Obesity Is Indirectly Associated with Sudden Cardiac Arrest through Various Risk Factors
Abstract
1. Introduction
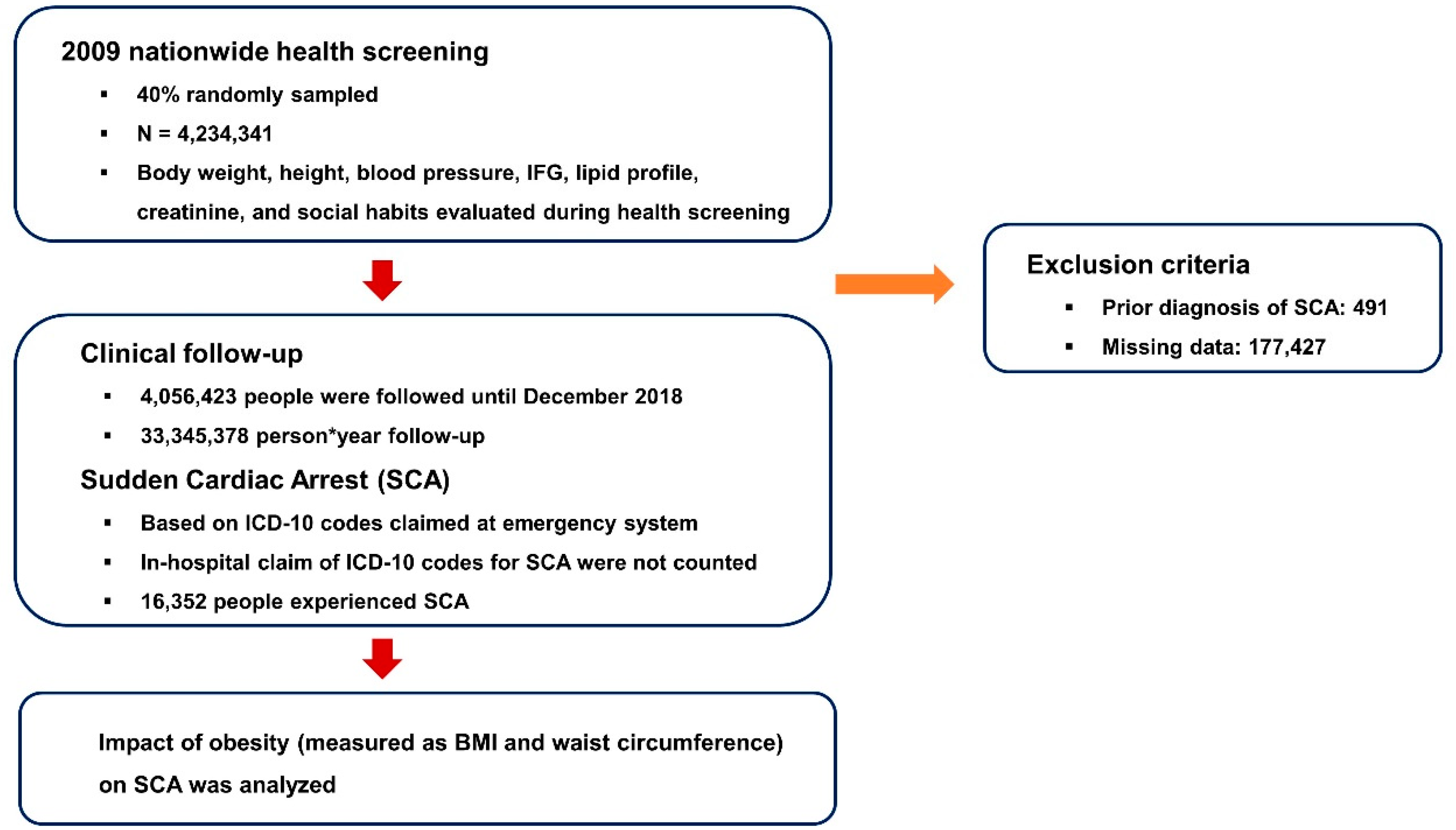
2. Materials and Methods
2.1. K-NHIS Database
2.2. Participants
2.3. Primary Outcome
2.4. Definitions
2.5. Statistical Analysis
3. Results
3.1. Study Population
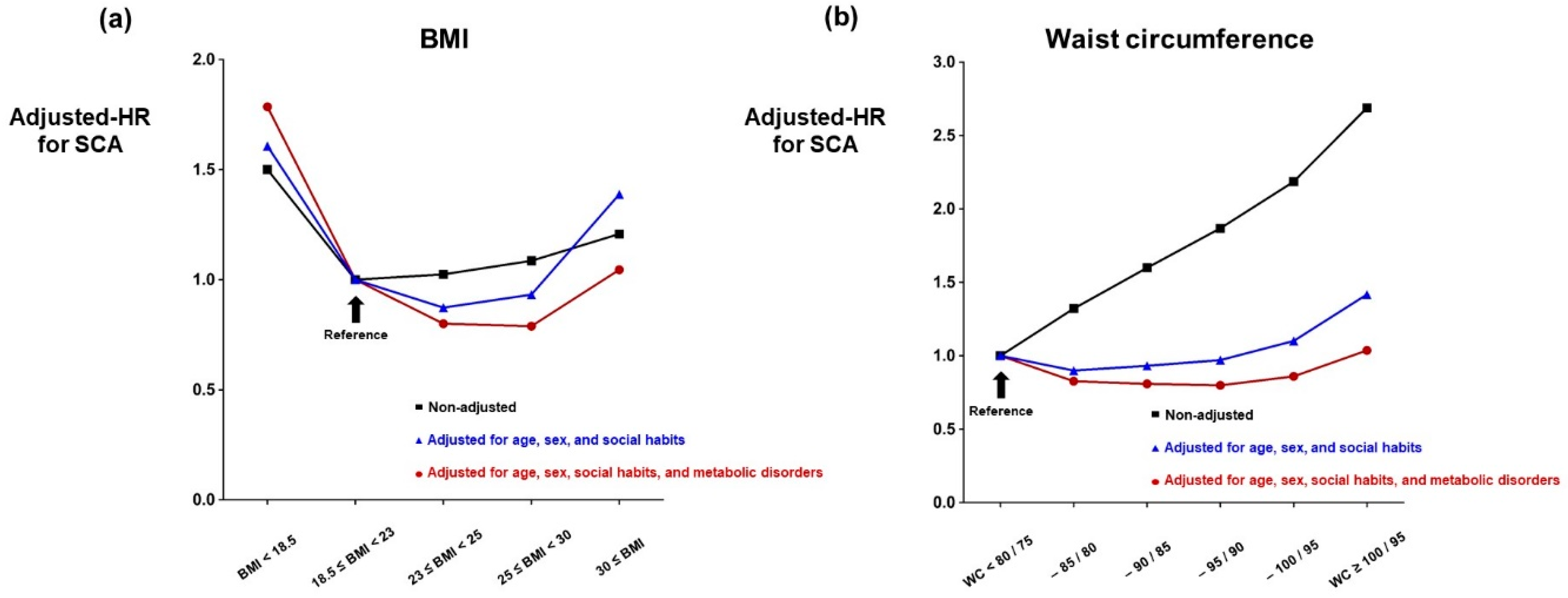
3.2. BMI and SCA
3.3. Waist Circumference and SCA
3.4. Obesity, Metabolic Syndrome and SCA
3.5. Multivariate Model
4. Discussion
4.1. Obesity and SCA
4.2. Prevention of SCA
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Hall, J.E.; da Silva, A.A.; do Carmo, J.M.; Dubinion, J.; Hamza, S.; Munusamy, S.; Smith, G.; Stec, D.E. Obesity-induced hypertension: Role of sympathetic nervous system, leptin, and melanocortins. J. Biol. Chem. 2010, 285, 17271–17276. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; Nathan, D.M. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Stecker, T.; Sparks, S. Prevalence of obese patients in a primary care setting. Obesity 2006, 14, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.J.; Rexrode, K.M.; Hu, F.B.; Glynn, R.J.; Caspard, H.; Manson, J.E.; Willett, W.C.; Rimm, E.B. Body mass index, waist circumference, and risk of coronary heart disease: A prospective study among men and women. Obes. Res. Clin. Pract. 2010, 4, e171–e181. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef]
- Twig, G.; Yaniv, G.; Levine, H.; Leiba, A.; Goldberger, N.; Derazne, E.; Ben-Ami Shor, D.; Tzur, D.; Afek, A.; Shamiss, A.; et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N. Engl. J. Med. 2016, 374, 2430–2440. [Google Scholar] [CrossRef]
- Calle, E.E.; Thun, M.J.; Petrelli, J.M.; Rodriguez, C.; Heath, C.W., Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N. Engl. J. Med. 1999, 341, 1097–1105. [Google Scholar] [CrossRef]
- Jee, S.H.; Sull, J.W.; Park, J.; Lee, S.Y.; Ohrr, H.; Guallar, E.; Samet, J.M. Body-mass index and mortality in Korean men and women. N. Engl. J. Med. 2006, 355, 779–787. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Moore, S.C.; Jacobs, E.J.; Kitahara, C.M.; Rosenberg, P.S.; Adami, H.O.; Ebbert, J.O.; English, D.R.; Gapstur, S.M.; Giles, G.G.; et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin. Proc. 2014, 89, 335–345. [Google Scholar] [CrossRef]
- Snijder, M.B.; van Dam, R.M.; Visser, M.; Seidell, J.C. What aspects of body fat are particularly hazardous and how do we measure them? Int. J. Epidemiol. 2006, 35, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rimm, E.B.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am. J. Clin. Nutr. 2005, 81, 555–563. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L.; et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef]
- Myat, A.; Song, K.J.; Rea, T. Out-of-hospital cardiac arrest: Current concepts. Lancet 2018, 391, 970–979. [Google Scholar] [CrossRef]
