Relationship of Extravascular Lung Water and Pulmonary Vascular Permeability to Respiratory Mechanics in Patients with COVID-19-Induced ARDS
Abstract
1. Introduction
2. Materials and Methods
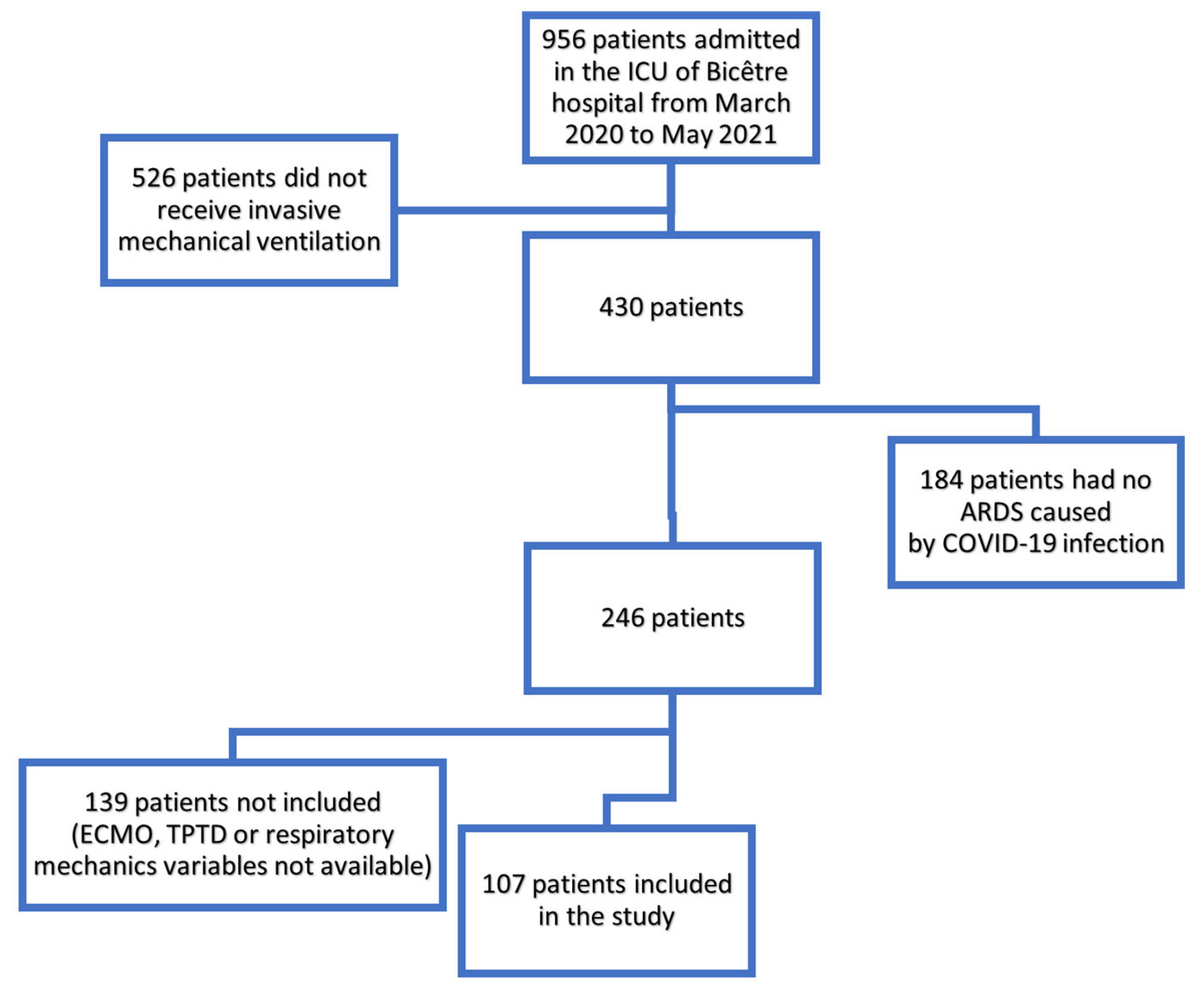
2.1. Study Design and Patients
2.2. Data Collection
2.3. Data Quality Control
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Outcome
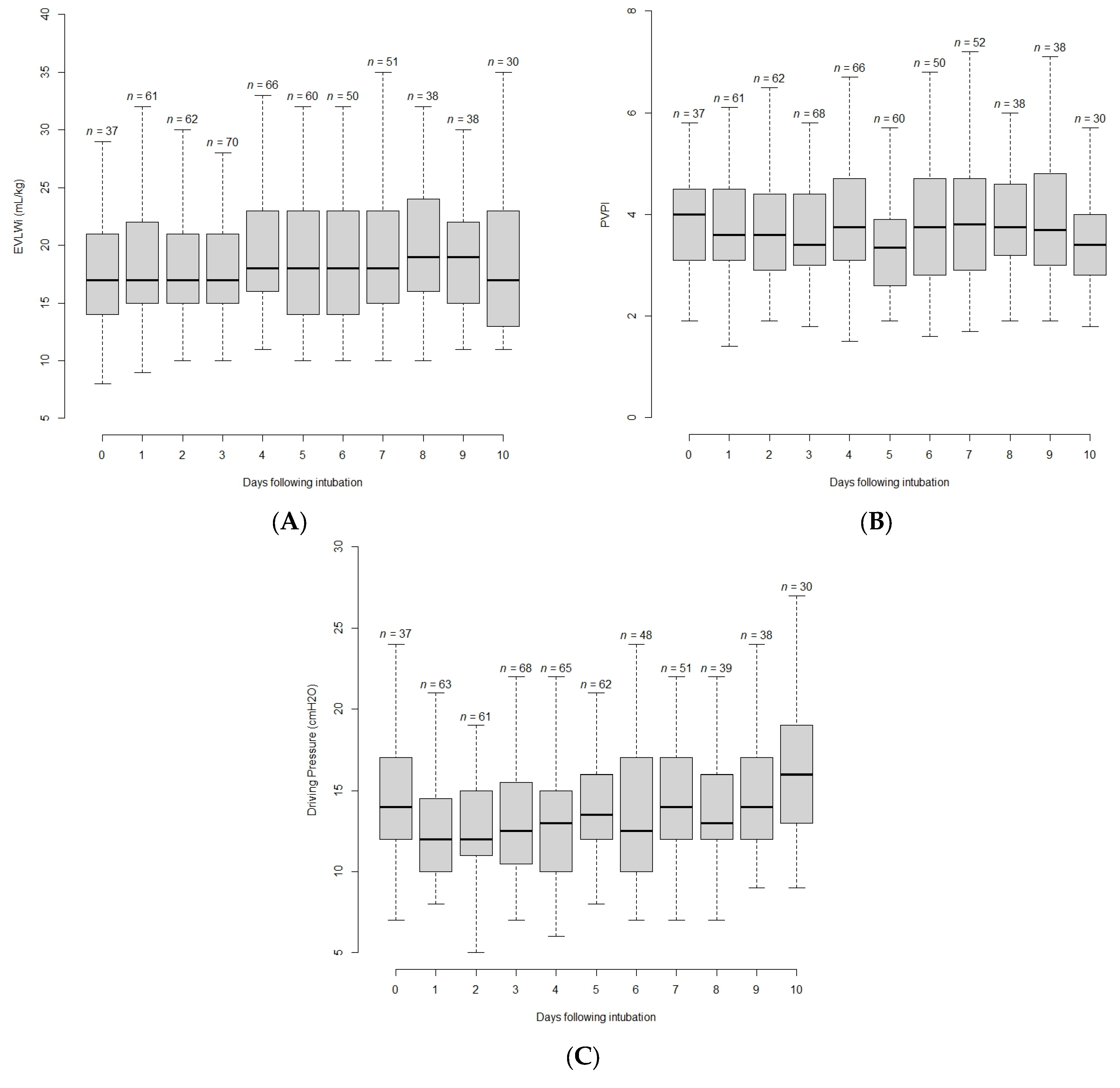
3.2. Evolution of Variables with Time
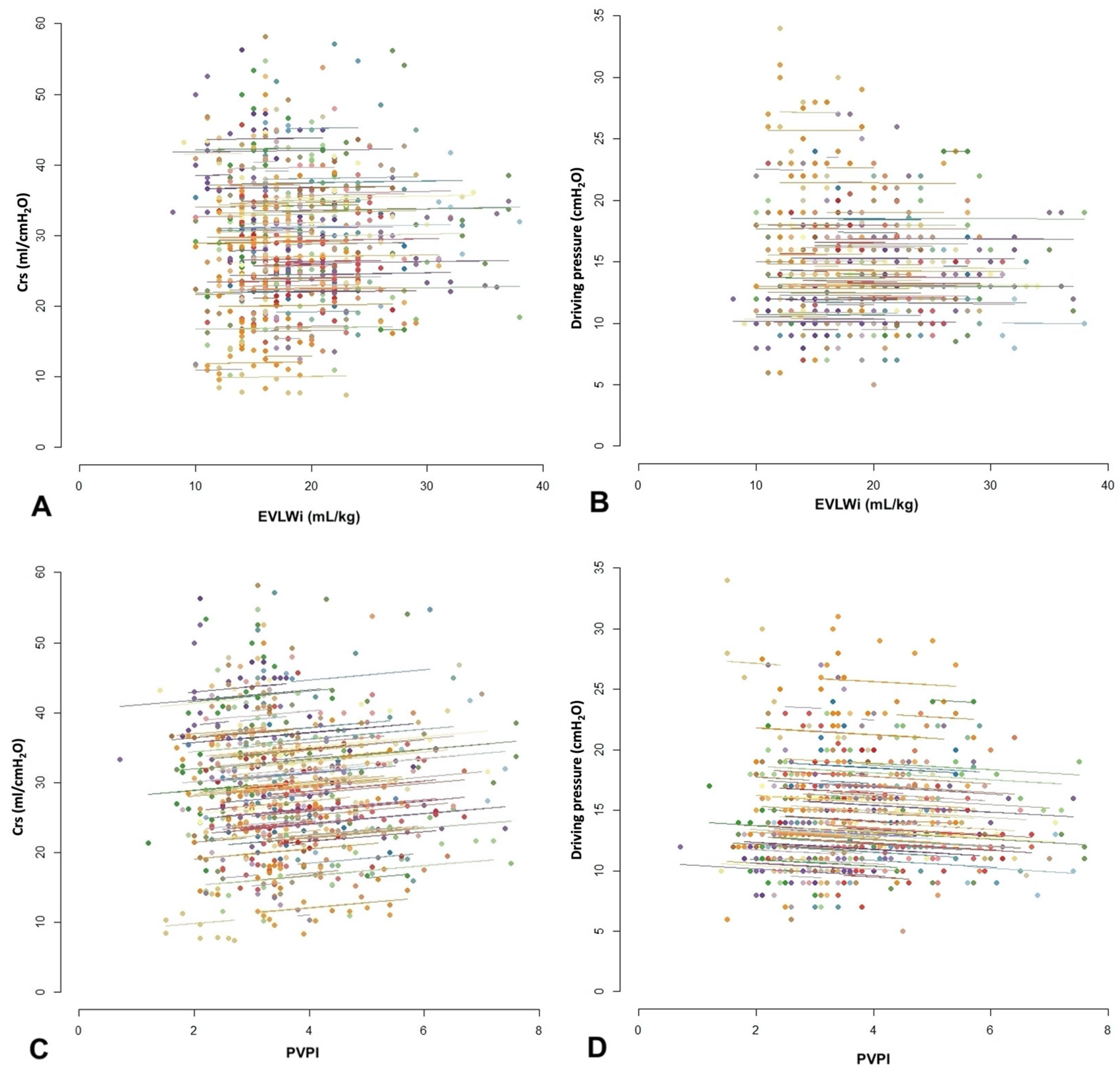
3.3. Correlation between EVLWi or PVPI and Respiratory Mechanics Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARDS | acute respiratory distress syndrome |
| COVID-19 | coronavirus disease 2019 |
| Crs | compliance of the respiratory system |
| EVLWi | extra-vascular lung water indexed for ideal body weight |
| FiO2 | inspired oxygen fraction |
| ICU | intensive care unit |
| PaO2 | arterial partial pressure of oxygen |
| PEEP | positive end-expiratory pressure |
| Pplat | plateau pressure |
| PVPI | pulmonary vascular permeability index |
| SAPS | simplified acute physiology score |
| SOFA | sepsis-related organ failure assessment |
| TPTD | transpulmonary thermodilution |
References
- Matthay, M.A.; Folkesson, H.G.; Clerici, C. Lung Epithelial Fluid Transport and the Resolution of Pulmonary Edema. Physiol. Rev. 2002, 82, 569–600. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.P. What is acute lung injury? What is ARDS? Chest 1995, 107, 1721–1726. [Google Scholar] [CrossRef]
- Tagami, T.; Sawabe, M.; Kushimoto, S.; Marik, P.E.; Mieno, M.N.; Kawaguchi, T.; Kusakabe, T.; Tosa, R.; Yokota, H.; Fukuda, Y. Quantitative Diagnosis of Diffuse Alveolar Damage Using Extravascular Lung Water. Crit. Care Med. 2013, 41, 2144–2150. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Kushimoto, S.; Yamamoto, Y.; Atsumi, T.; Tosa, R.; Matsuda, K.; Oyama, R.; Kawaguchi, T.; Masuno, T.; Hirama, H.; et al. Validation of extravascular lung water measurement by single transpulmonary thermodilution: Human autopsy study. Crit. Care 2010, 14, R162. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, M.; Teboul, J.-L.; Monnet, X. Extravascular lung water in critical care: Recent advances and clinical applications. Ann. Intensiv. Care 2015, 5, 38. [Google Scholar] [CrossRef]
