Perimount MAGNA Ease vs. INSPIRIS Resilia Valve: A PS-Matched Analysis of the Hemodynamic Performances in Patients below 70 Years of Age
Abstract
1. Introduction
2. Material and Methods
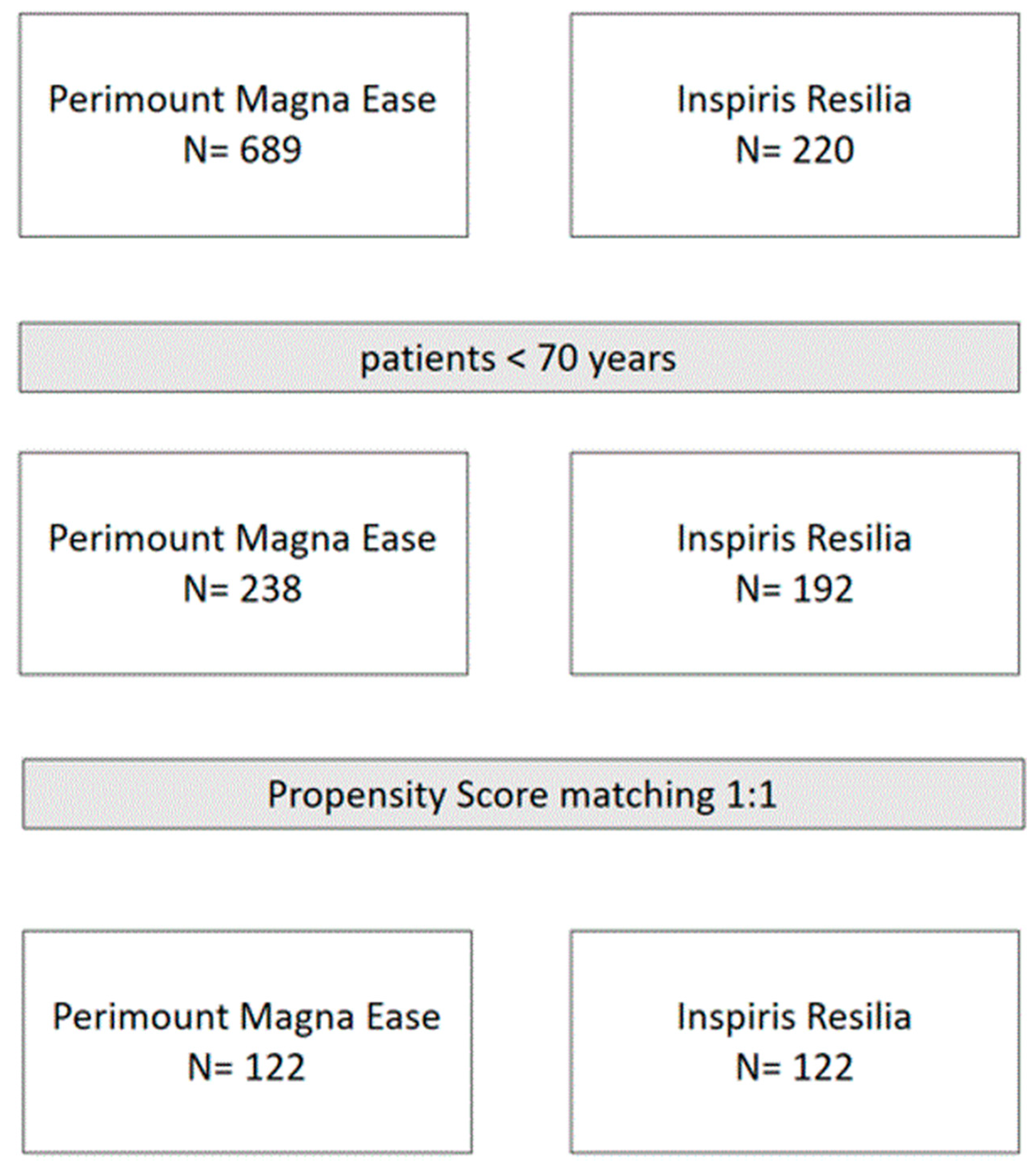
2.1. Study Design and Population
2.2. Endpoints
2.3. Data Management
2.4. Statistical Analysis
3. Results
3.1. Overall Population
3.2. Propensity-Matched Population
3.3. Prosthetic Hemodynamic Performance Evaluation
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attia, R.Q.; Raja, S.G. Surgical pericardial heart valves: 50 Years of evolution. Int. J. Surg. 2021, 94, 106121. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, T.; Bouquiaux-Stablo, A.L.; Candolfi, P.; Mirza, A.; Loardi, C.; May, M.A.; El-Khoury, R.; Marchand, M.; Aupart, M. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann. Thorac. Surg. 2015, 99, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, M.J.; Maríagonzález-Santos, J.; López-Rodríguez, J.; Bueno, M.; Arribas, A. The Carpentier-Edwards Perimount Magna aortic xenograft: A new design with an improved hemodynamic performance. Interact. Cardiovasc. Thorac. Surg. 2006, 5, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Thorp, S.D.; Khazaal, J.; Yu, G.; Parker, J.L.; Timek, T.A. Magna ease bioprosthetic aortic valve: Mid-term haemodynamic outcomes in 1126 patients. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 839–845. [Google Scholar] [CrossRef]
- Rajab, T.K.; Ali, J.M.; Hernández-Sánchez, J.; Mackie, J.; Grimaudo, V.; Sinichino, S.; Mills, C.; Rana, B.; Dunning, J.; Abu-Omar, Y. Mid-term follow-up after aortic valve replacement with the Carpentier Edwards Magna Ease prosthesis. J. Cardiothorac. Surg. 2020, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Piperata, A.; Fiocco, A.; Cavicchiolo, A.; Ponzoni, M.; Pesce, R.; Gemelli, M.; Evangelista, G.; Gastino, E.; Michelotti, S.; Mazzaro, E.; et al. Carpentier-Edwards Magna Ease bioprosthesis: A multicentre clinical experience and 12-year durability. Eur. J. Cardiothorac. Surg. 2022, 61, 888–896. [Google Scholar] [CrossRef]
- Alperi, A.; Hernandez-Vaquero, D.; Pascual, I.; Diaz, R.; Silva, I.; Alvarez-Cabo, R.; Avanzas, P.; Moris, C. Aortic valve replacement in young patients: Should the biological prosthesis be recommended over the mechanical? Ann. Transl. Med. 2018, 6, 183. [Google Scholar] [CrossRef]
