobial Intravenous-to-Oral Switch in Hospitalised Adult Populations: A Systematic Rapid Review. medRxiv 2022. [Google Scholar] [CrossRef]
- NHS Health Research Authority. Is My Study Research? 2022. Available online: http://www.hra-decisiontools.org.uk/research/result7.html (accessed on 20 September 2022).
- Public Health England. Start Smart—Then Focus: Antimicrobial Stewardship Toolkit for English Hospitals. 2015. Available online: https://www.gov.uk/government/publications/antimicrobial-stewardship-start-smart-then-focus (accessed on 20 September 2022).
- The AGREE Next Steps Consortium. Appraisal of Guidelines for Research and Evaluation II. 2017. Available online: https://www.agreetrust.org/resource-centre/agree-ii/ (accessed on 20 September 2022).
- Harvey, E.; Ashiru-Oredope, D. Criteria to achieve safe and effective antimicrobial intravenous to oral switch in hospitalised adults: A rapid review. PROSPERO 2022, CRD42022320343. [Google Scholar]
- Hsu, C.C.; Sandford, B.A. The Delphi technique: Making sense of consensus. Pract. Assess. Res. Eval. 2007, 12, 10. [Google Scholar]
- Page, A.; Potter, K.; Clifford, R.; McLachlan, A.; Etherton-Beer, C. Prescribing for Australians living with dementia: Study protocol using the Delphi technique. BMJ Open 2015, 5, e008048. [Google Scholar] [CrossRef]
- Ashiru-Oredope, D.; Hopkins, S.; Kessel, A.; Brown, B.; Brown, N.; Carter, S.; Charlett, A.; Cichowka, A.; Faulding, S.; Gallagher, R.; et al. Antimicrobial stewardship: English surveillance programme for antimicrobial utilization and resistance (ESPAUR). J. Antimicrob. Chemother. 2013, 68, 2421–2423. [Google Scholar] [CrossRef] [PubMed]
- Akhloufi, H.; Hulscher, M.; Melles, D.C.; Prins, J.M.; van der Sijs, H.; Verbon, A. Development of operationalized intravenous to oral antibiotic switch criteria. J. Antimicrob. Chemother. 2017, 72, 543–546. [Google Scholar] [CrossRef]
- Department of Health and Social Care. Record Numbers of Doctors and 14,813 More Nurses Working in the NHS. 2020. Available online: https://www.gov.uk/government/news/record-numbers-of-doctors-and-14813-more-nurses-working-in-the-nhs (accessed on 20 September 2022).
- Rawson, T.M.; Charani, E.; Moore, L.S.P.; Hernandez, B.; Castro-Sánchez, E.; Herrero, P.; Georgiou, P.; Holmes, A.H. Mapping the decision pathways of acute infection management in secondary care among UK medical physicians: A qualitative study. BMC Med. 2016, 14, 208. [Google Scholar] [CrossRef]
- Mostaghim, M.; Snelling, T.; McMullan, B.; Konecny, P.; Bond, S.; Adhikari, S.; Chubaty, A.; Lovell, C.; Bajorek, B. Nurses are underutilised in antimicrobial stewardship—Results of a multisite survey in paediatric and adult hospitals. Infect. Dis. Health 2017, 22, 57–64. [Google Scholar] [CrossRef]
- Polidori, P.; Vinci, D.L.; Adami, S.; Bianchi, S.; Faggiano, M.E.; Provenzani, A. Role of the hospital pharmacist in an Italian antimicrobial stewardship programme. Eur. J. Hosp. Pharm. 2021, 29, 95–100. [Google Scholar] [CrossRef]
- Van Den Bosch, C.M.A.; Geerlings, S.E.; Natsch, S.; Prins, J.M.; Hulscher, M.E. Quality indicators to measure appropriate antibiotic use in hospitalized adults. Clin. Infect. Dis. 2015, 60, 281–291. [Google Scholar] [CrossRef]
- Treacy, M.; Wong, G.; Odell, M.; Roberts, N. Understanding the use of the National Early Warning Score 2 in acute care settings: A realist review protocol. BMJ Open 2022, 12, e062154. [Google Scholar] [CrossRef] [PubMed]
- Iversen, K.; Ihlemann, N.; Gill, S.U.; Madsen, T.; Elming, H.; Jensen, K.T.; Bruun, N.E.; Høfsten, D.E.; Fursted, K.; Christensen, J.J.; et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N. Engl. J. Med. 2019, 380, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Li, H.K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M.; et al. Oral versus intravenous antibiotics for bone and joint infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef]
- Rx-info Define FY2021-22. Available online: https://rxinfo.thirdparty.nhs.uk/reports (accessed on 1 February 2023).