- Gavelli, F.; Shi, R.; Teboul, J.-L.; Azzolina, D.; Mercado, P.; Jozwiak, M.; Chew, M.S.; Huber, W.; Kirov, M.Y.; Kuzkov, V.V.; et al. Extravascular lung water levels are associated with mortality: A systematic review and meta-analysis. Crit. Care 2022, 26, 202. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-Induced Lung Injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef]
- Amato, M.B.P.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.V.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.S.; Mercat, A.; et al. Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef]
- Gattinoni, L.; Pesenti, A. The concept of “baby lung”. Intensiv. Care Med. 2005, 31, 776–784. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 2019, 5, 18. [Google Scholar] [CrossRef]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Schuller, D.; Calandrino, F.S.; Schuster, D.P. Improved Outcome Based on Fluid Management in Critically III Patients Requiring Pulmonary Artery Catheterization. Am. Rev. Respir. Dis. 1992, 145, 990–998. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann, H.P.; Wheeler, A.P.; Bernard, G.R.; Thompson, B.T.; Hayden, D.; Deboisblanc, B.; Connors, A.F.J.; Hite, R.D.; Harabin, A.L. Comparison of Two Fluid-Management Strategies in Acute Lung Injury. N. Engl. J. Med. 2006, 354, 2564–2575. [Google Scholar] [CrossRef]
- Craig, T.R.; Duffy, M.J.; Shyamsundar, M.; McDowell, C.; McLaughlin, B.; Elborn, J.; McAuley, D. Extravascular lung water indexed to predicted body weight is a novel predictor of intensive care unit mortality in patients with acute lung injury. Crit. Care Med. 2010, 38, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Mallat, J.; Pepy, F.; Lemyze, M.; Barrailler, S.; Gasan, G.; Tronchon, L.; Thevenin, D. Extravascular