Abstract
The second-to-four digit ratio (2D:4D) has been proposed as a marker of prenatal hormonal exposure. It is suggested that prenatal exposure to androgens results in a shorter 2D:4D ratio, whereas a prenatal oestrogenic environment results in a longer one. In addition, previous research has shown an association between exposure to endocrine-disrupting chemicals and 2D:4D in animals and humans. On the endometriosis side, hypothetically, a longer 2D:4D ratio, reflecting a lower androgenic intrauterine milieu, could represent an indicator of the presence of the disease. In this light, we have designed a case-control study to compare 2D:4D measurements between women with and without endometriosis. Exclusion criteria included the presence of PCOS and previous trauma on the hand that could impact the measurement of the digit ratio. The 2D:4D ratio of the right hand was measured using a digital calliper. A total of 424 participants (endometriosis n = 212; controls n = 212) were recruited. The group of cases included 114 women with endometriomas and 98 patients with deep infiltrating endometriosis. The 2D:4D ratio was significantly higher in women with endometriosis compared to controls (p = 0.002). There is an association between a higher 2D:4D ratio and the presence of endometriosis. Our results support the hypothesis claiming potential influences of intrauterine hormonal and endocrine disruptors exposure on the onset of the disease.
1. Introduction
Endometriosis is a chronic, oestrogen-dependent, inflammatory disease characterised by the presence of endometrium-like epithelium and/or stroma outside the endometrium and myometrium [1,2]. Endometriosis affects about 5% of women of reproductive age [1]. The pathogenesis of the disease is still to be precisely defined. However, recently, the potential intrauterine origin of endometriosis has been gaining consensus [3,4]. An intrauterine hormonal environment characterised by an imbalance in favour of a low androgenic milieu and exposure to endocrine disruptors may be a risk factor for developing endometriosis in adult life [5,6,7,8,9,10,11,12,13]. The second-to-four digit ratio (2D:4D) has been proposed as a marker of prenatal hormonal exposure [14]. The 2D:4D ratio is a sexually dimorphic feature and represents the ratio between the length of the index finger (2D) and the length of the ring finger (4D) [15,16]. In recent decades, numerous studies have been conducted to establish whether the 2D:4D ratio is a reliable indicator of the effects of prenatal sex hormones on the body and a predictor of the development of multiple disorders [17]. This sexual dimorphism in 2D:4D ratios is apparent by two years of age and seems to be established early in life, possibly by the 14th week of gestation [18]. It is suggested that prenatal exposure to androgens results in a lower 2D:4D ratio, whereas a prenatal oestrogenic environment results in a higher one [15]. In addition, previous research showed an association between exposure to endocrine-disrupting chemicals and 2D:4D in animals and humans [19,20,21,22].
Regarding the potential association between the 2D:4D ratio and female reproductive disorders, previous studies prevalently focused their attention on polycystic ovary syndrome (PCOS). Numerous studies have evaluated this anthropometric biomarker in women with PCOS with contradictory results [22,23,24,25,26]. Interestingly, endometriosis and PCOS are both characterised by an altered function of the female hypothalamic–pituitary–gonadal axis (HPG). The function of the HPG could be influenced by different levels of prenatal androgens [27]. In line with this theory, Crespi [28] suggested that endometriosis and PCOS are expected to show a pattern of opposite causes and phenotypes due to high prenatal androgens increasing the risk of PCOS and low prenatal androgens increasing the risk of endometriosis.
On the endometriosis side, hypothetically, a longer 2D:4D ratio, reflecting a lower androgenic intrauterine milieu, could represent an indicator of the presence of the disease [28]. Only one study [25] has investigated the 2D:4D ratio in women with endometriosis without identifying any difference between cases and controls. However, the study was underpowered for definite conclusions. On the other hand, a recent Israeli study [19] identified an association between a higher 2D:4D ratio and heavier menses bleeding and dysmenorrhea.
To gain insight into the potential intrauterine origin of endometriosis, we have designed a large case-control study to compare 2D:4D measurements between women with and without the disease.