- Forcillo, J.; El Hamamsy, I.; Stevens, L.M.; Badrudin, D.; Pellerin, M.; Perrault, L.P.; Cartier, R.; Bouchard, D.; Carrier, M.; Demers, P. The perimount valve in the aortic position: Twenty-year experience with patients under 60 years old. Ann. Thorac. Surg. 2014, 97, 1526–1532. [Google Scholar] [CrossRef]
- Flameng, W.; Hermans, H.; Verbeken, E.; Meuris, B. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J. Thorac. Cardiovasc. Surg. 2015, 49, 340–345. [Google Scholar] [CrossRef]
- Sadri, V.; Trusty, P.M.; Madukauwa-David, I.D.; Yoganathan, A.P. Long-term durability of a new surgical aortic valve: A 1 billion cycle in vitro study. JTCVS Open 2022, 9, 59–69, Erratum in JTCVS Open 2022, 10, 168. [Google Scholar] [CrossRef]
- Tamagnini, G.; Bourguignon, T.; Rega, F.; Verbrugghe, P.; Lamberigts, M.; Langenaeken, T.; Meuris, B. Device profile of the Inspiris Resilia valve for aortic valve replacement: Overview of its safety and efficacy. Expert Rev. Med. Devices 2021, 18, 239–244. [Google Scholar] [CrossRef]
- Bavaria, J.E.; Griffith, B.; Heimansohn, D.A.; Rozanski, J.; Johnston, D.R.; Bartus, K.; Girardi, L.N.; Beaver, T.; Takayama, H.; Mumtaz, M.A.; et al. Five-year Outcomes of the COMMENCE Trial Investigating Aortic Valve Replacement with RESILIA Tissue. Ann. Thorac. Surg. 2022. [Google Scholar] [CrossRef]
- Meuris, B.; Borger, M.A.; Bourguignon, T.; Siepe, M.; Grabenwöger, M.; Laufer, G.; Binder, K.; Polvani, G.; Stefano, P.; Coscioni, E.; et al. Durability of bioprosthetic aortic valves in patients under the age of 60 years—Rationale and design of the international INDURE registry. J. Cardiothorac. Surg. 2020, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [PubMed]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Glaser, N.; Nilsson, J.; Friberg, Ö.; Franco-Cereceda, A.; Sartipy, U. Comparison of Long-term Performance of Bioprosthetic Aortic Valves in Sweden from 2003 to 2018. JAMA Netw. Open 2022, 5, e220962. [Google Scholar] [CrossRef]
- Theologou, T.; Harky, A.; Shaw, M.; Harrington, D.; Kuduvalli, M.; Oo, A.; Field, M. Mitroflow and Perimount Magna 10 years outcomes a direct propensity match analysis to assess reintervention rates and long follow-up mortality. J. Card. Surg. 2019, 34, 1279–1287. [Google Scholar] [CrossRef]