- Cummins, R.O.; Ornato, J.P.; Thies, W.H.; Pepe, P.E. Improving survival from sudden cardiac arrest: The “chain of survival” concept. A statement for health professionals from the Advanced Cardiac Life Support Subcommittee and the Emergency Cardiac Care Committee, American Heart Association. Circulation 1991, 83, 1832–1847. [Google Scholar] [CrossRef]
- Ong, M.E.H.; Perkins, G.D.; Cariou, A. Out-of-hospital cardiac arrest: Prehospital management. Lancet 2018, 391, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; van der Schouw, Y.T.; Spencer, E.; Moons, K.G.; Tjonneland, A.; et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Han, K.D.; Kim, D.Y.; Choi, Y.Y.; Choi, H.Y.; Roh, S.Y.; Shim, J.; Kim, J.S.; Choi, J.I.; Kim, Y.H. Different Influence of Blood Pressure on New-Onset Atrial Fibrillation in Pre- and Postmenopausal Women: A Nationwide Population-Based Study. Hypertension 2021, 77, 1500–1509. [Google Scholar] [CrossRef]
- Kim, Y.G.; Han, K.-D.; Choi, J.-I.; Choi, Y.Y.; Choi, H.Y.; Boo, K.Y.; Kim, D.Y.; Lee, K.-N.; Shim, J.; Kim, J.-S. Non-genetic risk factors for atrial fibrillation are equally important in both young and old age: A nationwide population-based study. Eur. J. Prev. Cardiol. 2021, 28, 666–676. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2018, 72, 1677–1749. [Google Scholar] [CrossRef]
- World Health Organization; Regional Office for the Western Pacific; the International Association for the Study of Obesity; the International Obesity Task Force. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; World Health Organization: Geneva, Switzerland, 2002; 56p. [Google Scholar]
- Seo, M.H.; Lee, W.Y.; Kim, S.S.; Kang, J.H.; Kang, J.H.; Kim, K.K.; Kim, B.Y.; Kim, Y.H.; Kim, W.J.; Kim, E.M.; et al. 2018 Korean Society for the Study of Obesity Guideline for the Management of Obesity in Korea. J. Obes. Metab. Syndr. 2019, 28, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Oh, S.K.; Choi, H.Y.; Choi, J.I. Inherited arrhythmia syndrome predisposing to sudden cardiac death. Korean J. Intern. Med. 2021, 36, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.-Y.; Choi, J.-I.; Park, S.H.; Kim, Y.G.; Shim, J.; Kim, J.-S.; Do Han, K.; Kim, Y.-H. The 10-year trend of out-of-hospital cardiac arrests: A Korean nationwide population-based study. Korean Circ. J. 2021, 51, 866–874. [Google Scholar] [CrossRef]
- Kim, Y.G.; Han, K.; Jeong, J.H.; Roh, S.-Y.; Choi, Y.Y.; Min, K.; Shim, J.; Choi, J.-I.; Kim, Y.-H. Metabolic Syndrome, Gamma-Glutamyl Transferase, and Risk of Sudden Cardiac Death. J. Clin. Med. 2022, 11, 1781. [Google Scholar] [CrossRef]
- Kim, Y.G.; Roh, S.Y.; Han, K.-D.; Jeong, J.H.; Choi, Y.Y.; Min, K.; Shim, J.; Choi, J.-I.; Kim, Y.-H. Hypertension and diabetes including their earlier stage are associated with increased risk of sudden cardiac arrest. Sci. Rep. 2022, 12, 12307. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; McAuley, P.A.; Church, T.S.; Milani, R.V.; Blair, S.N. Obesity and cardiovascular diseases: Implications regarding fitness, fatness, and severity in the obesity paradox. J. Am. Coll. Cardiol. 2014, 63, 1345–1354. [Google Scholar] [CrossRef]