2. Materials and Methods
This case-control study was conducted in the Fondazione Ca’ Granda Ospedale Maggiore Policlinico of Milano, which includes a tertiary referral centre for the study and management of endometriosis and its related infertility. Participants were recruited from July 2021 to October 2022. Cases included women with a past surgical diagnosis of endometriosis or with a current nonsurgical diagnosis of the disease. Nonsurgical diagnoses were based on previously published criteria [29,30,31,32]. Women with a history of superficial endometriosis (typically diagnosed at laparoscopy) were also excluded [33]. Women with endometriosis were subcategorised into two groups: deep endometriosis (DE) and ovarian endometrioma (OMA). The DE group included women with rectovaginal plaques, bowel lesions, intrinsic ureteral endometriosis, and deep endometriosis infiltrating the pouch of Douglas and parametria. Women with both DE and OMA were included in the group of DE, as the former lesions are considered more severe than the latter ones [34]. In the same period, women attending our outpatient clinics for periodic well-woman visits, contraception, severe male infertility, and cervical cancer screening programme and without a previous clinical or surgical diagnosis of endometriosis were enrolled as the control group. Endometriosis was excluded based on gynaecological history, pelvic transvaginal ultrasound, gynaecological bimanual examination, and visual inspection of the posterior vaginal fornix. Women reporting a previous trauma on the evaluated hand that could impact the measurement of the digit ratio were excluded from both study groups. Women with PCOS, according to the 2018 definition [35], were also excluded from both groups.
In women who agreed to participate, a resident in gynaecology measured the 2D:4D digit ratio of the right hand using a digital calliper (Borletti CDJB15 150-mm Digital Calliper). All measurements were made by only three residents. They were blinded to the condition of the woman when called for the measurement. The digit lengths were measured on the right hand’s ventral surface, from the digit’s basal crease to the finger’s tip in the midline (unit of measure millimeters) (Figure 1).

Figure 1.
The 2D:4D ratio is calculated by dividing the length of the index finger of a given hand by the length of the ring finger of the same hand.
The 2D:4D ratio was calculated by dividing the length of the index finger by the length of the ring finger. We decided to measure only the 2D:4D ratio on the right hand because previous studies suggested that the right hand is more sensitive to androgens [23,25]. In addition, data were collected on standardised forms, including demographic information and clinical characteristics.
The local Institutional Review Board (Comitato di Etica Milano Area B) approved the study (approval no. 980_2021bis). All participants provided written informed consent before starting the measurement of the biomarker.
The sample size (at least 150 women per group) was calculated setting type I and II errors and 0.05 and 0.20, expecting a 2D:4D ratio of 0.98 ± 0.03 in non-affected cases [25], and deeming interesting demonstrating that in women with endometriosis, the ratio could be more than 0.99. On these bases, we had to recruit about 150 women per group. However, given the relative weakness of the basic assumption used (regarding mean, SD, and distribution), we aimed for at least 200 women per group.
Data were analysed using the software Statistical Package for Social Sciences (SPSS 27.0, International Business Machines Corporation (IBM), IL, USA). All data were initially examined for normality using the Kolmogorov–Smirnov test: the normally distributed data were analysed with the Student’s t-test, while the non-normally distributed data were analysed with the Mann–Whitney test. The frequency of patients’ characteristics was compared with the Chi-square test. Data are presented as number (%), mean ± Standard Deviation (SD), and median interquartile range (IQR). p values below 0.05 were considered statistically significant.
3. Results
A total of 424 participants (endometriosis n = 212; controls n = 212) were recruited for this study. The group of cases included 114 women with OMAs and 98 patients with DE. The deep infiltrating endometriosis group comprised 50 patients with rectovaginal endometriotic plaques, 34 with deep lesions infiltrating the pouch of Douglas and parametria, 9 with full-thickness bowel lesions, and 5 with intrinsic ureteral endometriosis. In the endometriosis group, 79 participants (37%) had a surgical diagnosis of the disease. The characteristics of the 133 participants (63%) with a nonsurgical diagnosis are summarised in Table 1. One hundred-forty-eight (34.9%) women were seeking pregnancy (75 cases and 73 controls, respectively).

Table 1.
Characteristics of participants with a nonsurgical diagnosis of the disease.
The general characteristics of the participants are shown in Table 2. Although the median age in the endometriosis group was 37 years, significantly higher than controls (p < 0.01), the other variables did not differ between the study groups. Considering that the digit length does not modify during life except for very advanced age, the statistical difference in participants’ age should not be considered a bias in the study.

Table 2.
Distribution of baseline characteristics of women with endometriosis (n = 212) and controls (n = 212).