- Biancari, F.; Valtola, A.; Juvonen, T.; Husso, A.; Dahlbacka, S.; Laakso, T.; Jalava, M.P.; Tauriainen, T.; Ahvenvaara, T.; Kinnunen, E.-M.; et al. Trifecta Versus Perimount Magna Ease Aortic Valve Prostheses. Ann. Thorac. Surg. 2020, 110, 879–888. [Google Scholar] [CrossRef]
- Johnston, D.R.; Griffith, B.P.; Puskas, J.D.; Bavaria, J.E.; Svensson, L.G.; Blackstone, E.H.; Gammie, J.S.; Heimansohn, D.A.; Sadowski, J.; Bartus, K.; et al. Intermediate-term outcomes of aortic valve replacement using a bioprosthesis with a novel tissue. J. Thorac. Cardiovasc. Surg. 2021, 162, 1478–1485. [Google Scholar] [CrossRef]
- Anselmi, A.; Ruggieri, V.G.; Belhaj Soulami, R.; Flécher, E.; Langanay, T.; Corbineau, H.; Corbineau, H.; Leguerrier, A.; Verhoye, J.-P. Hemodynamic Results and Mid-term Follow-up of 850 19 to 23 mm Perimount Magna Ease Valves. Thorac. Cardiovasc. Surg. 2019, 67, 274–281, Erratum in Thorac. Cardiovasc. Surg. 2019, 67, e1. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Hjortdal, V.; Modrau, I.S.; Jensen, H.; Kimose, H.H.; Terp, K.; Poulsen, S.H.; Smerup, M.; Nielsen, S.L. Durability after aortic valve replacement with the Mitroflow versus the Perimount pericardial bioprosthesis: A single-centre experience in 2393 patients. Eur. J. Cardiothorac. Surg. 2016, 49, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.R.; Soltesz, E.G.; Vakil, N.; Rajeswaran, J.; Roselli, E.E.; Sabik, J.F., III; Smedira, N.G.; Svensson, L.G.; Lytle, B.W.; Blackstone, E.H. Long-term durability of bioprosthetic aortic valves: Implications from 12,569 implants. Ann. Thorac. Surg. 2015, 99, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Izumi, C.; Kitai, T.; Kume, T.; Onishi, T.; Yuda, S.; Hirata, K.; Yamashita, E.; Kawata, T.; Nishimura, K.; Takeuchi, M.; et al. Effect of Left Ventricular Reverse Remodeling on Long-term Outcomes After Aortic Valve Replacement. Am. J. Cardiol. 2019, 124, 105–112. [Google Scholar] [CrossRef]
- Corti, R.; Binggeli, C.; Turina, M.; Jenni, R.; Lüscher, T.F.; Turina, J. Predictors of long-term survival after valve replacement for chronic aortic regurgitation; is M-mode echocardiography sufficient? Eur. Heart J. 2001, 22, 866–873. [Google Scholar] [CrossRef] [PubMed]

| Total | Perimount Magna Ease | Inspiris Resilia | p-Value Cohens d | |
|---|---|---|---|---|
| n/N (%) or Mean ± SD/Median (IQR) n = 244 | n/N (%) or Mean ± SD/Median (IQR) n = 122 | n/N (%) or Mean ± SD/Median (IQR) n = 122 | ||
| Age, years | 57.4 ± 10.1 60 (52;65) | 57.7 ± 11.1 61 (52;64) | 57.0 ± 9.1 58 (52;64) | 0.098 |