- Zeller, M.; Steg, P.G.; Ravisy, J.; Lorgis, L.; Laurent, Y.; Sicard, P.; Janin-Manificat, L.; Beer, J.-C.; Makki, H.; Lagrost, A.-C.c. Relation between body mass index, waist circumference, and death after acute myocardial infarction. Circulation 2008, 118, 482–490. [Google Scholar] [CrossRef]
- Ravnskov, U. High cholesterol may protect against infections and atherosclerosis. Int. J. Med. 2003, 96, 927–934. [Google Scholar] [CrossRef]
- Cavaillon, J.; Fitting, C.; Haeffner-Cavaillon, N.; Kirsch, S.; Warren, H.S. Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Infect. Immun. 1990, 58, 2375–2382. [Google Scholar] [CrossRef]
- Kistorp, C.; Faber, J.; Galatius, S.; Gustafsson, F.; Frystyk, J.; Flyvbjerg, A.; Hildebrandt, P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation 2005, 112, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Csige, I.; Ujvárosy, D.; Szabó, Z.; Lőrincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The impact of obesity on the cardiovascular system. J. Diabetes Res. 2018, 2018, 3407306. [Google Scholar] [CrossRef]
- Lee, S.-H.; Chen, Y.-C.; Chen, Y.-J.; Chang, S.-L.; Tai, C.-T.; Wongcharoen, W.; Yeh, H.-I.; Lin, C.-I.; Chen, S.-A. Tumor necrosis factor-α alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 2007, 80, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ali, V.; Goodrick, S.; Bulmer, K.; Holly, J.M.; Yudkin, J.S.; Coppack, S.W. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am. J. Physiol. -Endocrinol. Metab. 1999, 277, E971–E975. [Google Scholar] [CrossRef] [PubMed]
- Goossens, C.; Marques, M.B.; Derde, S.; Vander Perre, S.; Dufour, T.; Thiessen, S.E.; Güiza, F.; Janssens, T.; Hermans, G.; Vanhorebeek, I.; et al. Premorbid obesity, but not nutrition, prevents critical illness-induced muscle wasting and weakness. J. Cachexia Sarcopenia Muscle 2017, 8, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.C.; Pastore, C.A.; Orlandi, S.P.; Heymsfield, S.B. Obesity paradox in cancer: New insights provided by body composition. Am. J. Clin. Nutr. 2014, 99, 999–1005. [Google Scholar] [CrossRef]
- Prado, C.M.; Gonzalez, M.C.; Heymsfield, S.B. Body composition phenotypes and obesity paradox. Curr. Opin. Clin. Nutr. 2015, 18, 535–551. [Google Scholar] [CrossRef]
- Bastien, M.; Poirier, P.; Lemieux, I.; Despres, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef]
- Parto, P.; Lavie, C.J. Obesity and Cardiovascular Diseases. Curr. Prob. Cardiol. 2017, 42, 376–394. [Google Scholar] [CrossRef]
- Penketh, J.; Nolan, J.P. In-hospital cardiac arrest: The state of the art. Crit. Care 2022, 26, 376. [Google Scholar] [CrossRef]
- Andersson, A.; Arctaedius, I.; Cronberg, T.; Levin, H.; Nielsen, N.; Friberg, H.; Lybeck, A. In-hospital versus out-of-hospital cardiac arrest: Characteristics and outcomes in patients admitted to intensive care after return of spontaneous circulation. Resuscitation 2022, 176, 1–8. [Google Scholar] [CrossRef] [PubMed]
| BMI | p-Value | |||||
|---|---|---|---|---|---|---|