lung water indexed or not to predicted body weight is a predictor of mortality in septic shock patients. J. Crit. Care 2012, 27, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kuzkov, V.; Kirov, M.; Sovershaev, M.A.; Kuklin, V.; Suborov, E.V.; Waerhaug, K.; Bjertnaes, L.J. Extravascular lung water determined with single transpulmonary thermodilution correlates with the severity of sepsis-induced acute lung injury. Crit. Care Med. 2006, 34, 1647–1653. [Google Scholar] [CrossRef]
- Zhang, F.; Li, C.; Zhang, J.-N.; Guo, H.-P.; Wu, D.-W. Comparison of quantitative computed tomography analysis and single-indicator thermodilution to measure pulmonary edema in patients with acute respiratory distress syndrome. Biomed. Eng. Online 2014, 13, 30. [Google Scholar] [CrossRef]
- Nirmalan, M.; Niranjan, M.; Willard, T.; Edwards, J.D.; Little, R.A.; Dark, P.M. Estimation of errors in determining intrathoracic blood volume using thermal dilution in pigs with acute lung injury and haemorrhage. Br. J. Anaesth. 2004, 93, 546–551. [Google Scholar] [CrossRef]
- Li, W.; Xue, Q.; Liu, K.; Hong, J.; Xu, J.; Wu, L.; Ji, G.; Wang, Z.; Zhang, Y. Effects of MIAVS on Early Postoperative ELWI and Respiratory Mechanics. Med. Sci. Monit. 2016, 22, 1085–1092. [Google Scholar] [CrossRef]
- The ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.; Zollner, C.; Manert, W.; Briegel, J.; Kilger, E.; Polasek, J.; Hummel, T.; Forst, H.; Peter, K. Thermodilution cardiac output may be incorrect in patients on venovenous extracorporeal lung assist. Am. J. Respir. Crit. Care Med. 1995, 152, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Teboul, J.-L. Transpulmonary thermodilution: Advantages and limits. Crit. Care 2017, 21, 147. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Persichini, R.; Ktari, M.; Jozwiak, M.; Richard, C.; Teboul, J.-L. Precision of the transpulmonary thermodilution measurements. Crit. Care 2011, 15, R204. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Stelfox, H.T.; Johnson, J.; McDermid, R.C.; Rolfson, D.B.; Tsuyuki, R.T.; Ibrahim, Q.; Majumdar, S.R. Long-Term Association Between Frailty and Health-Related Quality of Life Among Survivors of Critical Illness. Crit. Care Med. 2015, 43, 973–982. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistics notes: Calculating correlation coefficients with repeated observations: Part 1—Correlation within subjects. BMJ 1995, 310, 446. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistics notes: Calculating correlation coefficients with repeated observations: Part 2—Correlation between subjects. BMJ 1995, 310, 633. [Google Scholar] [CrossRef]
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef]
- Noble, W.H.; Kay, J.C.; Obdrzalek, J. Lung mechanics in hypervolemic pulmonary edema. J. Appl. Physiol. 1975, 38, 681–687. [Google Scholar] [CrossRef]
- Snapper, J.R. Lung Mechanics in Pulmonary Edema. Clin. Chest Med. 1985, 6, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Fishel, R.S.; Are, C.; Barbul, A. Vessel injury and capillary leak. Crit. Care Med. 2003, 31, S502–S511. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.O.; Bräu, N.; Waters, M.; Go, R.C.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Aziz, M.S.; Cooper, N.; Douglas, I.S.; et al. Tocilizumab in Hospitalized Patients with Severe COVID-19 Pneumonia. N. Engl. J. Med. 2021, 384, 1503–1516. [Google Scholar] [CrossRef]
- Chang, R.; Elhusseiny, K.M.; Yeh, Y.-C.; Sun, W.-Z. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes—A systematic review and meta-analysis. PLoS ONE 2021, 16, e0246318. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated with Mortality Among Patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef]
- Chiumello, D.; Busana, M.; Coppola, S.; Romitti, F.; Formenti, P.; Bonifazi, M.; Pozzi, T.; Palumbo, M.M.; Cressoni, M.; Herrmann, P.; et al. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: A matched cohort study. Intensive Care Med. 2020, 46, 2187–2196. [Google Scholar] [CrossRef]