| Female gender | 55 (22.5) | 24 (19.7) | 31 (25.4) | 0.284 |
| BMI, kg/m2 | 26.9 ± 4.6 | 26.6 ± 4.2 | 27.1 ± 5.1 | 0.939 |
| Diabetes mellitus | 16 (6.6) | 11 (9.0) | 5 (4.1) | 0.121 |
| COPD | 32 (13.1) | 21 (17.2) | 11 (9.0) | 0.058 |
| Creatinine, mg/dL | 1.0 ± 0.8 238 | 1.1 ± 1.1 116 | 1.0 ± 0.4 122 | 0.291 |
| Dialysis | 4 (1.6) | 4 (3.3) | 0 (0) | 0.122 |
| NYHA | ||||
| I | 51 (20.9) | 4 (3.3) | 47 (38.5) | <0.001 |
| II | 116 (47.5) | 81 (66.4) | 35 (28.7) | |
| III | 40 (16.4) | 10 (8.2) | 30 (24.6) | |
| IV | 37 (15.2) | 27 (22.1) | 10 (8.2) | |
| NYHA III/IV | 77 (31.6) | 37 (30.3) | 40 (32.8) | 0.679 |
| EuroScore II, % | 2.7 ± 2.4 | 2.7 ± 2.4 | 2.7 ± 2.4 | 0.788 |
| Hypertension | 133 (54.5) | 68 (55.7) | 65 (53.3) | 0.700 |
| Dyslipidemia | 78 (32.0) | 40 (32.8) | 38 (31.1) | 0.784 |
| Active/former smoker | 101 (41.4) | 45 (36.9) | 56 (45.9) | 0.153 |
| Peripheral vascular disease | 28 (11.5) | 18 (14.8) | 10 (8.2) | 0.159 |
| History of stroke | 9 (3.7) | 6 (4.9) | 3 (2.5) | 0.500 |
| Atrial fibrillation | ||||
| Paroxysmal | 15 (6.1) | 12 (9.8) | 3 (2.5) | 0.004 |
| Persistent | 30 (12.3) | 20 (16.4) | 10 (8.2) | |
| Pacemaker | 4 (1.6) | 3 (2.5) | 1 (0.8) | 0.622 |
| Prev. MI | 15 (6.1) | 9 (7.4) | 6 (4.9) | 0.424 |
| Prior PCI | 15 (6.1) | 8 (6.6) | 7 (5.7) | 0.790 |
| Prior cardiac surgery | 25 (10.2) | 16 (13.1) | 9 (7.4) | 0.139 |
| Coronary artery disease | 47 (19.3) | 22 (18.0) | 25 (20.5) | 0.626 |
| Endocarditis | 20 (8.2) | 10 (8.2) | 10 (8.2) | 1.000 |
| Ejection fraction, % | 56.8 ± 10.4 60 (55;64) | 57.1 ± 10.0 58.6 (55;65) | 56.4 ± 10.9 60 (54;63) | 0.932 |
| Preoperative Echo | ||||
| Bicuspid valve | 109 (44.7) | 50 (41.0) | 59 (48.4) | 0.246 |
| End-diastolic volume, mL | 153.9 ± 57.7 140 | 176.9 ± 64.8 35 | 146.2 ± 53.3 105 | 0.012 −0.545 |
| End-diastolic diameter, mm | 59.8 ± 18.4 65 | 59.8 ± 10.2 36 | 59.8 ± 25.3 29 | 0.134 |
| Interventricular septum, mm | 13.0 ± 2.2 125 | 13.2 ± 2.2 50 | 12.8 ± 2.1 75 | 0.484 |
| Peak gradient, mmHg | 64.9 ± 26.7 99 | 62.8 ± 23.0 29 | 65.8 ± 28.2 70 | 0.623 |
| Mean gradient, mmHg | 43.3 ± 17.1 104 | 41.7 ± 17.3 29 | 43.9 ± 17.1 75 | 0.545 |
| Vmax, cm/sec | 3.8 ± 1.0 27 | 4.4 ± 0.9 2 | 3.7 ± 1.0 25 | 0.399 |
| AVA, cm2 | 0.74 ± 0.36 67 | 0.46 ± 0.52 13 | 0.81 ± 0.27 54 | 0.165 |
| Pulmonary artery pressure systolic, mmHg | 38.6 ± 14.0 77 | 40.0 ± 11.6 22 | 38.0 ± 14.9 55 | 0.283 |