- Maki, D.G.; Kluger, D.M.; Crnich, C.J. The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Mertz, D.; Koller, M.; Haller, P.; Lampert, M.L.; Plagge, H.; Hug, B.; Koch, G.; Battegay, M.; Flückiger, U.; Bassetti, S. Outcomes of early switching from intravenous to oral antibiotics on medical wards. J. Antimicrob. Chemother. 2009, 64, 188–199. [Google Scholar] [CrossRef]
- Van Zanten, A.R.H.; Engelfriet, P.M.; Van Dillen, K.; Van Veen, M.; Nuijten, M.J.C.; Polderman, K.H. Importance of nondrug costs of intravenous antibiotic therapy. Crit. Care 2003, 7, R184. [Google Scholar] [CrossRef] [PubMed]
- Mouwen, A.M.A.; Dijkstra, J.A.; Jong, E.; Buijtels, P.; Jong, P.P.-D.; Nagtegaal, J. Early switching of antibiotic therapy from intravenous to oral using a combination of education, pocket-sized cards and switch advice: A practical intervention resulting in reduced length of hospital stay. Int. J. Antimicrob. Agents 2020, 55, 105769. [Google Scholar] [CrossRef]
- Ehrenkranz, N.J.; Nerenberg, D.E.; Shultz, J.M.; Slater, K.C. Intervention to discontinue parenteral antimicrobial therapy in patients hospitalized with pulmonary infections: Effect on shortening patient stay. Infect. Control. Hosp. Epidemiol. 1992, 13, 21–32. [Google Scholar] [CrossRef]
- Engel, M.F.; Postma, D.F.; Hulscher, M.E.J.L.; van Berkhout, F.T.; Emmelot-Vonk, M.H.; Sankatsing, S.; Gaillard, C.A.; Bruns, A.H.; Hoepelman, A.I.; Oosterheert, J.J. Barriers to an early switch from intravenous to oral antibiotic therapy in hospitalised patients with CAP. Eur. Respir. J. 2013, 41, 123–130. [Google Scholar] [CrossRef]
- Nathwani, D.; Lawson, W.; Dryden, M.; Stephens, J.; Corman, S.; Solem, C.; Li, J.; Charbonneau, C.; Baillon-Plot, N.; Haider, S.; et al. Implementing criteria-based early switch/early discharge programmes: A European perspective. Clin. Microbiol. Infect. 2015, 21 (Suppl. S2), S47–S55. [Google Scholar] [CrossRef]
- Akhloufi, H.; Hulscher, M.; Van Der Hoeven, C.P.; Prins, J.M.; Van Der Sijs, H.; Melles, D.C.; Verbon, A. A clinical decision support system algorithm for intravenous to oral antibiotic switch therapy: Validity, clinical relevance and usefulness in a three-step evaluation study. J. Antimicrob. Chemother. 2018, 73, 2201–2206. [Google Scholar] [CrossRef] [PubMed]
- Cash, P.; Gamundi, X.V.; Echstrøm, I.; Daalhuizen, J. Method Use in Behavioural Design: What, How, and Why? Int. J. Des. 2022, 16, 1–21. [Google Scholar]
- Krockow, E.M.; Harvey, E.J.; Ashiru-Oredope, D. Addressing long-term and repeat antibiotic prescriptions in primary care: Considerations for a behavioural approach. BMJ Qual. Saf. 2022, 31, 782–786. [Google Scholar] [CrossRef]
- NHS England. 2023/24 CQUIN. 2022. Available online: https://www.england.nhs.uk/nhs-standard-contract/cquin/cquin-23-24/ (accessed on 21 January 2023).