- Ferrando, C.; Aguilar, G.; Piqueras, L.; Soro, M.; Moreno, J.; Belda, F.J. Sevoflurane, but not propofol, reduces the lung inflammatory response and improves oxygenation in an acute respiratory distress syndrome model: A randomised laboratory study. Eur. J. Anaesthesiol. 2013, 30, 455–463. [Google Scholar] [CrossRef]
- Shi, R.; Lai, C.; Teboul, J.-L.; Dres, M.; Moretto, F.; De Vita, N.; Pham, T.; Bonny, V.; Mayaux, J.; Vaschetto, R.; et al. COVID-19 ARDS is characterized by higher extravascular lung water than non-COVID-19 ARDS: The PiCCOVID study. Crit. Care 2021, 25, 186. [Google Scholar] [CrossRef]
- Pham, T.; Telias, I.; Beitler, J.R. Esophageal Manometry. Respir. Care 2020, 65, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Van Mourik, N.; Metske, H.A.; Hofstra, J.J.; Binnekade, J.M.; Geerts, B.F.; Schultz, M.J.; Vlaar, A.P.J. Cumulative fluid balance predicts mortality and increases time on mechanical ventilation in ARDS patients: An observational cohort study. PLoS ONE 2019, 14, e0224563. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Patients n = 107 Mean ± SD or n (%) | Non-Survivors n = 62 Mean ± SD or n (%) | Survivors n = 45 Mean ± SD or n (%) | p-Value | n |
|---|---|---|---|---|---|
| Age, years | 64 ± 11 | 64 ± 11 | 64 ± 12 | 0.993 | 107 |
| Male | 82 (76.6%) | 50 (80.6%) | 32 (71.1%) | 0.358 | 107 |
| Height, cm | 171 ± 9 | 171 ± 9 | 171 ± 10 | 0.981 | 107 |
| Weight, kg | 84.0 ± 15.1 | 83.5 ± 15.3 | 84.6 ± 15.0 | 0.714 | 105 |
| BMI, kg/m2 | 28.6 ± 4.9 | 28.5 ± 5.3 | 28.7 ± 4.4 | 0.831 | 105 |
| Obesity, BMI > 30 kg/m2 | 33 (30.8%) | 19 (30.6%) | 14 (31.1%) | 1.000 | 105 |
| SOFA Score | 5.3 ± 3.2 | 5.5 ± 3.5 | 5.2 ± 2.7 | 0.425 | 107 |
| SAPS II Score | 38.6 ± 15.1 | 40.6 ± 16.6 | 36.0 ± 12.6 | 0.113 | 105 |
| Frailty score | 4 (3.7%) | 2 (3.2%) | 2 (4.4%) | 1.000 | 107 |
| Alcohol abuse | 6 (5.6%) | 3 (4.8%) | 3 (6.7%) | 0.694 | 107 |
| Smoking | 5 (4.7%) | 1 (1.6%) | 4 (8.9%) | 0.159 | 107 |
| COPD | 8 (7.5%) | 4 (6.5%) | 4 (8.9%) | 0.718 | 107 |
| Asthma | 9 (8.4%) | 4 (6.5%) | 5 (11.1%) | 0.488 | 107 |