| BMI < 18.5 | 18.5 ≤ BMI < 23 | 23 ≤ BMI < 25 | 25 ≤ BMI < 30 | 30 ≤ BMI | ||
| 148,460 | 1,579,653 | 1,001,394 | 1,182,398 | 144,518 | ||
| Male | 49,452 (33.3%) | 750,152 (47.5%) | 602,974 (60.2%) | 749,493 (63.4%) | 81,460 (56.4%) | <0.001 |
| Age (years) | 40.5 ± 16.6 | 45.2 ± 14.4 | 48.6 ± 13.4 | 49.1 ± 13.3 | 46.2 ± 13.9 | <0.001 |
| Age group | <0.001 | |||||
| 20–29 | 52,975 (35.7%) | 262,941 (16.7%) | 85,034 (8.5%) | 83,743 (7.1%) | 16,923 (11.7%) | |
| 30–39 | 33,026 (22.3%) | 315,592 (20.0%) | 175,039 (17.5%) | 219,742 (18.6%) | 35,411 (24.5%) | |
| 40–49 | 23,966 (16.1%) | 424,880 (26.9%) | 272,812 (27.2%) | 309,454 (26.2%) | 34,747 (24.0%) | |
| 50–59 | 13,701 (9.2%) | 293,562 (18.6%) | 241,153 (24.1%) | 284,426 (24.1%) | 28,106 (19.5%) | |
| 60–69 | 10,819 (7.3%) | 171,096 (10.8%) | 151,983 (15.2%) | 194,509 (16.5%) | 20,081 (13.9%) | |
| 70–79 | 10,593 (7.1%) | 93,783 (5.9%) | 66,893 (6.7%) | 81,888 (6.9%) | 8526 (5.9%) | |
| 80– | 3380 (2.3%) | 17,799 (1.1%) | 8480 (0.9%) | 8636 (0.7%) | 724 (0.5%) | |
| Waist circumference (cm) | 66.2 ± 6.1 | 74.3 ± 6.8 | 81.2 ± 6.5 | 87.2 ± 6.5 | 96.8 ± 8.5 | <0.001 |
| Smoking | <0.001 | |||||
| Never-smoker | 104,399 (70.3%) | 1,009,981 (63.9%) | 569,292 (56.9%) | 641,966 (54.3%) | 81,957 (56.7%) | |
| Ex-smoker | 10,274 (6.9%) | 176,612 (11.2%) | 166,249 (16.6%) | 211,763 (17.9%) | 19,715 (13.6%) | |
| Current-smoker | 33,787 (22.8%) | 393,060 (24.9%) | 265,853 (26.6%) | 328,669 (27.8%) | 42,846 (29.7%) | |
| Alcohol consumption | <0.001 | |||||
| Non-drinker | 84,650 (57.0%) | 844,228 (53.4%) | 502,676 (50.2%) | 580,170 (49.1%) | 74,863 (51.8%) | |
| Mild-drinker | 57,034 (38.4%) | 635,906 (40.3%) | 415,595 (41.5%) | 483,904 (40.9%) | 54,251 (37.5%) | |
| Heavy-drinker | 6776 (4.6%) | 99,519 (6.3%) | 83,123 (8.3%) | 118,324 (10.0%) | 15,404 (10.7%) | |
| Regular exercise | 14,083 (9.5%) | 260,130 (16.5%) | 201,750 (20.2%) | 235,736 (19.9%) | 25,058 (17.3%) | <0.001 |
| Income (lowest 20%) | 26,986 (18.2%) | 285,175 (18.1%) | 170,960 (17.1%) | 198,438 (16.8%) | 26,103 (18.1%) | <0.001 |
| Diabetes mellitus | 5032 (3.4%) | 88,229 (5.6%) | 89,877 (9.0%) | 145,104 (12.3%) | 25,156 (17.4%) | <0.001 |
| Diabetes mellitus stage | <0.001 | |||||
| Non-diabetic | 123,405 (83.1%) | 1,201,431 (76.1%) | 672,234 (67.1%) | 710,909 (60.1%) | 76,152 (52.7%) | |
| Impaired fasting glucose | 20,023 (13.5%) | 289,993 (18.4%) | 239,283 (23.9%) | 326,385 (27.6%) | 43,210 (30.0%) | |
| New onset diabetes | 2145 (1.4%) | 30,453 (1.9%) | 29,509 (3.0%) | 49,482 (4.2%) | 8994 (6.2%) | |
| Diabetic < 5 years | 1339 (0.9%) | 25,901 (1.6%) | 29,589 (3.0%) | 52,562 (4.5%) | 10,096 (7.0%) | |
| Diabetic ≥ 5 years | 1548 (1.0%) | 31,875 (2.0%) | 30,779 (3.1%) | 43,060 (3.6%) | 6066 (4.2%) | |
| Glucose (mg/dL) | 90.9 ± 20.9 | 94.0 ± 21.3 | 97.9 ± 23.8 | 100.9 ± 25.7 | 104.9 ± 30.2 | <0.001 |
| Hypertension | 14,751 (9.9%) | 271,106 (17.2%) | 280,148 (28.0%) | 451,800 (38.2%) | 73,908 (51.1%) | <0.001 |
| Hypertension stage | <0.001 | |||||
