Antiplatelet Strategies for Older Patients with Acute Coronary Syndromes: Finding Directions in a Low-Evidence Field
Abstract
1. Introduction
2. Invasive versus Conservative Strategy
3. Dual Antiplatelet Therapy in Elderly ACS Patients: Comparative Efficacy and Safety among Different P2Y12 Inhibitors
4. Bleeding and Thrombotic Risk in Elderly ACS Patients
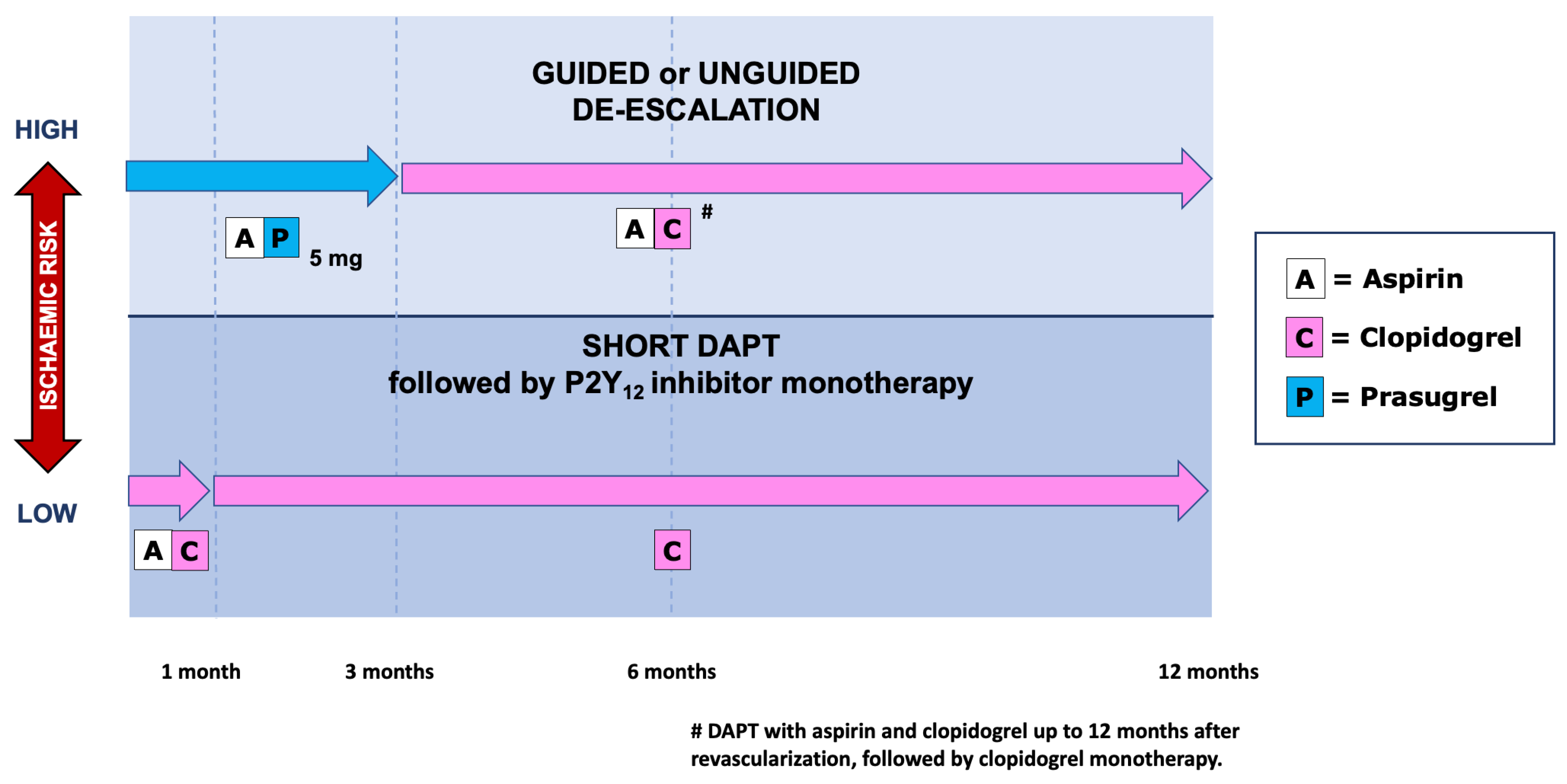
5. Antiplatelet Strategies in Elderly ACS Patients
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Lorenzo, E.; Sauro, R.; Varricchio, A.; Capasso, M.; Lanzillo, T.; Manganelli, F.; Carbone, G.; Lanni, F.; Pagliuca, M.R.; Stanco, G.; et al. Randomized comparison of everolimus-eluting stents and sirolimus-eluting stents in patients with ST elevation myocardial infarction: RACES-MI trial. JACC Cardiovasc. Interv. 2014, 7, 849–856. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Schaffer, A.; Wirianta, J.; Suryapranata, H. Comprehensive meta-analysis of radial vs femoral approach in primary angioplasty for STEMI. Int. J. Cardiol. 2013, 168, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Suryapranata, H.; Stone, G.W.; Antoniucci, D.; Tcheng, J.E.; Neumann, F.-J.; Bonizzoni, E.; Topol, E.J.; Chiariello, M. Relationship between patient’s risk profile and benefits in mortality from adjunctive abciximab to mechanical revascularization for ST-segment elevation myocardial infarction: A meta-regression analysis of randomized trials. J. Am. Coll. Cardiol. 2006, 47, 685–686. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Cassetti, E.; Piccolo, R.; Galasso, G.; Marino, P.; Sinigaglia, F.; De Luca, G. Benefits from new ADP antagonists as compared with clopidogrel in patients with stable angina or acute coronary syndrome undergoing invasive management: A meta-analysis of randomized trials. J. Cardiovasc. Pharmacol. 2014, 63, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Montalto, C.; Branca, M.; Hong, S.J.; Watanabe, H.; Franzone, A.; Vranckx, P.; Hahn, J.Y.; Gwon, H.C.; Feres, F.; et al. Dual antiplatelet therapy duration after percutaneous coronary intervention in high bleeding risk: A meta-analysis of randomized trials. Eur. Heart J. 2022, 250, ehac706. [Google Scholar] [CrossRef]
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe: Epidemiological update. Eur. Heart J. 2013, 34, 3028–3034. [Google Scholar] [CrossRef]
- Silverio, A.; Cancro, F.P.; Di Maio, M.; Bellino, M.; Esposito, L.; Centore, M.; Carrizzo, A.; Di Pietro, P.; Borrelli, A.; De Luca, G.; et al. Lipoprotein(a) levels and risk of adverse events after myocardial infarction in patients with and without diabetes. J. Thromb. Thrombolysis 2022, 54, 382–392. [Google Scholar] [CrossRef]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Bellomo, G.; Marino, P.; Sinigaglia, F.; Suryapranata, H.; De Luca, G. Impact of age on mean platelet volume and its relationship with coronary artery disease: A single-centre cohort study. Exp. Gerontol. 2015, 62, 32–36. [Google Scholar] [CrossRef]
- De Luca, G.; Verdoia, M.; Cassetti, E.; Schaffer, A.; Cavallino, C.; Bolzani, V.; Marino, P. High fibrinogen level is an independent predictor of presence and extent of coronary artery disease among Italian population. J. Thromb. Thrombolysis 2011, 31, 458–463. [Google Scholar] [CrossRef]
- Verdoia, M.; Barbieri, L.; Di Giovine, G.; Marino, P.; Suryapranata, H.; De Luca, G. Neutrophil to Lymphocyte Ratio and the Extent of Coronary Artery Disease: Results from a Large Cohort Study. Angiology 2016, 67, 75–82. [Google Scholar] [CrossRef]
- De Luca, L.; Marini, M.; Gonzini, L.; Boccanelli, A.; Casella, G.; Chiarella, F.; De Servi, S.; Di Chiara, A.; Di Pasquale, G.; Olivari, Z.; et al. Contemporary trends and age-specific sex differences in management and outcome for patients with ST-segment elevation myocardial infarction. J. Am. Heart Assoc. 2016, 5, e004202. [Google Scholar] [CrossRef]