| Hypertension | 53 (49.5%) | 33 (53.2%) | 20 (44.4%) | 0.483 | 107 |
| Diabetes mellitus | 34 (31.8%) | 21 (33.9%) | 13 (28.9%) | 0.737 | 107 |
| Chronic heart failure | 2 (1.9%) | 2 (3.2%) | 0 (0.0%) | 0.508 | 107 |
| Chronic kidney disease | 13 (12.1%) | 8 (12.9%) | 5 (11.1%) | 1.000 | 107 |
| Liver failure | 1 (0.9%) | 0 (0.0%) | 1 (2.2%) | 0.421 | 107 |
| Neuromuscular disease | 1 (0.9%) | 1 (1.6%) | 0 (0.0%) | 1.000 | 107 |
| Variables | All Patients n = 107 Median [IQR] or Mean ± SD or n (%) | Non-Survivors n = 62 Median [IQR] or Mean ± SD or n (%) | Survivors n = 45 Median [IQR] or Mean ± SD or n (%) | p-Value | n |
|---|---|---|---|---|---|
| Respiratory and hemodynamics variables at baseline (Day 1) | |||||
| PEEPtot, cmH2O | 15 [12;15] | 15 [12;15] | 14 [12;15] | 0.696 | 106 |
| Driving pressure, cmH2O | 12 [11;16] | 13 [11;16] | 12 [10;15] | 0.140 | 105 |
| Plateau pressure, cmH2O | 27 [25;30] | 28 [26;30] | 26 [25;30] | 0.065 | 105 |
| Crs, mL/cmH2O | 32 [25;38] | 31 [24;36] | 33 [26;38] | 0.254 | 105 |
| EVLWi, mL/kg PBW | 17 [15;22] | 19 [15;22] | 17 [14;21] | 0.151 | 100 |
| PVPI | 3.6 [3.1;4.5] | 4.0 [3.1;4.7] | 3.4 [2.6;4.1] | 0.028 | 100 |
| CI, L/min/m2 | 2.80 [2.16;3.39] | 2.70 [2.09;3.33] | 2.87 [2.24;3.40] | 0.503 | 100 |
| CVP, cmH2O | 11.0 [8.00;12.8] | 11.5 [8.00;14.0] | 10.0 [8.00;12.0] | 0.462 | 62 |
| PaO2/FiO2, mmHg | 153 ± 79 | 142 ± 81 | 169 ± 73 | 0.085 | 104 |
| TV, mL/kg PBW | 6 ± 0.6 | 5.9 ± 0.5 | 6.0 ± 0.7 | 0.178 | 106 |
| Maximal/minimal values of respiratory and hemodynamics variables | |||||
| PEEP max, cmH20 | 15 [14;16] | 15 [15;16] | 15 [14;16] | 0.238 | 106 |
| DP max, cmH20 | 17 [14;21] | 18 [15;22] | 16 [13;18] | 0.013 | 106 |
| Crs min, mL/cmH20 | 24 [19;28] | 24 [17;27] | 26 [20;30] | 0.035 | 106 |
| EVLWi max, mL/kg PBW | 22 [18;27] | 24 [21;28] | 19 [16;24] | 0.001 | 100 |
| PVPI max | 4.7 [3.7;5.7] | 5.1 [4.0;6.1] | 4.1 [3.5;5.2] | 0.005 | 100 |
| CI min, L/min/m2 | 2.30 [1.94;2.71] | 2.35 [1.96;2.70] | 2.24 [1.85;2.75] | 0.396 | 100 |