The right hand 2D:4D digit ratio was not normally distributed. Therefore, we opted for non-parametric statistics. The ratio was significantly higher in women with endometriosis compared to controls (Figure 2 and Table 3) (p = 0.002).
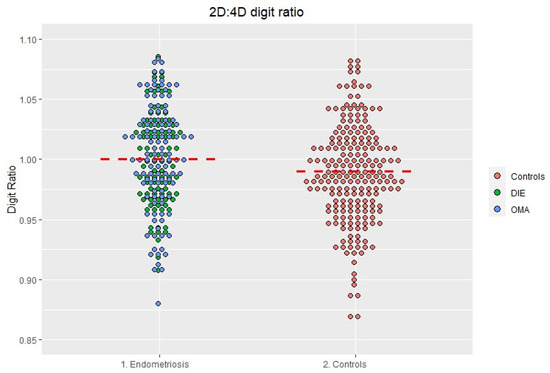
Figure 2.
Distribution of the 2D:4D ratio in the endometriosis and in the control group. The red line represents the median.

Table 3.
Comparison of right hand 2D:4D digit ratio of women with endometriosis and controls.
The significant association remained when exclusively focussing on women with OMAs (p = 0.002). In contrast, the association was no more significant when the analysis was restricted to women with DE forms (p = 0.07). In addition, the association was still not significant when analysing women with solely DE forms (i.e., excluding those who have associated forms OMA + DE; p = 0.08, Table 4). Finally, we performed a sub-analysis comparing women with a surgical diagnosis of endometriosis and controls (p = 0.001; Table 4).

Table 4.
Comparison of right hand 2D:4D digit ratio of women with surgical diagnosis of endometriosis and controls and with different subtypes of the disease and controls.
4. Discussion
In the present study, the 2D:4D ratio was significantly higher in the endometriosis group, particularly in women with OMAs. The association remained when exclusively focussing on women with OMAs but was lost when focussing on those with DE. We interpreted this latter finding as a type II error. The type II error could be explained by the small numerosity of the sample size and could be solved by increasing the number of women with DE.
Overall, the results of our study support the tested hypothesis (i.e.,: longer 2D:4D in the endometriosis group).
Endometriosis is a complex disease with undefined pathophysiology involving genetic factors and environmental influences [36]. Recent findings suggest that the disease may originate due to endocrine exposure during intrauterine life [37]. A low ratio of testosterone-to-estradiol during foetal life may play a crucial role in endometriosis onset and progression [4,28,37]. Of relevance here is that previous studies have already demonstrated an association between the disease and specific phenotypic characteristics, particularly in women with deep endometriosis forms [8,38,39]. For example, an association with pigmentary traits, i.e., higher numbers of naevi and freckles and the presence of blue eyes have been documented [8,38]. Furthermore, women with endometriosis had a lower body mass index (BMI) and waist-to-hip ratio (WHR) compared to women without the disease [40,41,42,43,44]. All the above findings support the potential role of oestrogen in the pathogenesis of the disease, already from the intrauterine life. Our findings also support the recent hypothesis of a group of evolutionary biologists who were persuaded that endometriosis could derive from a lower intrauterine exposure to androgens [28]. Evidence in favour of this theory is, however, not univocal. Another biomarker of intrauterine exposure to sex steroids or endocrine disruptors is represented by anogenital distance (AGD). A shorter AGD, the distance measured from the anus to the genital tubercle [10], has been linked to a more oestrogenic uterine milieu, and to a higher endometriosis risk, particularly with deep infiltrating forms [45,46,47,48]. Notably, in a previous study, we were not able to confirm this association [49].
To our knowledge, only one study [25] has previously evaluated the potential association between 2D:4D and endometriosis. Peters et al. [25] evaluated both AGD and the 2D:4D digit ratio in 172 women (endometriosis n = 43; Mayer–Rokitansky–Kuster-Hauser syndrome n = 43; PCOS n = 43; controls n = 43). The authors observed an association between a shorter AGD and the presence of endometriosis, whereas the digit ratio did not differ between the groups. The study was, however, underpowered for definite conclusions [28]. In 2020 [19], an Israelian study on 187 pregnant women revealed an association between a higher digit ratio and heavier menses bleeding and dysmenorrhea, two of the most frequently reported symptoms in endometriosis patients.
