Abstract
Objective: The present study aimed to evaluate the association between hematocrit (HCT) levels and all-cause mortality in geriatric hip fractures. Methods: Older adult patients with hip fractures were screened between January 2015 and September 2019. The demographic and clinical characteristics of these patients were collected. Linear and nonlinear multivariate Cox regression models were used to identify the association between HCT levels and mortality. Analyses were performed using EmpowerStats and the R software. Results: A total of 2589 patients were included in this study. The mean follow-up period was 38.94 months. Eight hundred and seventy-five (33.8%) patients died due to all-cause mortality. Linear multivariate Cox regression models showed that HCT level was associated with mortality (hazard ratio [HR] = 0.97, 95% confidence interval [CI]: 0.96–0.99, p = 0.0002) after adjusting for confounding factors. However, the linear association was unstable and nonlinearity was identified. A HCT level of 28% was the inflection point for prediction. A HCT level of <28% was associated with mortality (HR = 0.91, 95% CI: 0.87–0.95, p < 0.0001), whereas a HCT level > 28% was not a risk factor for mortality (HR = 0.99, 95% CI: 0.97–1.01, p = 0.3792). We found that the nonlinear association was very stable in the propensity score-matching sensitivity analysis. Conclusions: The HCT level was nonlinearly associated with mortality in geriatric hip fracture patients and could be considered a predictor of mortality in these patients. Registration: ChiCTR2200057323.
1. Introduction
Hip fractures are common injuries seen in older adults; they are especially common in women who often suffer from osteoporosis and multiple comorbidities [1]. In the United States, more than 320,000 patients are hospitalized per annum with hip fractures [2]. The global annual number of hip fractures is predicted to increase to 6.5 million fractures by 2050 [3]. A similar upward trend in the incidence of hip fractures has been projected for China, where it is estimated that the number will rise to 1.079 million by 2050 [4]. Although hip fractures comprise only 14% of geriatric fractures, they account for 72% of the total cost of orthopedic fracture care in older adults [5]. Most geriatric hip fractures are caused by falls, and they are associated with an increased mortality. The mean one-year global mortality rate in geriatric hip fracture patients is 22% [6]. Given the aging population and high incidence of hip fractures, the costs of geriatric hip fractures are projected to increase dramatically and consume more medical and health resources [7].
The prognosis of geriatric hip fractures is poor, and only about one-fifth of them return to their preinjury functional status one year after surgery [8]. Multiple factors have been proven to directly affect morbidity and mortality after hip surgery; these include delay in surgery, cancer, coronary heart disease, stroke, pneumonia, urinary tract infection, fluid and electrolyte imbalances, and perioperative anemia [9,10]. In addition, a systematic review indicated that malignancy, nursing home residence, time to surgery > two days, pulmonary disease, diabetes, and cardiovascular disease significantly increased the risk of mortality after hip fracture surgery [11]. Song also reported that frailty can predict adverse outcomes effectively in geriatric hip fracture patients [12].
Hematocrit (HCT), is a test that measures the volume of packed red blood cells relative to whole blood [13]. This test can identify conditions such as anemia or polycythemia, monitor response to treatment [14], and as act as a surrogate marker for factors affecting mortality after hip fracture [15].
Anemia, defined by hemoglobin levels in most of these studies, is common in older adults and may increase the risk of death [16]. It was reported that an estimated 40% of geriatric hip fracture patients were anemic on admission, and nearly all of them were anemic postoperatively [17]. Previous studies have identified anemia as a significant prognostic risk factor for patients who undergo surgery for hip fracture [18,19,20,21,22]. Several studies [21,23,24,25] have reported that anemia on admission was associated with short and long-term mortality. A study from Lancet has reported that preoperative low HCT was independently associated with an increased risk of 30-day morbidity and mortality in patients undergoing major surgery [26]. However, as an indicator of anemia, the role of HCT on mortality in hip fracture patients was unclear. Therefore, the specific relationship between HCT levels and the prognosis of patients with hip fractures needs to be further explored.