| CVP max (cmH2O) | 14.0 [11.0;17.0] | 15.0 [12.0;18.0] | 13.0 [11.0;16.0] | 0.179 | 62 |
| PaO2/FiO2 min (mmHg) | 107 ± 57 | 89.1 ± 42 | 133 ± 65 | <0.001 | 104 |
| TV max, mL/kg PBW | 6.3 ± 0.8 | 6.3 ± 0.7 | 6.5 ± 0.9 | 0.265 | 106 |
| Maximal/minimal values of respiratory and hemodynamic variables during the first week | |||||
| PEEP max, cmH2O | 15 [14;16] | 15 [15;16] | 15 [14;15] | 0.180 | 102 |
| DP max, cmH2O | 16 [13;19] | 17 [14;19] | 14 [12;17] | 0.003 | 102 |
| Crs min, mL/cmH20 | 26 [20;32] | 24 [19;29] | 28 [22;33] | 0.022 | 102 |
| EVLWi max, mL/kg PBW | 22 [18;27] | 24 [20;28] | 20 [16;24] | 0.005 | 97 |
| PVPI max | 4.7 [3.6;5.7] | 5.0 [4,0;6.1] | 4.2 [3.5;5.1] | 0.008 | 97 |
| CI min, L/min/m2 | 2.31 [1.94;2.79] | 2.44 [1.99;2.71] | 2.24 [1.90;2.80] | 0.412 | 97 |
| CVP max, cmH2O | 13.0 [11.0;15.5] | 14.0 [12.0;16.0] | 12.0 [10.5;15.0] | 0.103 | 59 |
| PaO2/FiO2 min, mmHg | 112 ± 56 | 98 ± 43 | 133 ± 65 | 0.004 | 100 |
| TV max, mL/kg PBW | 6.3 ± 0.8 | 6.2 ± 0.7 | 6.4 ± 0.9 | 0.372 | 102 |
| Outcome characteristics | |||||
| Duration of MV, days | 17.5 [9.00;28.8] | 14.5 [8.00;26.5] | 18.5 [11.0;34.2] | 0.134 | 106 |
| MV free days, days | 0.00 [0.00;4.00] | 0.00 [0.00;0.00] | 9.50 [0.00;17.0] | <0.001 | 106 |
| ICU length of stay, days | 19.0 [11.0;32.0] | 16.0 [9.00;29.0] | 22.0 [14.0;39.5] | 0.023 | 105 |
| Hospital length of stay, days | 30.0 [19.0;49.5] | 25.0 [14.0;33.0] | 44.5 [23.0;69.5] | <0.001 | 83 |
| Correlation Coefficient [CI 95%] | r2 | p-Value | |
|---|---|---|---|
| Correlation between respiratory variables | |||
| Pplat and Crs | −0.677 [−0.718; −0.631] | 0.458 | <0.001 |
| Pplat and DP | 0.826 [0.787; 0.841] | 0.682 | <0.001 |
| Pplat and PEEP | 0.264 [0.188; 0.338] | 0.070 | <0.001 |
| Crs and DP | −0.825 [−0.849; −0.797] | 0.681 | <0.001 |
| Crs and PEEP | 0.275 [0.200; 0.349] | 0.076 | <0.001 |
| DP and PEEP | −0.342 [−0.411; −0.270] | 0.117 | <0.001 |
| Correlation between hemodynamic variables | |||
| PVPI and EVLWi | 0.77 [0.745; 0.808] | 0.593 | <0.001 |