Strengths of our study include the large sample size and a direct measure of digit length, as an indirect measure may distort the 2D:4D ratio [19,50,51]. As for any case-control study, the choice of controls may represent a source of bias. In the present study, endometriosis was ruled out based on gynaecological and ultrasonographic examination. Therefore, we cannot exclude having inadvertently included some cases among controls. However, the prevalence of asymptomatic disease in the general population is modest, and misdiagnosis should be more likely for superficial peritoneal forms, a condition of uncertain clinical value [1,33]. In addition, we decided to enrol both women with a surgical and sonographic diagnosis of endometriosis. One could argue that in the latter group, a definitive diagnosis based on histological findings is lacking. However, as recently suggested by ESHRE guidelines [52], diagnostic laparoscopy should no longer be used as the first-line approach in the diagnosis of the disease. The use of imaging techniques (i.e., ultrasonography) has been repeatedly demonstrated to be highly accurate and reliable [53,54,55].
Our results provide evidence of a potential association between a biomarker of the hormonal prenatal environment in women and the presence of endometriosis. Our findings, if confirmed, could serve as predictors of the disease and have implications for endometriosis in terms of prevention and clinical practice.
In conclusion, there is an association between a higher 2D:4D ratio and the presence of endometriosis. Our results could suggest the hypothesis claiming potential influences of intrauterine hormonal and endocrine disruptors exposure on the onset of the disease. However, further evidence is needed to replicate our results and to explore further this fascinating pathogenic hypothesis.
Author Contributions
Conceptualisation, L.B. (Laura Buggio) and L.B. (Laura Benaglia); methodology, L.B. (Laura Benaglia) and P.V.; validation, L.B. (Laura Buggio) and D.D.; formal analysis, L.B. (Laura Buggio) and M.R.; investigation, F.G., G.G. and A.C.; resources, M.R.; data curation, M.R.; writing—original draft preparation, L.B. (Laura Buggio); writing—review and editing, E.S., D.D., L.B. (Laura Buggio) and F.G.; visualisation, L.B. (Laura Benaglia); supervision, P.V. and E.S.; project administration, L.B. (Laura Buggio) and D.D.; funding acquisition, L.B. (Laura Benaglia). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health (ENDO-2021-12371933) following peer review in the competitive grant “Bando Endometriosi 2021”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and the local Institutional Review Board (Comitato Etico Milano Area B) approved the study (approval no. 980_2021bis).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study before starting the measurement of the biomarker.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Vercellini, P.; Viganò, P. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 29, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, C.; Johnson, N.P.; Petrozza, J.; Abrao, M.S.; I Einarsson, J.; Horne, A.W.; Lee, T.T.M.; Missmer, S.; Vermeulen, N.; Zondervan, K.T.; et al. An international terminology for endometriosis, 2021. Hum. Reprod. Open 2021, 2021, hoab029. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Ottolina, J.; Schimberni, M. Early-life factors, in-utero exposures and endometriosis risk: A meta-analysis. Reprod. Biomed. Online 2020, 41, 279–289. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Hediger, M.L. Intrauterine exposures and risk of endometriosis. Hum. Reprod. 2007, 22, 3232–3236. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M.; Peterson, C.M. Bisphenol A and phthalates and endometriosis: The Endometriosis: Natural History, Diagnosis and Outcomes Study. Fertil. Steril. 2013, 100, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Rizner, T.L. Estrogen metabolism and action in endometriosis. Mol. Cell. Endocrinol. 2009, 307, 8–18. [Google Scholar] [CrossRef]
- Somigliana, E.; Viganò, P. Perinatal environment and endometriosis. Gynecol. Obstet. Investig. 2011, 72, 135–140. [Google Scholar] [CrossRef]
- Wolff, E.F.; Sun, L. In utero exposures and endometriosis: The Endometriosis, Natural History, Disease, Outcome (ENDO) Study. Fertil. Steril. 2013, 99, 790–795. [Google Scholar] [CrossRef]