| Non-hypertensive | 91,041 (61.3%) | 725,291 (45.9%) | 307,165 (30.7%) | 245,829 (20.8%) | 16,651 (11.5%) | |
| Pre-hypertension | 42,668 (28.7%) | 583,256 (36.9%) | 414,081 (41.4%) | 484,769 (41.0%) | 53,959 (37.3%) | |
| Hypertension | 5325 (3.6%) | 90,481 (5.7%) | 84,145 (8.4%) | 131,823 (11.2%) | 24,305 (16.8%) | |
| Hypertension with medication | 9426 (6.4%) | 180,625 (11.4%) | 196,003 (19.6%) | 319,977 (27.1%) | 49,603 (34.3%) | |
| Systolic blood pressure (mmHg) | 113.7 ± 14.1 | 118.6 ± 14.4 | 123.4 ± 14.4 | 126.8 ± 14.5 | 131.2 ± 15.2 | <0.001 |
| Diastolic blood pressure (mmHg) | 71.3 ± 9.3 | 74.0 ± 9.6 | 76.8 ± 9.7 | 79.0 ± 9.8 | 82.0 ± 10.5 | <0.001 |
| Dyslipidemia | 8133 (5.5%) | 187,002 (11.8%) | 196,442 (19.6%) | 300,990 (25.5%) | 45,026 (31.2%) | <0.001 |
| Dyslipidemia stage | <0.001 | |||||
| Total cholesterol < 240 (mg/dL) | 140,327 (94.5%) | 1,392,651 (88.2%) | 804,952 (80.4%) | 881,408 (74.5%) | 99,492 (68.8%) | |
| Total cholesterol ≥ 240 | 4679 (3.2%) | 95,287 (6.0%) | 92,427 (9.2%) | 136,280 (11.5%) | 19,999 (13.8%) | |
| Total cholesterol ≥ 240 with medication | 3454 (2.3%) | 91,715 (5.8%) | 104,015 (10.4%) | 164,710 (13.9%) | 25,027 (17.3%) | |
| Cholesterol (mg/dL) | 177.8 ± 35.1 | 188.8 ± 39.1 | 197.9 ± 41.4 | 202.6 ± 42.1 | 206.3 ± 42.1 | <0.001 |
| High-density lipoprotein (mg/dL) | 64.3 ± 37.7 | 59.7 ± 34.3 | 55.2 ± 30.4 | 53.0 ± 32.3 | 51.7 ± 29.6 | <0.001 |
| Low-density lipoprotein (mg/dL) | 115.8 ± 351.8 | 119.1 ± 252.9 | 122.3 ± 181.8 | 123.3 ± 158.1 | 124.6 ± 152.8 | <0.001 |
| Chronic kidney disease | 8198 (5.5%) | 95,435 (6.0%) | 71,342 (7.1%) | 92,362 (7.8%) | 11,257 (7.8%) | <0.001 |
| eGFR (mL/min/1.73 m2) | 93.1 ± 47.9 | 89.2 ± 44.7 | 86.5 ± 44.8 | 85.6 ± 44.6 | 87.0 ± 45.3 | <0.001 |
| ɣ-GTP * | 18.7 (18.7–18.8) | 21.3 (21.3–21.4) | 27.4 (27.4–27.4) | 33.8 (33.7–33.8) | 40.5 (40.3–40.6) | <0.001 |
| Waist Circumference (Male/Female; cm) | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| WC < 80/75 | 80/75 ≤ WC < 85/80 | 85/80 ≤ WC < 90/85 | 90/85 ≤ WC < 95/90 | 95/90 ≤ WC < 100/95 | 100/95 ≤ WC | ||
| 1,490,892 | 965,616 | 803,708 | 471,183 | 209,692 | 115,332 | ||
| Male | 660,775 (44.3%) | 598,889 (62.0%) | 499,969 (62.2%) | 293,667 (62.3%) | 120,066 (57.3%) | 60,165 (52.2%) | <0.001 |
| Age (years) | 42.4 ± 13.6 | 47.6 ± 13.2 | 50.2 ± 13.4 | 51.8 ± 13.6 | 52.7 ± 14.2 | 52.0 ± 15.3 | <0.001 |
| Age group | <0.001 | ||||||
| 20–29 | 318,255 (21.4%) | 88,707 (9.2%) | 49,348 (6.1%) | 24,681 (5.2%) | 11,448 (5.5%) | 9177 (8.0%) | |
| 30–39 | 335,158 (22.5%) | 187,253 (19.4%) | 133,262 (16.6%) | 72,134 (15.3%) | 31,459 (15.0%) | 19,544 (17.0%) | |
| 40–49 | 415,596 (27.9%) | 272,408 (28.2%) | 206,013 (25.6%) | 108,027 (22.9%) | 42,727 (20.4%) | 21,088 (18.3%) | |
| 50–59 | 242,128 (16.2%) | 225,760 (23.4%) | 202,887 (25.2%) | 117,630 (25.0%) | 48,855 (23.3%) | 23,688 (20.5%) | |
| 60–69 | 113,085 (7.6%) | 128,347 (13.3%) | 140,559 (17.5%) | 95,715 (20.3%) | 46,428 (22.1%) | 24,354 (21.1%) | |