- Leonardi, S.; Montalto, C.; Carrara, G.; Casella, G.; Grosseto, D.; Galazzi, M.; Repetto, A.; Tua, L.; Portolan, M.; Ottani, F.; et al. Clinical governance of patients with acute coronary syndromes. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Savonitto, S.; Cavallini, C.; Petronio, A.S.; Murena, E.; Antonicelli, R.; Sacco, A.; Steffenino, G.; Bonechi, F.; Mossuti, E.; Manari, A.; et al. Early aggressive versus initially conservative treatment in elderly patients with non-ST-segment elevation acute coronary syndrome: A randomized controlled trial. JACC Cardiovasc. Interv. 2012, 5, 906–916. [Google Scholar] [CrossRef]
- Morici, N.; Savonitto, S.; Murena, E.; Antonicelli, R.; Piovaccari, G.; Tucci, D.; Tamburino, C.; Fontanelli, A.; Bolognese, L.; Menozzi, M.; et al. Causes of death in patients ≥75 years of age with non-ST-segment elevation acute coronary syndrome. Am. J. Cardiol. 2013, 112, 1–7. [Google Scholar] [CrossRef]
- Morici, N.; De Servi, S.; De Luca, L.; Crimi, G.; Montalto, C.; De Rosa, R.; De Luca, G.; Rubboli, A.; Valgimigli, M.; Savonitto, S. Management of acute coronary syndromes in older adults. Eur. Heart J. 2022, 43, 1542–1553. [Google Scholar] [CrossRef]
- Ekerstad, N.; Swahn, E.; Janzon, M.; Alfredsson, J.; Löfmark, R.; Lindenberger, M.; Carlsson, P. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011, 124, 2397–2404. [Google Scholar] [CrossRef]
- Dodson, J.A.; Hochman, J.S.; Roe, M.T.; Chen, A.Y.; Chaudhry, S.I.; Katz, S.; Zhong, H.; Radford, M.J.; Udell, J.A.; Bagai, A.; et al. The association of frailty with in-hospital bleeding among older adults with acute myocardial infarction: Insights from the ACTION Registry. JACC Cardiovasc. Interv. 2018, 11, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.A.; Forman, D.E.; Wang, T.Y.; Chikwe, J.; Kunadian, V.; Rich, M.W.; Young, B.A.; Page, R.L., 2nd; DeVon, H.A.; Alexander, K.P.; et al. Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement From the American Heart Association. Circulation 2022, 147, e32–e62. [Google Scholar] [PubMed]
- De Luca, G.; Dirksen, M.T.; Spaulding, C.; Kelbæk, H.; Schalij, M.; Thuesen, L.; van der Hoeven, B.; Vink, M.A.; Kaiser, C.; Musto, C.; et al. Impact of diabetes on long-term outcome after primary angioplasty: Insights from the DESERT cooperation. Diabetes Care 2013, 36, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Verdoia, M.; Savonitto, S.; Piatti, L.; Grosseto, D.; Morici, N.; Bossi, I.; Sganzerla, P.; Tortorella, G.; Cacucci, M.; et al. Impact of diabetes on clinical outcome among elderly patients with acute coronary syndrome treated with percutaneous coronary intervention: Insights from the ELDERLY ACS 2 trial. J. Cardiovasc. Med. 2020, 21, 453–459. [Google Scholar] [CrossRef]
- Gu, S.Z.; Beska, B.; Chan, D.; Neely, D.; Batty, J.A.; Adams-Hall, J.; Mossop, H.; Qiu, W.; Kunadian, V. Cognitive decline in older patients with Non-ST elevation acute coronary syndrome. J. Am. Heart Assoc. 2019, 8, e011218. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with STsegment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Bueno, H.; Betriu, A.; Heras, M.; Alonso, J.J.; Cequier, A.; Garcia, E.J.; Lopez-Sendon, J.L.; Macaya, C.; Hernandez-Antolin, R.; Bueno, H.; et al. Primary angioplasty vs. fibrinolysis in very old patients with acute myocardial infarction: TRIANA (TRatamiento del Infarto Agudo de miocardio eN Ancianos) randomized trial and pooled analysis with previous studies. Eur. Heart J. 2011, 32, 51–60. [Google Scholar] [CrossRef] [PubMed]