| Aortic regurgitation | ||||
| No | 70 (28.7) | 36 (29.5) | 34 (27.9) | 0.174 |
| Mild | 47 (19.3) | 18 (14.8) | 29 (23.8) | |
| Moderate | 40 (16.4) | 18 (14.8) | 22 (18.0) | |
| Severe | 87 (35.7) | 50 (41.0) | 37 (30.3) | |
| Moderate/severe | 127 (52.0) | 68 (55.7) | 59 (48.4) | 0.249 |
| Mitral regurgitation | ||||
| No | 67/166 (40.4) | 38/62 (61.3) | 29/104 (27.9) | <0.001 |
| Mild | 71/166 (42.8) | 14/62 (22.6) | 57/104 (54.8) | |
| Moderate | 20/166 (12.0) | 8/62 (12.9) | 12/104/(11.5) | |
| Severe | 8/166 (4.8) | 2/62 (3.2) | 6/104 (5.8) | |
| Moderate/severe | 28/166 (16.9) | 10/62 (16.1) | 18/104 (17.3) | 0.844 |
| Tricuspid regurgitation | ||||
| No | 66/113 (58.4) | 28/30 (93.3) | 38/83 (45.8) | <0.001 |
| Mild | 37/113 (32.7) | 2/30 (6.7) | 35/83 (42.2) | |
| Moderate | 6/113 (5.3) | 0/30 (0) | 6/83 (7.2) | |
| Severe | 4/113 (3.5) | 0/30 (0) | 4/83 (4.8) | |
| Moderate/severe | 10/113 (8.8) | 0/30(0) | 10/83 (12.0) | 0.046 |
| Total | Perimount Magna Ease | Inspiris Resilia | p-Value Cohens d | |
|---|---|---|---|---|
| n/N (%) or Mean ± SD n = 244 | n/N (%) or Mean ± SD n = 122 | n/N (%) or Mean ± SD n = 122 | ||
| One-year echo | ||||
| Ejection fraction, % | 57.2 ± 9.7 203 | 56.5 ± 9.7 86 | 57.6 ± 9.7 117 | 0.514 |
| End-diastolic volume, mL | 110.3 ± 34.6 121 | 120.3 ± 41.7 39 | 105.5 ± 29.8 82 | 0.091 |
| End-diastolic diameter, mm | 50.1 ± 7.6 78 | 51.3 ± 6.8 32 | 49.2 ± 8.1 46 | 0.081 |
| Interventricular septum, mm | 12.5 ± 2.1 123 | 13.2 ± 2.1 44 | 12.1 ± 2.0 79 | 0.011 −0.54 |
| Peak gradient, mmHg | 20.9 ± 7.9 203 | 20.2 ± 6.7 87 | 21.3 ± 8.7 116 | 0.624 |
| Mean gradient, mmHg | 11.7 ± 4.7 206 | 11.3 ± 3.5 87 | 11.9 ± 5.4 119 | 0.794 |
| Vmax, cm/sec | 2.23 ± 0.43 204 | 2.20 ± 0.39 87 | 2.26 ± 0.45 117 | 0.300 |
| Pulmonary artery pressure systolic, mmHg | 29.8 ± 9.0 63 | 34.3 ± 9.3 25 | 26.8 ± 7.6 38 | 0.001 −0.902 |
| Prosthesis stenosis | ||||
| No | 203/205 (99.0) | 83/83 (100) | 120/122 (98.4) | 0.516 |
| Mild | 2/205 (1.0) | 0/83 (0) | 2/122 (1.6) | |
| Moderate | 0/205 (0) | 0/83 (0) | 0/1222 (0) | |
| Severe | 0/205 (0) | 0/83 (0) | 0/1222 (0) | |
| Moderate/severe | 0/205 (0) | 0/83 (0) | 0/1222 (0) | n.a. |
| Prosthesis regurgitation | ||||
| No | 199/206 (96.6) | 80/84 (95.2) | 119/122 (97.5) | 0.447 |
| Mild | 7/206 (3.4) | 4/84 (4.8) | 3/122 (2.5) | |