| 70–79 | 55,993 (3.8%) | 55,007 (5.7%) | 63,160 (7.9%) | 46,864 (10.0%) | 25,367 (12.1%) | 15,292 (13.3%) | |
| 80– | 10,677 (0.7%) | 8134 (0.8%) | 8479 (1.1%) | 6132 (1.3%) | 3408 (1.6%) | 2189 (1.9%) | |
| Body mass index (kg/m2) | 21.1 ± 2.1 | 23.5 ± 1.9 | 25.0 ± 2.0 | 26.5 ± 2.2 | 28.0 ± 2.4 | 30.7 ± 3.3 | <0.001 |
| Smoking | <0.001 | ||||||
| Never-smoker | 986,822 (66.2%) | 532,393 (55.1%) | 440,387 (54.8%) | 257,592 (54.7%) | 120,894 (57.7%) | 69,507 (60.3%) | |
| Ex-smoker | 148,357 (10.0%) | 156,878 (16.3%) | 142,207 (17.7%) | 85,813 (18.2%) | 35,049 (16.7%) | 16,309 (14.1%) | |
| Current-smoker | 355,713 (23.9%) | 276,345 (28.6%) | 221,114 (27.5%) | 127,778 (27.1%) | 53,749 (25.6%) | 29,516 (25.6%) | |
| Alcohol consumption | <0.001 | ||||||
| Non-drinker | 793,738 (53.2%) | 470,369 (48.7%) | 402,489 (50.1%) | 240,337 (51.0%) | 113,560 (54.2%) | 66,094 (57.3%) | |
| Mild-drinker | 612,902 (41.1%) | 412,931 (42.8%) | 326,697 (40.7%) | 182,237 (38.7%) | 74,397 (35.5%) | 37,526 (32.5%) | |
| Heavy-drinker | 84,252 (5.7%) | 82,316 (8.5%) | 74,522 (9.3%) | 48,609 (10.3%) | 21,735 (10.4%) | 11,712 (10.2%) | |
| Regular exercise | 245,521 (16.5%) | 190,060 (19.7%) | 156,820 (19.5%) | 88,629 (18.8%) | 37,125 (17.7%) | 18,602 (16.1%) | <0.001 |
| Income (lowest 20%) | 276,206 (18.5%) | 162,310 (16.8%) | 133,818 (16.7%) | 78,807 (16.7%) | 36,187 (17.3%) | 20,334 (17.6%) | <0.001 |
| Diabetes mellitus | 54,778 (3.7%) | 75,069 (7.8%) | 89,473 (11.1%) | 69,195 (14.7%) | 38,400 (18.3%) | 26,483 (23.0%) | <0.001 |
| Diabetes mellitus stage | <0.001 | ||||||
| Non-diabetic | 1,183,986 (79.4%) | 665,077 (68.9%) | 502,496 (62.5%) | 268,100 (56.9%) | 109,731 (52.3%) | 54,741 (47.5%) | |
| Impaired fasting glucose | 252,128 (16.9%) | 225,470 (23.4%) | 211,739 (26.4%) | 133,888 (28.4%) | 61,561 (29.4%) | 34,108 (29.6%) | |
| New onset diabetes | 22,574 (1.5%) | 27,518 (2.9%) | 30,030 (3.7%) | 21,671 (4.6%) | 11,218 (5.4%) | 7572 (6.6%) | |
| Diabetic < 5 years | 15,676 (1.1%) | 23,454 (2.4%) | 30,162 (3.8%) | 24,940 (5.3%) | 14,740 (7.0%) | 10,515 (9.1%) | |
| Diabetic ≥ 5 years | 16,528 (1.1%) | 24,097 (2.5%) | 29,281 (3.6%) | 22,584 (4.8%) | 12,442 (5.9%) | 8396 (7.3%) | |
| Glucose (mg/dL) | 92.3 ± 18.7 | 97.1 ± 23.1 | 100.0 ± 25.6 | 102.5 ± 27.5 | 104.8 ± 29.8 | 108.0 ± 33.4 | <0.001 |
| Hypertension | 195,526 (13.1%) | 240,734 (24.9%) | 276,994 (34.5%) | 203,635 (43.2%) | 107,014 (51.0%) | 67,810 (58.8%) | <0.001 |
| Hypertension stage | <0.001 | ||||||
| Non-hypertensive | 746,795 (50.1%) | 314,141 (32.5%) | 196,915 (24.5%) | 86,969 (18.5%) | 29,483 (14.1%) | 11,674 (10.1%) | |
| Pre-hypertension | 548,571 (36.8%) | 410,741 (42.5%) | 329,799 (41.0%) | 180,579 (38.3%) | 73,195 (34.9%) | 35,848 (31.1%) | |
| Hypertension | 75,002 (5.0%) | 80,335 (8.3%) | 81,614 (10.2%) | 54,905 (11.7%) | 26,803 (12.8%) | 17,420 (15.1%) | |
| Hypertension with medication | 120,524 (8.1%) | 160,399 (16.6%) | 195,380 (24.3%) | 148,730 (31.6%) | 80,211 (38.3%) | 50,390 (43.7%) | |