- de Boer, M.J.; Ottervanger, J.P.; van’t Hof, A.W.J.; Hoornethe, A.; Suryapranata, H.; Zijlstra, F.; on behalf of the Zwolle Myocardial Infarction Study Group. Reperfusion therapy in elderly patients with acute myocardial infarction. A randomized comparison of primary angioplasty and thrombolytic therapy. J. Am. Coll. Cardiol. 2002, 39, 1723–1728. [Google Scholar] [CrossRef]
- Grines, C. SENIOR PAMI: A prospective randomized trial of primary angioplasty and thrombolytic therapy in elderly patients with acute myocardial infarction. Presented at the Transcatheter Cardiovascular Therapeutics, Washington, DC, USA; 2005. Available online: https://www.acc.org (accessed on 2 February 2023).
- Puymirat, E.; Aissaoui, N.; Cayla, G.; Lafont, A.; Riant, E.; Mennuni, M.; Saint-Jean, O.; Blanchard, D.; Jourdain, P.; Elbaz, M.; et al. Changes in one-year mortality in elderly patients admitted with acute myocardial infarction in relation with early management. Am. J. Med. 2017, 130, 555–563. [Google Scholar] [CrossRef]
- Perl, L.; Franzé, A.; D’Ascenzo, F.; Golomb, N.; Levi, A.; Vaknin-Assa, H.; Greenberg, G.; Assali, A.; De Ferrari, G.M.; Kornowski, R. Elderly Suffering from ST-Segment Elevation Myocardial Infarction-Results from a Database Analysis from Two Mediterranean Medical Centers. J. Clin. Med. 2021, 10, 2435. [Google Scholar] [CrossRef]
- Bach, R.G.; Cannon, C.P.; Weintraub, W.S.; Weintraub, W.S.; DiBattiste, P.M.; Demopoulos, L.A.; Anderson, H.V.; De Lucca, P.T.; Mahoney, E.M.; Murphy, S.A.; et al. The effect of routine, early invasive management on outcome for elderly patients with non-ST-segment elevation acute coronary syndromes. Ann. Intern. Med. 2004, 141, 186–195. [Google Scholar] [CrossRef]
- Damman, P.; Clayton, T.; Wallentin, L.; Lagerqvist, B.; Fox, K.A.; Hirsch, A.; Windhausen, F.; Swahn, E.; Pocock, S.J.; Tijssen, J.G.; et al. Effects of age on long-term outcomes after a routine invasive or selective invasive strategy in patients presenting with non-ST segment elevation acute coronary syndromes: A collaborative analysis of individual data from the FRISC II-ICTUS -RITA-3 (FIR) trials. Heart 2012, 98, 207–213. [Google Scholar]
- Galasso, G.; De Servi, S.; Savonitto, S.; Strisciuglio, T.; Piccolo, R.; Morici, N.; Murena, E.; Cavallini, C.; Petronio, A.S.; Piscione, F. Effect of an invasive strategy on outcome in patients ≥75 years of age with non-ST-elevation acute coronary syndrome. Am. J. Cardiol. 2015, 115, 576–580. [Google Scholar] [CrossRef]
- Tegn, N.; Abdelnoor, M.; Aaberge, L.; Endresen, K.; Smith, P.; Aakhus, S.; Gjertsen, E.; Dahl-Hofseth, O.; Ranhoff, A.H.; Gullestad, L.; et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): An open-label randomised controlled trial. Lancet 2016, 387, 1057–1065. [Google Scholar] [CrossRef]
- de Belder, A.; Myat, A.; Blaxill, J.; Haworth, P.; O’Kane, P.; Hatrick, R.; Aggarwal, R.K.; Davie, A.; Smith, W.; Gerber, R.; et al. Revascularisation or medical therapy in elderly patients with acute anginal syndromes: The RINCAL randomised trial. EuroIntervention 2021, 17, 67–74. [Google Scholar] [CrossRef]
- Garg, A.; Garg, L.; Agarwal, M.; Rout, A.; Raheja, H.; Agrawal, S.; Rao, S.V.; Cohen, M. Routine invasive versus selective invasive strategy in elderly patients older than 75 years with Non-ST elevation acute coronary syndrome: A systematic review and meta-analysis. Mayo Clin. Proc. 2018, 93, 436–444. [Google Scholar] [CrossRef]
- Kaura, A.; Sterne, J.A.C.; Trickey, A.; Abbott, S.; Mulla, A.; Glampson, B.; Panoulas, V.; Davies, J.; Woods, K.; Mayet, J.; et al. Invasive versus non-invasive management of older patients with non-ST elevation myocardial infarction (SENIOR-NSTEMI): A cohort study based on routine clinical data. Lancet 2020, 396, 623–634. [Google Scholar] [CrossRef]
- The British Heart Foundation SENIOR-RITA Trial (SENIOR-RITA). Available online: https://clinicaltrials.gov/ct2/show/NCT03052036 (accessed on 7 December 2022).