| Moderate | 0/206 (0) | 0/84 (0) | 0/122 (0) | |
| Severe | 0/206 (0) | 0/84 (0) | 0/122 (0) | |
| Moderate/severe | 0/206 (0) | 0/84 (0) | 0/122 (0) | n.a. |
| Paravalvular leak | ||||
| No | 205/208 (98.6) | 84/86 (97.7) | 121/122 (99.2) | 0.571 |
| Mild | 3/208 (1.4) | 2/86 (2.3) | 1/122 (0.8) | |
| Moderate | 0/208 (0) | 0/86 (0) | 0/122 (0) | |
| Severe | 0/208 (0) | 0/86 (0) | 0/122 (0) | |
| Moderate/severe | 0/208 (0) | 0/86 (0) | 0/122 (0) | n.a. |
| Mitral regurgitation | ||||
| No | 126/203 (62.1) | 40/83 (48.2) | 86/120 (71.7) | <0.001 |
| Mild | 73/203 (36.0) | 39/83 (47.0) | 34/120 (28.3) | |
| Moderate | 4/203 (2.0) | 4/83 (4.8) | 0/120 (0) | |
| Severe | 0/203 (0) | 0/83 (0) | 0/120 (0) | |
| Moderate/severe | 4/203 (2.0) | 4/83 (4.8) | 0/120 (0) | 0.027 |
| Tricuspid regurgitation | ||||
| No | 99/163 (60.7) | 52/80 (65.0) | 47/83 (56.6) | 0.664 |
| Mild | 61/163 (37.4) | 27/80 (33.8) | 34/83 (41.0) | |
| Moderate | 2/163 (1.2) | 1(80 (1.3) | 1/83 (1.2) | |
| Severe | 1/163 (0.6) | 0/80 (0) | 1/83 (1.2) | |
| Moderate/severe | 3/163 (1.8) | 1/80 (1.3) | 2/83 (2.4) | 1.000 |
| 3-years echo | ||||
| Ejection fraction, % | 58.7 ± 8.8 113 | 58.1 ± 9.5 88 | 61.1 ± 5.1 25 | 0.166 |
| End-diastolic volume, mL | 112.9 ± 35.6 108.5 (86.0;136.0) 68 | 116.1 ± 36.4 112.0 (87.0;137.0) 51 | 103.0 ± 32.1 100.0 (79.0;117.75) 17 | 0.189 |
| End-diastolic diameter, mm | 50.3 ± 5.9 57 | 50.5 ± 6.3 49 | 49.1 ± 3.4 8 | 0.552 |
| Interventricular septum, mm | 12.1 ± 2.2 73 | 12.3 ± 2.2 58 | 11.1 ± 1.8 15 | 0.050 |
| Peak gradient, mmHg | 22.8 ± 13.2 109 | 22.8 ± 14.2 84 | 22.7 ± 9.1 25 | 0.762 |
| Mean gradient, mmHg | 12.5 ± 8.2 110 | 12.5 ± 8.8 84 | 12.6 ± 5.5 26 | 0.680 |
| Vmax, cm/sec | 2.28 ± 0.55 109 | 2.26 ± 0.57 84 | 2.33 ± 0.46 25 | 0.621 |
| Pulmonary artery pressure systolic, mmHg | 31.0 ± 7.1 50 | 31.7 ± 7.9 37 | 28.9 ± 3.8 13 | 0.227 |
| Prosthesis stenosis | ||||
| No | 105/114 (92.1) | 81/88 (92.0) | 24/26 (92.3) | 0.799 |
| Mild | 7/114 (6.1) | 5/88 (5.7) | 2/26 (7.7) | |
| Moderate | 0/114 ((0) | 0/88 (0) | 0/26 (0) | |
| Severe | 2/114 (1.8) | 2/88 (2.3) | 0/26 (0) | |
| Moderate/severe | 2/114 (1.8) | 2/88 (2.3) | 0/26 (0) | 1.000 |
| Prosthesis regurgitation | ||||
| No | 106/114 (93.0) | 80/88 (90.9) | 26/26 (100) | 0.604 |
| Mild | 6/114 (5.3) | 6/88 (6.8) | 0/26 (0) | |
| Moderate | 1/114 (0.9) | 1/88 (1.1) | 0/26 (0) | |
| Severe | 1/114 (0.9) | 1/88 (1.1) | 0/26 (0) | |