| Systolic blood pressure (mmHg) | 117.3 ± 13.9 | 122.7 ± 14.3 | 125.4 ± 14.5 | 127.6 ± 14.8 | 129.5 ± 15.1 | 131.8 ± 15.7 | <0.001 |
| Diastolic blood pressure (mmHg) | 73.3 ± 9.5 | 76.5 ± 9.7 | 78.1 ± 9.8 | 79.3 ± 10.0 | 80.3 ± 10.2 | 81.7 ± 10.7 | <0.001 |
| Dyslipidemia | 142,044 (9.5%) | 170,261 (17.6%) | 187,884 (23.4%) | 130,610 (27.7%) | 66,440 (31.7%) | 40,354 (35.0%) | <0.001 |
| Dyslipidemia stage | <0.001 | ||||||
| Total cholesterol < 240 (mg/dL) | 1,348,848 (90.5%) | 795,355 (82.4%) | 615,824 (76.6%) | 340,573 (72.3%) | 143,252 (68.3%) | 74,978 (65.0%) | |
| Total cholesterol ≥ 240 | 80,387 (5.4%) | 85,808 (8.9%) | 85,795 (10.7%) | 54,982 (11.7%) | 26,340 (12.6%) | 15,360 (13.3%) | |
| Total cholesterol ≥ 240 with medication | 61,657 (4.1%) | 84,453 (8.8%) | 102,089 (12.7%) | 75,628 (16.1%) | 40,100 (19.1%) | 24,994 (21.7%) | |
| Cholesterol (mg/dL) | 186.6 ± 37.4 | 196.6 ± 40.8 | 201.1 ± 42.8 | 203.2 ± 43.3 | 204.9 ± 43.3 | 206.2 ± 44.2 | <0.001 |
| High-density lipoprotein (mg/dL) | 60.6 ± 33.7 | 55.8 ± 32.6 | 53.9 ± 31.9 | 52.6 ± 31.9 | 52.3 ± 31.9 | 52.2 ± 32.1 | <0.001 |
| Low-density lipoprotein (mg/dL) | 120.3 ± 292.0 | 120.6 ± 167.8 | 122.0 ± 142.0 | 122.5 ± 143.4 | 122.8 ± 132.4 | 123.2 ± 117.3 | <0.001 |
| Chronic kidney disease | 77,727 (5.2%) | 65,384 (6.8%) | 62,037 (7.7%) | 40,381 (8.6%) | 20,738 (9.9%) | 12,327 (10.7%) | < 0.001 |
| eGFR (mL/min/1.73 m2) | 90.1 ± 45.0 | 86.9 ± 43.9 | 86.0 ± 46.3 | 85.2 ± 44.6 | 84.7 ± 43.4 | 85.4 ± 44.8 | <0.001 |
| ɣ-GTP * | 20.1 (20.1–20.1) | 27.1 (27.1–27.2) | 31.2 (31.1–31.2) | 34.8 (34.7–34.9) | 36.6 (36.5–36.7) | 38.9 (38.7–39.0) | <0.001 |
| n | SCA | Follow-Up Duration (Person-Years) | Incidence | Hazard Ratio with 95% Confidence Interval | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate 1 | Multivariate 2 | Multivariate 3 | Multivariate 4 | Multivariate 5 | |||||
| BMI | ||||||||||
| BMI < 18.5 | 148,460 | 830 | 1,196,986 | 0.69 | 1.50 (1.40–1.61) | 1.70 (1.58–1.83) | 1.61 (1.49–1.73) | 1.78 (1.65–1.91) | 1.79 (1.66–1.92) | 1.79 (1.66–1.92) |
| 18.5 ≤ BMI < 23 | 1,579,653 | 6016 | 12,966,752 | 0.46 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 23 ≤ BMI < 25 | 1,001,394 | 3924 | 8,249,250 | 0.48 | 1.02 (0.98–1.07) | 0.85 (0.81–0.88) | 0.87 (0.84–0.91) | 0.80 (0.77–0.84) | 0.80 (0.77–0.83) | 0.78 (0.75–0.81) |
| 25 ≤ BMI < 30 | 1,182,398 | 4915 | 9,743,125 | 0.50 | 1.09 (1.05–1.13) | 0.90 (0.86–0.93) | 0.93 (0.90–0.97) | 0.80 (0.76–0.83) | 0.79 (0.76–0.82) | 0.74 (0.71–0.77) |
| 30 ≤ BMI | 144,518 | 667 | 1,189,264 | 0.56 | 1.21 (1.12–1.31) | 1.36 (1.25–1.47) | 1.39 (1.28–1.50) | 1.06 (0.97–1.14) | 1.05 (0.96–1.13) | 0.94 (0.87–1.02) |
| Waist circumference (male/female; cm) | ||||||||||
| <80/75 | 1,490,892 | 4286 | 12,283,975 | 0.35 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| –85/80 | 965,616 | 3665 | 7,945,666 | 0.46 | 1.32 (1.26–1.38) | 0.88 (0.84–0.92) | 0.90 (0.86–0.94) | 0.83 (0.79–0.87) | 0.83 (0.79–0.87) | 0.80 (0.76–0.83) |
| –90/85 | 803,708 | 3690 | 6,603,475 | 0.56 | 1.60 (1.53–1.67) | 0.91 (0.87–0.95) | 0.93 (0.89–0.97) | 0.81 (0.78–0.85) | 0.81 (0.77–0.85) | 0.76 (0.73–0.80) |