This study was to assess the influence of HCT level on all-cause mortality in geriatric patients with hip fractures over a long-term follow-up period. we hypothesized that there would be either a linear or a nonlinear association between HCT levels and mortality. In this prospective cohort study, we aimed to identify the role of HCT levels on hip fractures.
2. Materials and Methods
2.1. Study Design
Older adult patients who had a hip fracture between 1 January 2015, and 30 September 2019, and admitted to the largest trauma center in Northwest China, were enrolled in this study.
This prospective study was approved by the Ethics Committee of Xi’an Honghui Hospital (No. 202201009). All procedures involving human participants were performed in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
2.2. Participants
Data in-hospital, demographic and clinical data of the patients were obtained from their original medical records. The inclusion criteria were as follows: (1) age ≥ 65 years; (2) a radiographic or computed tomography diagnosis of the femoral neck, intertrochanteric, or subtrochanteric fracture; (3) receiving surgical or conservative treatment in a hospital; (4) availability of clinical data during hospitalization; and (5) availability of contact via telephone. Patients who could not be contacted were excluded from this study.
2.3. Hospital Treatment
Patients were examined using blood tests on admission, including HCT level. The ultrasonography of cardiac and lower extremity veins to prepare for surgery. Intertrochanteric fractures are often managed with closed/open reduction and internal fixation (ORIF) using a proximal femoral nail anti-rotation device. Femoral neck fractures are often treated with hemiarthroplasty (HA) or total hip arthroplasty (THA) according to the patient’s age. Prophylaxis for deep vein thrombosis was initiated on admission. Upon discharge, the patients were asked to return monthly for the assessment of fracture union or function.
2.4. Follow-Up
After discharge, patients’ family members were contacted by telephone between January 2022 and March 2022, to record data on survival, survival time, and activities of daily living. Follow-up was conducted by two medical professionals (Wen-Wen Cao and Shao-Hua Chen) who were trained in follow-up skills for two weeks. Three attempts were made to get in contact with patients. Failure of family members of the patients to maintain channels of communication with the team, resulted in patients being recorded as lost to follow-up.
2.5. Endpoint Events
The endpoint event in this study was all-cause mortality after treatment. We defined all-cause mortality as death reported by patients’ family members.
2.6. Variables
The variables in our study were: age, sex, occupation, history of allergy, injury mechanism, fracture classification, presence of hypertension, diabetes, coronary heart disease (CHD), arrhythmia, hemorrhagic stroke, ischemic stroke, cancer, multiple injuries, dementia, chronic obstructive pulmonary disease (COPD), hepatitis, gastritis, age-adjusted Charlson comorbidity index (aCCI), time from injury to admission, time from admission to surgery, HCT level, operation time, treatment strategy, blood loss, infusion, transfusion, length of hospital, and follow-up. The dependent variable was all-cause mortality, while the independent variable was the HCT level. The other variables were potential confounding factors.
2.7. Statistics Analysis
Continuous variables are reported as mean ± standard deviation (Gaussian distribution) or median (range, skewed distribution). Categorical variables are presented as numbers with proportions. Chi-square (categorical variables), one-way analysis of variance (normal distribution), and Kruskal–Wallis H test (skewed distribution) were used to detect differences between different HCT levels. Univariate and multivariate Cox proportional hazards regression models (three models) were used to test the association between HCT levels and mortality. Model 1 was not adjusted for covariates, Model 2 was minimally adjusted for sociodemographic variables, and Model 3 was fully adjusted for all covariates. To test the robustness of our results, we performed sensitivity analysis. We converted the HCT level into a categorical variable according to the anemia criteria and calculated the p-value for the trend to verify the results obtained using HCT level as the continuous variable; we also examined the possibility of nonlinearity. Because Cox proportional hazards regression model-based methods are often suspected to be unable to deal with nonlinear models, the nonlinearity between HCT and mortality was assessed using a Cox proportional hazards regression model with cubic spline functions and smooth curve fitting (the penalized spline method). If nonlinearity was detected, we first calculated the inflection point using a recursive algorithm and subsequently constructed a two-piecewise Cox proportional hazards regression model on both sides of the inflection point. In addition, propensity score matching (PSM) was introduced for comparison between matched groups, and confounding factors were adjusted for in the PSM models.