| Correlation between respiratory and hemodynamic variables | |||
| Pplat and PVPI | 0.059 [−0.022; 0.140] | 0.004 | 0.139 |
| Pplat and EVLWi | 0.123 [0.043; 0.202] | 0.015 | 0.003 |
| PVPI and Crs | 0.072 [−0.090; 0.153] | 0.005 | 0.080 |
| PVPI and DP | 0.051 [−0.131; 0.035] | 0.003 | 0.219 |
| PVPI and PEEP | 0.22 [0.141; 0.293] | 0.048 | <0.001 |
| EVLWi and Crs | −0.003 [−0.084; 0.079] | 0.000 | 0.951 |
| EVLWi and DP | 0.017 [−0.064; 0.098] | 0.000 | 0.674 |
| EVLWi and PEEP | 0.203 [0.126; 0.278] | 0.041 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lardet, F.; Monnet, X.; Teboul, J.-L.; Shi, R.; Lai, C.; Fossé, Q.; Moretto, F.; Gobé, T.; Jelinski, L.; Combet, M.; et al. Relationship of Extravascular Lung Water and Pulmonary Vascular Permeability to Respiratory Mechanics in Patients with COVID-19-Induced ARDS. J. Clin. Med. 2023, 12, 2028. https://doi.org/10.3390/jcm12052028
Lardet F, Monnet X, Teboul J-L, Shi R, Lai C, Fossé Q, Moretto F, Gobé T, Jelinski L, Combet M, et al. Relationship of Extravascular Lung Water and Pulmonary Vascular Permeability to Respiratory Mechanics in Patients with COVID-19-Induced ARDS. Journal of Clinical Medicine. 2023; 12(5):2028. https://doi.org/10.3390/jcm12052028
Chicago/Turabian StyleLardet, Florian, Xavier Monnet, Jean-Louis Teboul, Rui Shi, Christopher Lai, Quentin Fossé, Francesca Moretto, Thibaut Gobé, Ludwik Jelinski, Margot Combet, and et al. 2023. "Relationship of Extravascular Lung Water and Pulmonary Vascular Permeability to Respiratory Mechanics in Patients with COVID-19-Induced ARDS" Journal of Clinical Medicine 12, no. 5: 2028. https://doi.org/10.3390/jcm12052028
APA StyleLardet, F., Monnet, X., Teboul, J.-L., Shi, R., Lai, C., Fossé, Q., Moretto, F., Gobé, T., Jelinski, L., Combet, M., Pavot, A., Guérin, L., & Pham, T. (2023). Relationship of Extravascular Lung Water and Pulmonary Vascular Permeability to Respiratory Mechanics in Patients with COVID-19-Induced ARDS. Journal of Clinical Medicine, 12(5), 2028. https://doi.org/10.3390/jcm12052028