- García-Blas, S.; Cordero, A.; Diez-Villanueva, P.; Martinez-Avial, M.; Ayesta, A.; Ariza-Solé, A.; Mateus-Porta, G.; Martínez-Sellés, M.; Escribano, D.; Gabaldon-Perez, A.; et al. Acute Coronary Syndrome in the Older Patient. J. Clin. Med. 2021, 10, 4132. [Google Scholar] [CrossRef]
- Llaó, I.; Ariza-Solé, A.; Sanchis, J.; Alegre, O.; López-Palop, R.; Formiga, F.; Marín, F.; Vidán, M.T.; Martínez-Sellés, M.; Sionis, A.; et al. Invasive strategy and frailty in very elderly patients with acute coronary syndromes. EuroIntervention 2018, 14, e336–e342. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.; Bandeen-Roche, K.; Forman, D.; Gerstenblith, G.; Huang, J.; Moscucci, M.; Resar, J.; Varadhan, R.; Walston, J.; Segal, J. Frailty as an effect measure modifier in older adults with acute myocardial infarction. J. Am. Coll. Cardiol. 2019, 73, 20. [Google Scholar] [CrossRef]
- Ricci, B.; Cenko, E.; Vasiljevic, Z. Impact of the age of frailty on outcomes after percutaneous coronary intervention in acute coronary syndromes. J. Am. Coll. Cardiol. 2018, 71 (Suppl. S11), A211. [Google Scholar] [CrossRef]
- Verdoia, M.; Pergolini, P.; Rolla, R.; Nardin, M.; Schaffer, A.; Barbieri, L.; Marino, P.; Bellomo, G.; Suryapranata, H.; De Luca, G. Advanced age and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. J. Thromb. Haemost. 2016, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- De Servi, S.; Goedicke, J.; Schirmer, A.; Widimsky, P. Clinical outcomes for prasugrel versus clopidogrel in patients with unstable angina or non-ST-elevation myocardial infarction: An analysis from the TRITON-TIMI 38 trial. Eur. Heart J. Acute Cardiovasc. Care 2014, 4, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Husted, S.; James, S.; Becker, R.C.; Horrow, J.; Katus, H.; Storey, R.F.; Cannon, C.P.; Heras, M.; Lopes, R.D.; Morais, J.; et al. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: A substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 680–688. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, R.; Morici, N.; De Servi, S.; De Luca, G.; Galasso, G.; Piscione, F.; Ferri, L.A.; Piatti, L.; Grosseto, D.; Tortorella, G.; et al. Impact of renal dysfunction and acute kidney injury on outcome in elderly patients with acute coronary syndrome undergoing percutaneous coronary intervention. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 1160–1169. [Google Scholar] [CrossRef]
- Montalto, C.; Crimi, G.; Morici, N.; Piatti, L.; Grosseto, D.; Sganzerla, P.; Tortorella, G.; De Rosa, R.; De Luca, L.; De Luca, G.; et al. Bleeding risk prediction in elderly patients managed invasively for acute coronary syndromes: External validation of the PRECISE-DAPT and PARIS scores. Int. J. Cardiol. 2021, 328, 22–28. [Google Scholar] [CrossRef]
- De Luca, G.; Verdoia, M.; Morici, N.; Ferri, L.A.; Piatti, L.; Grosseto, D.; Bossi, I.; Sganzerla, P.; Tortorella, G.; Cacucci, M.; et al. Impact of hemoglobin levels at admission on outcomes among elderly patients with acute coronary syndrome treated with low-dose Prasugrel or clopidogrel: A sub-study of the ELDERLY ACS 2 trial. Int. J. Cardiol. 2022, 369, 5–11. [Google Scholar] [CrossRef]
- Savonitto, S.; Ferri, L.A.; Piatti, L.; Grosseto, D.; Piovaccari, G.; Morici, N.; Bossi, I.; Sganzerla, P.; Tortorella, G.; Cacucci, M.; et al. Comparison of reduced-dose prasugrel and standard-dose clopidogrel in elderly patients with acute coronary syndromes undergoing early percutaneous revascularization. Circulation 2018, 137, 2435–2445. [Google Scholar] [CrossRef]
- Crimi, G.; Morici, N.; Ferrario, M.; Ferri, L.A.; Piatti, L.; Grosseto, D.; Cacucci, M.; Mandurino Mirizzi, A.; Toso, A.; Piscione, F.; et al. Time course of ischemic and bleeding burden in elderly patients with acute coronary syndromes randomized to low-dose prasugrel or clopidogrel. J. Am. Heart Assoc. 2019, 8, e010956. [Google Scholar] [CrossRef]
- Roe, M.T.; Goodman, S.G.; Ohman, E.M.; Stevens, S.R.; Hochman, J.S.; Gottlieb, S.; Martinez, F.; Dalby, A.J.; Boden, W.E.; White, H.D.; et al. Elderly patients with acute coronary syndromes managed without revascularization: Insights into the safety of long-term dual antiplatelet therapy with reduced-dose prasugrel versus standard-dose clopidogrel. Circulation 2013, 128, 823–833. [Google Scholar] [CrossRef]
- Cayla, G.; Cuisset, T.; Silvain, J.; Leclercq, F.; Manzo-Silberman, S.; Saint-Etienne, C.; Delarche, N.; Bellemain-Appaix, A.; Range, G.; El Mahmoud, R.; et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): An open label, blinded-endpoint, randomized controlled superiority trial. Lancet 2016, 388, 2015–2022. [Google Scholar] [CrossRef]