All analyses were performed using statistical software packages R (http://www.R-project.org, accessed on 1 January 2023, R Foundation) and EmpowerStats (http://www.empowerstats.com, accessed on 1 January 2023, X&Y Solutions Inc., Boston, MA, USA). The hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. A p-value < 0.05 (two-sided) was considered statistically significant.
3. Results
3.1. Patient Characteristics
From the initial sample of 2887 participants who had hip fractures between January 2015 and September 2019, 2589 met the study criteria and were enrolled in our study. The 1-year mortality was 11.05% (286/2303). The mean follow-up period was 38.94 months. Of these, 875 (33.8%) patients died due to all-cause mortality. The HCT levels were divided into four groups. Table 1 lists the demographic and clinical characteristics of all enrolled patients, including comorbidities, factors associated with injuries, and treatment.

Table 1.
The Demographic and clinical characteristics (N = 2589).
3.2. Univariate Analysis of Association between Variables and Mortality
To identify potential confounding factors and the relationship between variables and mortality, we performed univariate analysis. According to the criteria of p < 0.1, the following variables were considered in the multivariate Cox regression: age (HR = 1.08; 95% CI: 1.06–1.09); p < 0.0001), sex (HR = 0.74; 95% CI: 0.65–0.85); p < 0.0001), time to admission (HR = 1.00; 95% CI: 1.00, 1.00; p = 0.0531), hypertension (HR = 1.13; 95% CI: 0.99–1.29; p = 0.0643), CHD (HR = 1.32; 95% CI: 1.15–1.51; p < 0.0001), arrhythmia (HR = 1.32; 95% CI: 1.15–1.51; p < 0.0001), ischemic stroke (HR = 1.42; 95% CI: 1.24–1.64; p < 0.0001), cancer (HR = 1.77; 95% CI: 1.28–2.44; p = 0.0005), dementia (HR = 2.62; 95% CI: 2.03–3.38; p < 0.0001), COPD (HR = 1.55; 95% CI: 1.23–1.95; p = 0.0002), hepatitis (HR = 1.62; 95% CI: 1.17–2.23; p = 0.0033), aCCI (HR = 1.51; 95% CI: 1.43–1.61; p < 0.0001), time to operation (HR = 1.03; 95% CI: 1.00–1.05; p < 0.0481), treatment strategy (ORIF, HA and THA p < 0.0001 compared to conservation, respectively), operation time (HR = 1.00; 95% CI: 1.00, 1.00; p = 0.0433), infusion (HR = 1.00; 95% CI: 1.00, 1.00; p = 0.4246), and length in hospital (HR = 1.02; 95% CI: 1.01–1.04; p = 0.0041).
3.3. Multivariate Analysis of Association between HCT and Mortality
We used three models (Table 2) to correlate the HCT levels and mortality. Linear regression was observed when HCT level was a continuous variable. The fully adjusted model showed a decrease in mortality risk of 3% (HR = 0.97, 95% CI: 0.96–0.99, p = 0.0002) when HCT level increased by 1% after controlling for confounding factors. When HCT level was used as a categorical variable, we found significant differences in HCT levels among the three models (p < 0.05). In addition, the p-value for the trend also showed a linear correlation in the three models (p < 0.05).

Table 2.
Univariate and multivariate results by cox regression (N = 2589).
We found the interval to be abnormal in the subgroup with an HCT level above the third quartile (Table 2). This instability indicates the possibility of a nonlinear correlation.
As shown in Figure 1, there was a curved association between the HCT levels and mortality after adjusting for confounding factors. We compared two fitting models to explain this association (Table 3). Interestingly, we observed an inflection point. A HCT level < 28% was associated with mortality (HR = 0.91, 95% CI: 0.87–0.95, p < 0.0001). At a HCT level of >28%, mortality did not change (HR = 0.99, 95% CI: 0.97–1.01, p = 0.3792).