- Mendiola, J.; Sánchez-Ferrer, M.L. Endometriomas and deep infiltrating endometriosis in adulthood are strongly associated with anogenital distance, a biomarker for prenatal hormonal environment. Hum. Reprod. 2016, 31, 2377–2383. [Google Scholar] [CrossRef]
- Hediger, M.L.; Hartnett, H.J. Association of endometriosis with body size and figure. Fertil. Steril. 2005, 84, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, S.; Lazzeri, L. Potential influence of in utero and early neonatal exposures on the later development of endometriosis. Fertil. Steril. 2016, 105, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sancho, G.; Ploteau, S. Human epidemiological evidence about the associations between exposure to organochlorine chemicals and endometriosis: Systematic review and meta-analysis. Environ. Int. 2019, 123, 209–223. [Google Scholar] [CrossRef]
- Lutchmaya, S.; Baron-Cohen, S.; Raggatt, P.; Knickmeyer, R.; Manning, J. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum. Dev. 2004, 77, 23–28. [Google Scholar] [CrossRef]
- Manning, J.T.; Scutt, D. The ratio of 2nd to 4th digit length: A predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum. Reprod. 1998, 13, 3000–3004. [Google Scholar] [CrossRef] [PubMed]
- Galis, F.; Ten Broek, C.M. Sexual dimorphism in the prenatal digit ratio (2D:4D). Arch. Sex. Behav. 2010, 39, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A.; Eker, H. Is there a correlation between the second to fourth digit ratio and vitiligo? A cross-sectional study. J. Cosmet. Dermatol. 2022, 21, 3146–3151. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T. Is the Second to Fourth Digit Ratio (2D:4D) a Biomarker of Sex-Steroids Activity? Pediatr. Endocrinol. Rev. 2017, 14, 378–386. [Google Scholar] [CrossRef]
- Tabachnik, M.; Sheiner, E. The association between second to fourth digit ratio, reproductive and general health among women: Findings from an Israeli pregnancy cohort. Sci. Rep. 2020, 10, 6341. [Google Scholar] [CrossRef]
- Wainstock, T.; Pearce, B. Exposure to PBB-153 and Digit Ratio. Early Hum. Dev. 2016, 103, 33–35. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, M. Effects of prenatal exposure to polybrominated diphenyl ethers (PBDEs) on the second to fourth digit ratio in children aged 4 years. Int. J. Hyg. Environ. Health 2021, 231, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Moriya, K. Association of exposure to prenatal phthalate esters and bisphenol A and polymorphisms in the ESR1 gene with the second to fourth digit ratio in school-aged children: Data from the Hokkaido study. Steroids 2020, 159, 108637. [Google Scholar] [CrossRef]
- Cattrall, F.R.; Vollenhoven, B.J. Anatomical evidence for in utero androgen exposure in women with polycystic ovary syndrome. Fertil. Steril. 2005, 84, 1689–1692. [Google Scholar] [CrossRef]
- Lujan, M.E.; Bloski, T.G. Digit ratios do not serve as anatomical evidence of prenatal androgen exposure in clinical phenotypes of polycystic ovary syndrome. Hum. Reprod. 2010, 25, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.E.; Laeven, C.H.C. Anthropometric biomarkers for abnormal prenatal reproductive hormone exposure in women with Mayer-Rokitanksy-Küster-Hauser syndrome, polycystic ovary syndrome, and endometriosis. Fertil. Steril. 2020, 114, 1297–1305. [Google Scholar] [CrossRef]
- Deepika, V.; Preethy, P. Evaluation of Body Fat Composition and Digit Ratio (2D:4D) in Polycystic Ovary Syndrome in Adolescents. Curr. Health Sci. J. 2021, 47, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, N.L.; Crespi, B.J. Endometriosis and polycystic ovary syndrome are diametric disorders. Evol. Appl. 2021, 14, 1693–1715. [Google Scholar] [CrossRef]
- Crespi, B. Variation among human populations in endometriosis and PCOS A test of the inverse comorbidity model. Evol. Med. Public Health 2021, 9, 295–310. [Google Scholar] [CrossRef]
- Eskenazi, B.; Warner, M. Validation study of nonsurgical diagnosis of endometriosis. Fertil. Steril. 2001, 76, 929–935. [Google Scholar] [CrossRef]
- Vercellini, P.; Bracco, B. Norethindrone acetate or dienogest for the treatment of symptomatic endometriosis: A before and after study. Fertil. Steril. 2016, 105, 734–743. [Google Scholar] [CrossRef]