- Gimbel, M.; Qaderdan, K.; Willemsen, L.; Hermanides, R.; Bergmeijer, T.; de Vrey, E.; Heestermans, T.; Tjon Joe Gin, M.; Waalewijn, R.; Hofma, S.; et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-STelevation acute coronary syndrome (POPular AGE): The randomised, open label, non-inferiority trial. Lancet 2020, 395, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Szummer, K.; Montez-Rath, M.E.; Alfredsson, J.; Erlinge, D.; Lindahl, B.; Hofmann, R.; Ravn-Fischer, A.; Svensson, P.; Jernberg, T. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: Insights from the SWEDEHEART registry. Circulation 2020, 142, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Elia, E.; de Filippo, O.; Manai, R.; Breviario, S.; Bruno, F.; Iannaccone, M.; Wańha, W.; Bianco, M.; Patti, G.; et al. PRAISE study group. Net clinical benefit of different strategies of dual antiplatelet therapy in elderly patients: Data from the praise registry. Int. J. Cardiol. 2022, 353, 9–14. [Google Scholar] [CrossRef]
- De Servi, S.; Landi, A.; Savonitto, S. Antiplatelet Therapy in Elderly Patients with Acute Coronary Syndromes: The Clopidogrel Revenge: Possible Reasons for a bright comeback. Cardiovasc. Drugs Ther. 2021, 35, 399–401. [Google Scholar] [CrossRef]
- De Servi, S.; Crimi, G.; Calabrò, P.; Piscione, F.; Cattaneo, M.; Maffeo, D.; Toso, A.; Bartorelli, A.; Palmieri, C.; De Carlo, M.; et al. Relationship between diabetes, platelet reactivity, and the SYNTAX score to one-year clinical outcome in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention. EuroIntervention 2016, 12, 312–318. [Google Scholar] [CrossRef]
- De Luca, G.; Dirksen, M.T.; Spaulding, C.; Kelbaek, H.; Schalij, M.; Thuesen, L.; van der Hoeven, B.; Vink, M.A.; Kaiser, C.; Musto, C.; et al. Drug-eluting vs bare-metal stents in primary angioplasty: A pooled patient-level meta-analysis of randomized trials. Arch. Intern. Med. 2012, 172, 611–621. [Google Scholar] [CrossRef]
- De Luca, G.; Smits, P.; Hofma, S.H.; Di Lorenzo, E.; Vlachojannis, G.J.; Van’t Hof, A.W.J.; van Boven, A.J.; Kedhi, E.; Stone, G.W.; Suryapranata, H.; et al. Everolimus eluting stent vs. first generation drug-eluting stent in primary angioplasty: A pooled patient-level meta-analysis of randomized trials. Int. J. Cardiol. 2017, 244, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar]
- De Luca, G.; Damen, S.A.; Camaro, C.; Benit, E.; Verdoia, M.; Rasoul, S.; Liew, H.B.; Polad, J.; Ahmad, W.A.; Zambahari, R.; et al. Final results of the randomised evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with a new-generation stent (REDUCE trial). EuroIntervention 2019, 15, e990–e998. [Google Scholar] [CrossRef]
- Kedhi, E.; Verdoia, M.; Suryapranata, H.; Damen, S.; Camaro, C.; Benit, E.; Barbieri, L.; Rasoul, S.; Liew, H.B.; Polad, J.; et al. Impact of age on the comparison between short-term vs 12-month dual antiplatelet therapy in patients with acute coronary syndrome treated with the COMBO dual therapy stent: 2-Year follow-up results of the REDUCE trial. Atherosclerosis 2021, 321, 39–44. [Google Scholar] [CrossRef]
- Costa, F.; van Klaveren, D.; James, S.; Heg, D.; Räber, L.; Feres, F.; Pilgrim, T.; Hong, M.K.; Kim, H.S.; Colombo, A.; et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017, 389, 1025–1034. [Google Scholar] [CrossRef]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the Academic Research Consortium for High Bleeding Risk. Eur. Heart J. 2019, 40, 2632–2653. [Google Scholar] [CrossRef]
- Fortuni, F.; Crimi, G.; Morici, N.; De Luca, G.; Alberti, L.P.; Savonitto, S.; De Servi, S. Assessing bleeding risk in acute coronary syndrome using the Bleeding Academic Research Consortium definition. J. Cardiovasc. Med. 2019, 20, 818–824. [Google Scholar] [CrossRef]
- Gragnano, F.; Heg, D.; Franzone, A.; McFadden, E.P.; Leonardi, S.; Piccolo, R.; Vranckx, P.; Branca, M.; Serruys, P.W.; Benit, E.; et al. PRECISE-DAPT score for bleeding risk prediction in patients on dual or single antiplatelet regimens: Insights from the GLOBAL LEADERS/and GLASSY. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Montalto, C.; Crimi, G.; Morici, N.; Savonitto, S.; De Servi, S. Use of clinical risk score in an elderly population: Need for ad hoc validation and calibration. J. Am. Coll. Cardiol. 2019, 74, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Corpataux, N.; Spirito, A.; Gragnano, F.; Vaisnora, L.; Galea, R.; Svab, S.; Gargiulo, G.; Zanchin, T.; Zanchin, C.; Siontis, G.C.M.; et al. Validation of high bleeding risk criteria and definition as proposed by the academic research consortium for high bleeding risk. Eur. Heart J. 2020, 41, 3743–3749. [Google Scholar] [CrossRef] [PubMed]