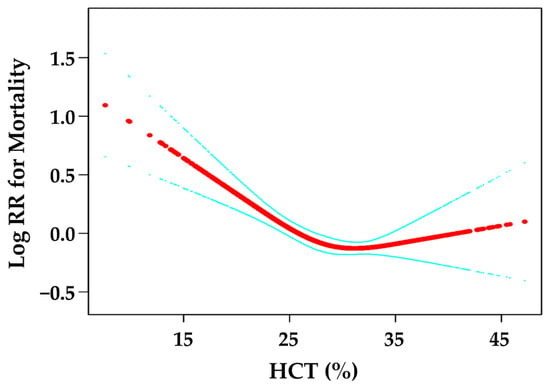
Figure 1.
Curve fitting between HCT and mortality. The red line is the fitting curve, and the blue lines are 95% CI.

Table 3.
Nonlinearity of HCT and mortality (N = 2589).
3.4. Propensity Score Matching (PSM)
To test the robustness of our results, we performed sensitivity analysis using PSM. Overall, 1400 patients (54.07%) were successfully matched. Age (p < 0.0001) and aCCI (p < 0.0001) did not match between the two groups. In the multivariate Cox regression results under the PSM and PSM-adjusted models, the results were stable, and the inflection point was 29.7% (Table 4).

Table 4.
Multivariate results by cox regression in PSM model (N = 1400).
The Kaplan–Meier survival curve is shown in Figure 2.
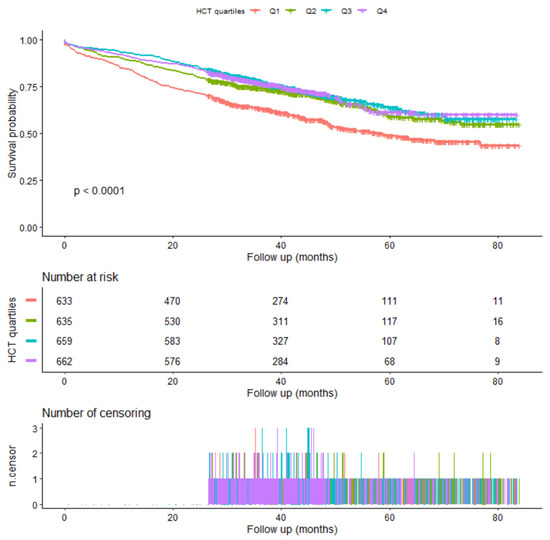
Figure 2.
Kaplan-Meier survival.
4. Discussion
A few systematic reviews have indicated many risk factors for mortality in geriatric hip fracture patients [11,12,27,28,29], and anemia was an important prognostic risk factor [18,19,20,21,22]. Even though previous studies have proven the association between preoperative hemoglobin and mortality in hip fracture, the results were an almost linear relationship and did not find nonlinearity. In addition, several high-quality studies assessed the effect of preoperative HCT on mortality in patients undergoing major surgery [26,30,31], but the role of HCT on mortality in hip fracture has not been addressed. Therefore, the specific relationship between HCT levels and the prognosis of patients with hip fractures is needed.
Our study showed a nonlinear association between HCT levels and all-cause mortality in geriatric patients after hip fracture treatment. When the HCT level was <28%, geriatric hip fracture patients with lower HCT levels had greater odds of mortality (HR = 0.91, 95% CI: 0.87–0.95; p < 0.0001). This result suggests that a 1% increase in HCT level was associated with a 9% decrease in mortality in geriatric patients with hip fractures. However, when the HCT level was >28%, no association was found between HCT level and mortality in geriatric hip fracture patients (HR = 0.99, 95% CI: 0.97–1.01; p = 0.3792). Consequently, the HCT level on admission can be used as a clinical predictor of all-cause mortality in geriatric patients with hip fracture.