| Moderate/severe | 2/114 (1.8) | 2/88 (2.3) | 0/26 (0) | 1.000 |
| Paravalvular leak | ||||
| No | 110/114 (96.5) | 84/88 (95.5) | 26/26 (100) | 0.572 |
| Mild | 4/114 (3.5) | 4/88 (4.5) | 0/26 (0) | |
| Moderate | 0/114 (0) | 0/88 (0) | 0/26 (0) | |
| Severe | 0/114 (0) | 0/88 (0) | 0/26 (0) | |
| Moderate/severe | 0/114 (0) | 0/88 (0) | 0/26 (0) | n.a. |
| Mitral regurgitation | ||||
| No | 45/114 (39.5) | 29/88 (33.0) | 16/26 (61.5) | 0.016 |
| Mild | 59/114 (51.8) | 49/88 (55.7) | 10/26 (38.5) | |
| Moderate | 10/114 (8.8) | 10/88 (11.4) | 0/26 (0) | |
| Severe | 0/114 (0) | 0/88 (0) | 0/26 (0) | |
| Moderate/severe | 10/114 (8.8) | 10/88 (11.4) | 0/26 (0) | 0.113 |
| Tricuspid regurgitation | ||||
| No | 55/101 (54.5) | 47/85 (55.3) | 8/16 (50.0) | 0.848 |
| Mild | 44/101 (43.6) | 36/85 (42.4) | 8/16 (50.0) | |
| Moderate | 2/101 (2.0) | 2/85 (2.4) | 0/16 (0) | |
| Severe | 0/101 (0) | 0/85 (0) | 0/16 (0) | |
| Moderate/severe | 2/101 (2.0) | 2/85 (2.4) | 0/16 (0) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francica, A.; Tonelli, F.; Rossetti, C.; Galeone, A.; Perrone, F.; Luciani, G.B.; Onorati, F. Perimount MAGNA Ease vs. INSPIRIS Resilia Valve: A PS-Matched Analysis of the Hemodynamic Performances in Patients below 70 Years of Age. J. Clin. Med. 2023, 12, 2077. https://doi.org/10.3390/jcm12052077
Francica A, Tonelli F, Rossetti C, Galeone A, Perrone F, Luciani GB, Onorati F. Perimount MAGNA Ease vs. INSPIRIS Resilia Valve: A PS-Matched Analysis of the Hemodynamic Performances in Patients below 70 Years of Age. Journal of Clinical Medicine. 2023; 12(5):2077. https://doi.org/10.3390/jcm12052077
Chicago/Turabian StyleFrancica, Alessandra, Filippo Tonelli, Cecilia Rossetti, Antonella Galeone, Fabiola Perrone, Giovanni Battista Luciani, and Francesco Onorati. 2023. "Perimount MAGNA Ease vs. INSPIRIS Resilia Valve: A PS-Matched Analysis of the Hemodynamic Performances in Patients below 70 Years of Age" Journal of Clinical Medicine 12, no. 5: 2077. https://doi.org/10.3390/jcm12052077
APA StyleFrancica, A., Tonelli, F., Rossetti, C., Galeone, A., Perrone, F., Luciani, G. B., & Onorati, F. (2023). Perimount MAGNA Ease vs. INSPIRIS Resilia Valve: A PS-Matched Analysis of the Hemodynamic Performances in Patients below 70 Years of Age. Journal of Clinical Medicine, 12(5), 2077. https://doi.org/10.3390/jcm12052077