- Montalto, C.; Crimi, G.; Morici, N.; Palmerini, T.; Valgimigli, M.; Savonitto, S.; De Servi, S. Validation and additive predictive value of the academic research consortium—High bleeding risk criteria in older adults. Thromb. Haemost. 2021, 121, 1255–1257. [Google Scholar] [CrossRef]
- Piccolo, R.; Magnani, G.; Ariotti, S.; Gargiulo, G.; Marino, M.; Santucci, A.; Franzone, A.; Tebaldi, M.; Heg, D.; Windecker, S.; et al. Ischaemic and bleeding outcomes in elderly patients undergoing a prolonged versus shortened duration of dual antiplatelet therapy after percutaneous coronary intervention: Insights from the PRODIGY randomised trial. EuroIntervention 2017, 13, 78–86. [Google Scholar] [CrossRef]
- Urban, P.; Gregson, J.; Owen, R.; Mehran, R.; Windecker, S.; Valgimigli, M.; Varenne, O.; Krucoff, M.; Saito, S.; Baber, U.; et al. Assessing the risks of bleeding vs thrombotic events in patients at high bleeding risk after coronary stent implantation: The ARC–High Bleeding Risk Trade-off Model. JAMA Cardiol. 2021, 6, 410–419. [Google Scholar] [CrossRef]
- Morici, N.; Savonitto, S.; Ferri, L.A.; Grosseto, D.; Bossi, I.; Sganzerla, P.; Tortorella, G.; Cacucci, M.; Ferrario, M.; Crimi, G.; et al. Outcomes of Elderly Patients with ST-Elevation or Non-ST-Elevation Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Am. J. Med. 2019, 132, 209–216. [Google Scholar] [CrossRef]
- De Servi, S.; Landi, A.; Savonitto, S.; De Luca, L.; De Luca, G.; Morici, N.; Montalto, C.; Crimi, G.; Cattaneo, M. Tailoring oral antiplatelet therapy in acute coronary syndromes: From guidelines to clinical practice. J. Cardiovasc. Med. 2023, 24, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Frigoli, E.; Heg, D.; Tijssen, J.; Jüni, P.; Vranckx, P.; Ozaki, Y.; Morice, M.C.; Chevalier, B.; Onuma, Y.; et al. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N. Engl. J. Med. 2021, 385, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Kim, J.S.; Hong, S.J.; Lim, D.S.; Lee, S.Y.; Yun, K.H.; Park, J.K.; Kang, W.C.; Kim, Y.H.; Yoon, H.J.; et al. 1-Month dual-antiplatelet therapy followed by aspirin monotherapy after polymer-free drug-coated stent implantation. JACC Cardiovasc. Interv. 2021, 14, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Capranzano, P. One-month DAPT after acute coronary syndrome: Too short or not too short? EuroIntervention 2022, 18, 443–445. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; De Servi, S.; Musumeci, G.; Bolognese, L. Is ticagrelor safe in octogenarian patients with non-ST elevation acute coronary syndromes? Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 12–14. [Google Scholar] [CrossRef]
- Montalto, C.; Morici, N.; Munafò, A.R.; Mangieri, A.; Mandurino-Mirizzi, A.; D’Ascenzo, F.; Oreglia, J.; Latib, A.; Porto, I.; Colombo, A.; et al. Optimal P2Y12 inhibition in older adults with acute coronary syndromes: A network meta-analysis of randomized controlled trials. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 20–27. [Google Scholar] [CrossRef]
- Andò, G.; De Santis, G.A.; Greco, A.; Pistelli, L.; Francaviglia, B.; Capodanno, D.; De Caterina, R.; Capranzano, P. P2Y12 Inhibitor or Aspirin Following Dual Antiplatelet Therapy After Percutaneous Coronary Intervention: A Network Meta-Analysis. JACC Cardiovasc. Interv. 2022, 15, 2239–2249. [Google Scholar] [CrossRef]
- Lin, K.J.; De Caterina, R.; Rodriguez, L.A.G. Low-dose aspirin and upper gastrointestinal bleeding in primary versus secondary cardiovascular prevention: A population-based, nested case-control study. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 70–77. [Google Scholar] [CrossRef]
- Sundstrom, J.; Hedberg, J.; Thuresson, M.; Aarskog, P.; Johannesen, K.M.; Oldgren, J. Low-Dose Aspirin Discontinuation and Risk of Cardiovascular Events: A Swedish Nationwide, Population-Based Cohort Study. Circulation 2017, 136, 1183–1192. [Google Scholar] [CrossRef]
- Koo, B.K.; Kang, J.; Park, K.W.; Rhee, T.M.; Yang, H.M.; Won, K.B.; Rha, S.W.; Bae, J.W.; Lee, N.H.; Hur, S.H.; et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): An investigator-initiated, prospective, randomized, open-label, multicenter trial. Lancet 2021, 397, 2487–2496. [Google Scholar] [CrossRef]