There were two primary meanings provided in this paper. On the one hand, the HCT level on admission (<28%) can be used as a clinical predictor of all-cause mortality in geriatric patients with hip fractures. When a patient was on admission, the surgeons should notice that this person was at a high risk of a bad prognosis. On the other hand, we would note the importance of HCT from this study. The surgeons could undertake the intervention study on improving the prognosis by transfusion for HCT < 28% in the future. Specifically, rotational thromboelastometry is a laboratory method that is gaining ground on the evaluation of the hemostatic profile of hip fracture patients [32,33].
A number of previous studies have Investigated the effect of anemia on the prognosis of patients undergoing orthopedic surgery. A single-center retrospective study in Singapore reported that anemia was independently associated with prolonged hospitalization and increased perioperative blood transfusions [34]. Nissenholtz et al. [23] reported that anemia on admission was associated with short and long-term mortality, in addition to the length of stay, amount of blood transfusions, repeated hospitalizations, post-operative complications, poor functioning, and a reduced quality of life. In addition, few studies have examined the effects of anemia on patients with hip fractures. Ryan et al. [21] suggested that geriatric hip fracture patients with preoperative anemia are at an increased risk for morbidity and mortality, especially during the first 30 postoperative days. Similar to the findings of our study, Gruson et al. [24] reported that anemia was associated with a longer length of hospital stay and higher rates of 6-month mortality after surgery for hip fracture. Bolton et al. [25] reported that anemia was a statistically-significant risk factor for perioperative complications. However, most of these studies used hemoglobin levels to define anemia. The specific relationship between HCT levels and prognosis of patients with hip fractures remains unclear. In a systematic review, Sheehan et al. [35] reported frailty was the proposed mechanism for the association between anemia and functional outcome.
In this study, we found a linear relationship between HCT levels and mortality in geriatric hip fractures; however, we also observed that the relationship was unstable. Therefore, we surmised the possibility of a curvilinear relationship from subgroup analysis and curve fitting. Our study found an inflection point on the curve, which was stabilized by PSM sensitivity analysis. The curvilinear relationship more appropriately explains the association between HCT levels and mortality in geriatric hip fracture patients. Many studies have indicated that HCT levels are associated with organ senescence or complications in older population. In a cohort study of geriatric patients undergoing spinal procedures based on the ACS-NSQIP database, Almeida et al. [36] concluded that patients with lower HCT levels were at a greater risk for requiring transfusion, renal failure, and infectious complications. Gupta et al. [31] found that a HCT level of ≤39% is associated with an increased risk of 30-day mortality and adverse cardiac events in patients aged 65 years or older undergoing elective vascular procedures. Bodewes et al. [37] reported that mortality and major adverse events in patients with chronic limb-threatening ischemia who underwent infrainguinal bypass were inversely associated with preoperative HCT levels.
The patients in our study received blood tests on admission immediately, and the HCT is the first value at admission before treatment. Because of the nature of observational association, we did not give the identified or particular intervention to the patients. However, we gave the transfusion to patients with severe anemia after admission or during the operation in clinical practice. The total volume of the transfusion is 1.84 (U), 1.22 (U), 1.00 (U), and 0.63 (U) in groups Q1–Q4, respectively.
To identify confounders in the study and draw reliable conclusions, we identified factors that affect the HCT level as well as the prognosis of geriatric hip fractures. There were several factors very well known to contribute to mortality in hip fracture population. Age, sex, comorbidities, CHD, arrhythmia, cancer, dementia, time to operation, and treatment strategy were the risk factors for the prognosis of hip fracture which have been reported in previous studies [7,8,10,38,39,40,41,42,43]. The probability of mortality density for a period of 10 years following a hip fracture was 16% for women and 25% for men [44]. A recent systematic review from 81 articles showed that the comorbidities, delay in operation, and type of fractures were important predictors of poor functional outcomes and mortality for patients with hip fractures [28]. In addition, the univariate analysis also found some variables with p < 0.1 to be associated ischemic stroke, operation time, and infusion volume. Furthermore, considering factors affecting the HCT level, we included hepatitis, COPD, and cancer [45,46,47,48]. Consequently, we have controlled for the vast majority of confounders.