- Vercellini, P.; Ottolini, F. Is Shifting to a Progestin Worthwhile When Estrogen-Progestins Are Inefficacious for Endometriosis-Associated Pain? Reprod. Sci. 2018, 25, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Somigliana, E. “You can’t always get what you want”: From doctrine to practicability of study designs for clinical investigation in endometriosis. BMC Womens Health 2015, 22, 89. [Google Scholar] [CrossRef]
- Holt, V.L.; Weiss, N.S. Recommendations for the design of epidemiologic studies of endometriosis. Epidemiology 2000, 11, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Chopin, N. Deeply infiltrating endometriosis: Pathogenetic implications of the anatomical distribution. Hum. Reprod. 2006, 21, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulou, L.; Matalliotakis, M. Defining the genetic profile of endometriosis. Exp. Ther. Med. 2019, 17, 3267–3281. [Google Scholar] [CrossRef] [PubMed]
- Smarr, M.M.; Kannan, K. Endocrine disrupting chemicals and endometriosis. Fertil. Steril. 2016, 106, 959–966. [Google Scholar] [CrossRef]
- Vercellini, P.; Buggio, L. ‘Behind blue eyes’†: The association between eye colour and deep infiltrating endometriosis. Hum. Reprod. 2014, 29, 2171–2175. [Google Scholar] [CrossRef]
- Viganò, P.; Somigliana, E. Principles of phenomics in endometriosis. Hum. Reprod. Updat. 2012, 18, 248–259. [Google Scholar] [CrossRef]
- Lafay Pillet, M.C.; Schneider, A. Deep infiltrating endometriosis is associated with markedly lower body mass index: A 476 case-control study. Hum. Reprod. 2012, 27, 265–272. [Google Scholar] [CrossRef]
- Backonja, U.; Buck Louis, G.M. Overall Adiposity, Adipose Tissue Distribution, and Endometriosis: A Systematic Review. Nurs. Res. 2016, 65, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Backonja, U.; Hediger, M.L. Beyond Body Mass Index: Using Anthropometric Measures and Body Composition Indicators to Assess Odds of an Endometriosis Diagnosis. J. Womens Health 2017, 26, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W. Association between body mass index and endometriosis risk: A meta-analysis. Oncotarget 2017, 8, 46928–46936. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Peterson, C.M. Adiposity and Endometriosis Severity and Typology. J. Minim. Invasive Gynecol. 2020, 27, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ferrer, M.L.; Mendiola, J. Investigation of anogenital distance as a diagnostic tool in endometriosis. Reprod. Biomed. Online 2017, 34, 375–382. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, M.L.; Jiménez-Velázquez, R. Accuracy of anogenital distance and anti-Müllerian hormone in the diagnosis of endometriosis without surgery. Int. J. Gynecol. Obstet. 2019, 144, 90–96. [Google Scholar] [CrossRef]
- Crestani, A.; Arfi, A. Anogenital distance in adult women is a strong marker of endometriosis: Results of a prospective study with laparoscopic and histological findings. Hum. Reprod. Open 2020, 2020, hoaa023. [Google Scholar] [CrossRef]
- Crestani, A.; Abdel Wahab, C. A short anogenital distance on MRI is a marker of endometriosis. Hum. Reprod. Open 2021, 2021, hoab003. [Google Scholar] [CrossRef]
- Buggio, L.; Somigliana, E. Anogenital Distance and Endometriosis: Results of a Case-Control Study. Reprod. Sci. 2022, 29, 3508–3515. [Google Scholar] [CrossRef]
- Auger, J.; Eustache, F. Second to fourth digit ratios, male genital development and reproductive health: A clinical study among fertile men and testis cancer patients. Int. J. Androl. 2011, 34, 49–58. [Google Scholar] [CrossRef]
- Ribeiro, E., Jr.; Neave, N. Digit ratio (2D:4D), testosterone, cortisol, aggression, personality and hand-grip strength: Evidence for prenatal effects on strength. Early Hum. Dev. 2016, 100, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Bokor, A. ESHRE Endometriosis Guideline Group. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, S.; Saba, L. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 586–595. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2, CD009591. [Google Scholar] [CrossRef]
- Leonardi, M.; Espada, M. Transvaginal Ultrasound Can Accurately Predict the American Society of Reproductive Medicine Stage of Endometriosis Assigned at Laparoscopy. J. Minim. Invasive Gynecol. 2020, 27, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).