- Kang, J.; Park, K.W.; Lee, H.; Hwang, D.; Yang, H.M.; Rha, S.W.; Bae, J.W.; Lee, N.H.; Hur, S.H.; Han, J.K.; et al. Aspirin vs. Clopidogrel for Chronic Maintenance Monotherapy after Percutaneous Coronary Intervention: The HOST-EXAM Extended Study. Circulation 2023, 147, 108–117. [Google Scholar] [CrossRef]
- Nelson, T.A.; Parker, W.A.E.; Ghukasyan Lakic, T.; Westerbergh, J.; James, S.K.; Siegbahn, A.; Becker, R.C.; Himmelmann, A.; Wallentin, L.; Storey, R.F. Differential effect of clopidogrel and ticagrelor on leukocyte count in relation to patient characteristics, biomarkers and genotype: A PLATO substudy. Platelets 2022, 33, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, T.; Barozzi, C.; Tomasi, L.; Sangiorgi, D.; Marzocchi, A.; De Servi, S.; Ortolani, P.; Reggiani, L.B.; Alessi, L.; Lauria, G.; et al. A randomised study comparing the antiplatelet and antinflammatory effect of clopidogrel 150 mg/day versus 75 mg/day in patients with ST-segment elevation acute myocardial infarction and poor responsiveness to clopidogrel: Results from the DOUBLE study. Thromb. Res. 2010, 125, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Laudani, C.; Greco, A.; Occhipinti, G.; Ingala, S.; Calderone, D.; Scalia, L.; Agnello, F.; Legnazzi, M.; Mauro, M.S.; Rochira, C.; et al. Short duration of DAPT versus de-escalation after percutaneous coronary intervention for acute coronary syndromes. JACC Cardiovasc. Interv. 2022, 15, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Crimi, G.; De Rosa, R.; Mandurino-Mirizzi, A.; Morici, N.; Alberti, L.P.; Savonitto, S.; De Servi, S. De-escalating dual antiplatelet therapy in patients with acute coronary syndromes: The right strategy to harmonize time-dependent ischemic and bleeding risk in elderly patients? J. Cardiovasc. Med. 2020, 21, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Motovska, Z.; Hlinomaz, O.; Kala, P.; Hromadka, M.; Knot, J.; Varvarovsky, I.; Dusek, J.; Jarkovsky, J.; Miklik, R.; Rokyta, R.; et al. 1-Year Outcomes of Patients Undergoing Primary Angioplasty for Myocardial Infarction Treated with Prasugrel Versus Ticagrelor. J. Am. Coll. Cardiol. 2018, 71, 371–381. [Google Scholar] [CrossRef]
- Menichelli, M.; Neumann, F.J.; Ndrepepa, G.; Mayer, K.; Wöhrle, J.; Bernlochner, I.; Richardt, G.; Witzenbichler, B.; Sibbing, D.; Gewalt, S.; et al. Age- and Weight-Adapted Dose of Prasugrel Versus Standard Dose of Ticagrelor in Patients With Acute Coronary Syndromes: Results From a Randomized Trial. Ann. Intern. Med. 2020, 173, 436–444. [Google Scholar] [CrossRef]
- Rodriguez, F.; Harrington, R.A. Management of antithrombotic therapy after acute coronary syndromes. N. Engl. J. Med. 2021, 384, 452–460. [Google Scholar] [CrossRef]
- De Luca, G.; Dirksen, M.T.; Spaulding, C.; Kelbæk, H.; Schalij, M.; Thuesen, L.; van der Hoeven, B.; Vink, M.A.; Kaiser, C.; Musto, C.; et al. Time course, predictors and clinical implications of stent thrombosis following primary angioplasty. Insights from the DESERT cooperation. Thromb. Haemost. 2013, 110, 826–833. [Google Scholar]
| Study, Year of Publication, Ref. | Population | Number of Patients | Treatment Arms | Primary Endpoints | Main Results | Follow-up (Months) |
|---|---|---|---|---|---|---|
| STEMI | ||||||
| TRIANA trial, 2011 [24] | Patients ≥75 years of age with STEMI presenting within 6 h of symptoms onset. | 266 | pPCI | All-cause mortality, re-infarction, or disabling stroke. |
| 1 month |
| Fibrinolysis | ||||||
| Zwolle MI study group, 2002 [25] | STEMI patients of ≥75 years of age. | 87 | pPCI | Death, reinfarction or stroke at 30 days. |
| 1 year |
| Fibrinolysis | ||||||
| SENIOR PAMI, 2005 [26] | STEMI patients of ≥70 years of age. | 483 | pPCI | Death or disabling stroke at 30 days | No differences in the primary composite endpoint (11.3% vs. 13%, p = 0.57) or in-hospital major bleeding (5.6% vs. 6.2%, p = 0.79) | 30 days |
| Fibrinolysis | ||||||
| NSTEMI | ||||||
| TACTIS-TIMI 18, 2001 [29] | UA or NSTEMI patients (age ≥65 years in 43.5% of patients). | 2220 | Early invasive strategy (routine catheterization within 4 to 48 h and revascularization). | Death, nonfatal MI, and rehospitalization for ACS. |
| 6 months |
| Conservative strategy (catheterization was performed only in case of recurrent ischemia or an abnormal stress test). | ||||||
| FRISC II, 1999 [30] | NSTEMI patients (median age 66 years). | 2457 | Early invasive strategy (coronary angiography and, if appropriate, revascularisation, within 7 days from admission). | Death or MI. |