| –95/90 | 471,183 | 2520 | 3,859,942 | 0.65 | 1.87 (1.78–1.96) | 0.94 (0.90–0.99) | 0.97 (0.92–1.02) | 0.81 (0.77–0.85) | 0.80 (0.76–0.84) | 0.74 (0.70–0.77) |
| –100/95 | 209,692 | 1310 | 1,714,019 | 0.76 | 2.19 (2.06–2.33) | 1.07 (1.00–1.14) | 1.10 (1.03–1.17) | 0.87 (0.82–0.93) | 0.86 (0.81–0.92) | 0.78 (0.73–0.83) |
| ≥100/95 | 115,332 | 881 | 938,301 | 0.94 | 2.69 (2.50–2.89) | 1.39 (1.29–1.50) | 1.42 (1.32–1.53) | 1.05 (0.98–1.14) | 1.04 (0.96–1.12) | 0.92 (0.86–0.99) |
| Hazard Ratio with 95% Confidence Interval | p-Value | |
|---|---|---|
| Age (year) | 1.08 (1.08–1.08) | <0.001 |
| Sex | <0.001 | |
| Male | 2.35 (2.25–2.46) | |
| Female | 1 (reference) | |
| Smoking status | <0.001 | |
| Non-smoker | 1 (reference) | |
| Ex-smoker | 1.14 (1.09–1.20) | |
| Current-smoker | 1.81 (1.74–1.89) | |
| Alcohol consumption | <0.001 | |
| Non-drinker | 1 (reference) | |
| Mild-drinker | 0.77 (0.74–0.80) | |
| Heavy-drinker | 0.75 (0.71–0.79) | |
| Regular exercise | <0.001 | |
| No | 1 (reference) | |
| Yes | 0.89 (0.86–0.93) | |
| Income | <0.001 | |
| High | 1 (reference) | |
| Low | 1.09 (1.05–1.14) | |
| Hypertension | <0.001 | |
| Non-hypertension | 1 (reference) | |
| Pre-hypertension | 1.16 (1.11–1.21) | |
| Hypertension | 1.51 (1.44–1.59) | |
| Diabetes mellitus | <0.001 | |
| Non-DM | 1 (reference) | |
| IFG | 1.06 (1.02–1.10) | |
| DM | 1.74 (1.67–1.81) | |
| Dyslipidemia | <0.001 | |
| Total cholesterol < 240 (mg/dL) | 1 (reference) | |
| Total cholesterol ≥ 240 | 1.09 (1.03–1.15) | |
| Total cholesterol ≥ 240 with medication | 0.97 (0.93–1.01) | |
| Chronic kidney disease | 1.47 (1.41–1.53) | <0.001 |
| Waist circumference (cm; continuous) | 0.99 (0.99–1.00) | <0.001 |
| ɣ-GTP (unit; continuous) | 1.00 (1.00–1.00) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.G.; Jeong, J.H.; Roh, S.-Y.; Han, K.-D.; Choi, Y.Y.; Min, K.; Shim, J.; Choi, J.-I.; Kim, Y.-H. Obesity Is Indirectly Associated with Sudden Cardiac Arrest through Various Risk Factors. J. Clin. Med. 2023, 12, 2068. https://doi.org/10.3390/jcm12052068
Kim YG, Jeong JH, Roh S-Y, Han K-D, Choi YY, Min K, Shim J, Choi J-I, Kim Y-H. Obesity Is Indirectly Associated with Sudden Cardiac Arrest through Various Risk Factors. Journal of Clinical Medicine. 2023; 12(5):2068. https://doi.org/10.3390/jcm12052068
Chicago/Turabian StyleKim, Yun Gi, Joo Hee Jeong, Seung-Young Roh, Kyung-Do Han, Yun Young Choi, Kyongjin Min, Jaemin Shim, Jong-Il Choi, and Young-Hoon Kim. 2023. "Obesity Is Indirectly Associated with Sudden Cardiac Arrest through Various Risk Factors" Journal of Clinical Medicine 12, no. 5: 2068. https://doi.org/10.3390/jcm12052068
APA StyleKim, Y. G., Jeong, J. H., Roh, S.-Y., Han, K.-D., Choi, Y. Y., Min, K., Shim, J., Choi, J.-I., & Kim, Y.-H. (2023). Obesity Is Indirectly Associated with Sudden Cardiac Arrest through Various Risk Factors. Journal of Clinical Medicine, 12(5), 2068. https://doi.org/10.3390/jcm12052068