- Brouwers, M.C.; Kerkvliet, K.; Spithoff, K.; on behalf of the AGREE Next Steps Consortium. The AGREE Reporting Checklist: A tool to improve reporting of clinical practice guidelines. BMJ 2016, 352, i1152. [Google Scholar] [CrossRef]
- Goff, D.A.; Bauer, K.A.; Reed, E.E.; Stevenson, K.B.; Taylor, J.J.; West, J.E. Is the “low-hanging fruit” worth picking for antimicrobial stewardship programs? Clin Infect Dis 2012, 55, 587–592. [Google Scholar] [CrossRef] [PubMed]

| Profession | Count |
|---|---|
| General Pharmacist | 7 |
| Specialist Microbiology/Infectious Diseases Pharmacist | 6 |
| Microbiologist/Infectious Diseases Physician | 5 |
| Microbiology/Infectious Diseases Nurse | 2 |
| General Physician | 1 |
| Surgeon | 1 |
| General Nurse | 1 |
| Healthcare Scientist | 1 |
| Total | 24 |
| Profession of Respondents | Count |
|---|---|
| Pharmacist (Microbiology or Infection Specialist) | 65 |
| Hospital General Physician | 55 |
| Medical Microbiologist/Infection Diseases Doctor | 38 |
| Pharmacist (General or non-Infection Specialist) | 36 |
| Nurse/Midwife (General or non-Infection Specialist) | 22 |
| Surgeon | 8 |
| Nurse (Antimicrobial Stewardship) | 8 |
| Allied Health Professional | 3 |
| Nurse (Infection Prevention Control) | 3 |
| Physician Associate | 1 |
| Healthcare Scientist | 1 |
| Specialist Pharmacy Technician | 1 |
| Dentist or Dental Nurse | 1 |
| Total | 242 |
| Section | Feasible for Nursing Team to Prompt for IVOS (%) | Feasible for Pharmacy Team to Prompt for IVOS (%) |
|---|---|---|
| 75.6 | 92.1 |
| 69.5 | 54.1 |
| 34.3 | 73.1 |
| 81.4 | 75.3 |
| 30.2 | 67.8 |
| Format | Count (Percentage) |
|---|---|
| Electronic prescribing system prompt | 39 (81) |
| Sticker to add to drug chart and/or medical notes | 18 (38) |
| Poster for, e.g., treatment room | 12 (25) |
| Smartphone app | 12 (25) |
| Page to add to medical notes | 9 (19) |
| Other | 7 (15) |
| Pocket-sized card | 2 (0.4) |
| Section | Antimicrobial IVOS Criteria |
|---|---|
| IVOS should be considered within 48 h of the first dose of IV antimicrobial being administered |
| If no IVOS within first 48 h, review daily thereafter | |
| Clinical signs and symptoms of infection are improving |
| Temperature is between 36–38 °C for the past 24 h |
| Early Warning Score is decreasing | |
| White Cell Count is trending towards the normal range * | |
| C-Reactive Protein is decreasing * | |
| Gastrointestinal tract is functioning with no evidence of malabsorption |
| Safe swallow or enteral tube administration | |
| Suitable oral switch option available, considering oral bioavailability, any clinically significant drug interactions or patient allergies | |
| No significant concerns over patient adherence to oral treatment | |
| No vomiting within the last 24 h | |
| Does your patient have an infection that may require special consideration? Infections that may require special consideration include: Deep-seated infection; Infection requiring high tissue concentration; Infection requiring prolonged IV therapy; Critical infection with high risk of mortality. Specific infections for special consideration are:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harvey, E.J.; Hand, K.; Weston, D.; Ashiru-Oredope, D. Development of National Antimicrobial Intravenous-to-Oral Switch Criteria and Decision Aid. J. Clin. Med. 2023, 12, 2086. https://doi.org/10.3390/jcm12062086
Harvey EJ, Hand K, Weston D, Ashiru-Oredope D. Development of National Antimicrobial Intravenous-to-Oral Switch Criteria and Decision Aid. Journal of Clinical Medicine. 2023; 12(6):2086. https://doi.org/10.3390/jcm12062086
Chicago/Turabian StyleHarvey, Eleanor J., Kieran Hand, Dale Weston, and Diane Ashiru-Oredope. 2023. "Development of National Antimicrobial Intravenous-to-Oral Switch Criteria and Decision Aid" Journal of Clinical Medicine 12, no. 6: 2086. https://doi.org/10.3390/jcm12062086
APA StyleHarvey, E. J., Hand, K., Weston, D., & Ashiru-Oredope, D. (2023). Development of National Antimicrobial Intravenous-to-Oral Switch Criteria and Decision Aid. Journal of Clinical Medicine, 12(6), 2086. https://doi.org/10.3390/jcm12062086