| 6 months |
| Non-invasive conservative strategy. | ||||||
| ICTUS, 2005 [30] | NSTEMI patients (age ≥65 years in 44.5% of patients). | 1200 | Early invasive strategy (coronary angiography within 24 to 48 h and revascularization). | Death or MI. |
| 5 years |
| Selective invasive strategy (angiography and revascularization in case of refractory angina, hemodynamic or rhythmic instability, or clinically significant ischemia on the pre-discharge exercise test). | ||||||
| RITA-3, 2005 [30] | Patients with NSTE-ACS (mean age 62 years). | 1810 | Early intervention | Two co-primary endpoints:
|
| 1 year |
| Conservative strategy | ||||||
| MOSCA | NSTEMI aged ≥70 years of age with at least two additional comorbidities. | 106 | Invasive strategy | All-cause mortality, reinfarction and readmission for cardiac cause. |
| 2.5 years |
| Conservative strategy (coronary angiogram only if recurrent ischemia or heart failure). | ||||||
| Elderly ACS trial, 2012 [13,31] | NSTEACS patients ≥75 years of age | 313 | Invasive strategy (coronary angiography within 72 h and revascularization if indicated). | Death, MI, disabling stroke, and repeat hospitalisation for cardiovascular causes or severe bleeding. |
| 1 year |
| Conservative strategy (coronary angiography if they demonstrated persistent myocardial ischemia, heart failure, or ventricular arrhythmias) | ||||||
| After Eighty trial, 2016 [32] | UA or NSTEACS patients ≥80 years of age | 457 | Invasive strategy (including early coronary angiography with immediate assessment for PCI, CABG, and optimum medical treatment). | MI, need for urgent revascularisation, stroke, and death. |
| 1.5 years |
| Conservative strategy (optimum medical treatment alone). | ||||||
| RINCAL trial, 2021 [33] | NSTEACS patients ≥80 years of age | 251 | Intervention-guided strategy plus OMT | All-cause mortality and non-fatal MI. |
| 1 year |
| OMT alone | ||||||
| Elderly ACS 2 Trial [49] | Triton-Timi 38 [42] | Plato [45] | Popular Age [53] | |
|---|---|---|---|---|
| Year | 2018 | 2007 | 2009 | 2020 |
| Population | Elderly (>74 years of age) patients with ACS undergoing PCI. | ACS patients undergoing invasive management. | Sub-analysis of the PLATO trial in elderly (≥75 years) versus non-elderly (<75 years) patients. | Patients aged 70 years or older with NSTE-ACS. |
| Intervention(s) | Prasugrel 5mg + ASA (N = 2531) | ASA + prasugrel (N = 6813) | Ticagrelor 90 mg bid (N = 9333) | Clopidogrel 75 mg plus standard of care (N = 500) |
| Control | Clopidogrel 75 mg + ASA (N = 2514) | ASA + clopidogrel (N = 6795) | Clopidogrel 75 mg (N = 9291) | Ticagrelor 90 mg bid plus standard of care (N = 502) |
| Primary endpoint(s) | Death, MI, disabling stroke, or rehospitalization for CV causes or bleeding. | CV death, MI, stroke. | Death from vascular causes, MI, or stroke. | Net clinical benefit (all-cause death, MI, stroke and PLATO major or minor bleeding). |
| Safety endpoints | BARC 2, 3 or 5 bleeding. | Non-CABG-related TIMI major bleeding. | Trial-defined major bleeding. | PLATO major or minor bleeding. |
| Main results |
|
|
|
|
| Follow-up | 12 months | 15 months | 12 months | 12 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Servi, S.; Landi, A.; Savonitto, S.; Morici, N.; De Luca, L.; Montalto, C.; Crimi, G.; De Rosa, R.; De Luca, G. Antiplatelet Strategies for Older Patients with Acute Coronary Syndromes: Finding Directions in a Low-Evidence Field. J. Clin. Med. 2023, 12, 2082. https://doi.org/10.3390/jcm12052082
De Servi S, Landi A, Savonitto S, Morici N, De Luca L, Montalto C, Crimi G, De Rosa R, De Luca G. Antiplatelet Strategies for Older Patients with Acute Coronary Syndromes: Finding Directions in a Low-Evidence Field. Journal of Clinical Medicine. 2023; 12(5):2082. https://doi.org/10.3390/jcm12052082
Chicago/Turabian StyleDe Servi, Stefano, Antonio Landi, Stefano Savonitto, Nuccia Morici, Leonardo De Luca, Claudio Montalto, Gabriele Crimi, Roberta De Rosa, and Giuseppe De Luca. 2023. "Antiplatelet Strategies for Older Patients with Acute Coronary Syndromes: Finding Directions in a Low-Evidence Field" Journal of Clinical Medicine 12, no. 5: 2082. https://doi.org/10.3390/jcm12052082
APA StyleDe Servi, S., Landi, A., Savonitto, S., Morici, N., De Luca, L., Montalto, C., Crimi, G., De Rosa, R., & De Luca, G. (2023). Antiplatelet Strategies for Older Patients with Acute Coronary Syndromes: Finding Directions in a Low-Evidence Field. Journal of Clinical Medicine, 12(5), 2082. https://doi.org/10.3390/jcm12052082