Our study has a few limitations. First, due to the prospective design of our study, there was an inevitable risk for patients to be loss to follow-up. Patients or their families who could not be contacted initially were called two other times to obtain information regarding patients’ prognosis. Second, the causal relationship between the HCT level and prognosis of hip fractures was not identified in our study and requires further confirmation in future studies. It would be meaningful if future studies could establish a causal relationship between HCT levels and all-cause mortality in geriatric hip fractures. Furthermore, the HCT value was not only influenced by anemia, but also by other factors (hematological disorders or fluid intake). Therefore, generalizations of this conclusion for populations from other regions should be made with caution.
In summary, the HCT level was nonlinearly associated with mortality in geriatric hip fractures and could be considered a predictor of the risk of mortality.
Author Contributions
Conceived and designed the study: B.-F.Z. Performed the study: K.L., W.-W.C., S.-H.C. Analyzed the data: B.-F.Z. Wrote the manuscript: Y.-M.Z. and B.-F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Foundation of Xi’an Municipal Health Commission (Grant Number: 2021ms09), the Natural Science Foundation of Shaanxi Province (Grant Number: 2021JM-572).
Institutional Review Board Statement
The Ethics Committee of the Honghui Hospital, Xi’an Jiaotong University approved this study (No. 202201009).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data were provided by Xi’an Honghui Hospital. According to relevant regulations, the data cannot be shared, but could request from correspondence author.
Acknowledgments
The authors thank the Chinese Clinical Trial Registry (ChiCTR, http://www.chictr.org.cn/showproj.aspx?proj=152919, accessed on 8 March 2022 ) as number ChiCTR2200057323.
Conflicts of Interest
The authors declare that they have no competing interest.
Abbreviations
| aCCI | age-adjusted Charlson comorbidity index |
| CHD | coronary heart disease |
| CI | confidence interval |
| COPD | chronic obstructive pulmonary disease |
| HCT | hematocrit |
| HR | Hazard ratio |
| PSM | propensity score matching |
References
- Ramponi, D.R.; Kaufmann, J.; Drahnak, G. Hip Fractures. Adv. Emerg. Nurs. J. 2018, 40, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Mundi, S.; Pindiprolu, B.; Simunovic, N.; Bhandari, M. Similar mortality rates in hip fracture patients over the past 31 years. Acta Orthop. 2013, 85, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Broggi, M.S.; Oladeji, P.O.; Tahmid, S.; Hernandez-Irizarry, R.; Allen, J. Depressive Disorders Lead to Increased Complications After Geriatric Hip Fractures. Geriatr. Orthop. Surg. Rehabil. 2021, 12, 21514593211016252. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Winzenberg, T.M.; Jiang, Q.; Chen, M.; Palmer, A.J. Projection of osteoporosis-related fractures and costs in China: 2010–2050. Osteoporos. Int. 2015, 26, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef]
- Downey, C.; Kelly, M.; Quinlan, J.F. Changing trends in the mortality rate at 1-year post hip fracture—A systematic review. World J. Orthop. 2019, 10, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Ferris, H.; Coughlan, T.; Hurson, C.; Ahern, E.; Sorensen, J.; Brent, L. Trends in hip fracture care in the Republic of Ireland from 2013 to 2018: Results from the Irish Hip Fracture Database. Osteoporos. Int. 2021, 32, 727–736. [Google Scholar] [CrossRef]
- Chiang, M.-H.; Huang, Y.-Y.; Kuo, Y.-J.; Huang, S.-W.; Jang, Y.-C.; Chu, F.-L.; Chen, Y.-P. Prognostic Factors for Mortality, Activity of Daily Living, and Quality of Life in Taiwanese Older Patients within 1 Year Following Hip Fracture Surgery. J. Pers. Med. 2022, 12, 102. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Kuo, Y.-J.; Hung, S.-W.; Wen, T.-W.; Chien, P.-C.; Chiang, M.-H.; Maffulli, N.; Lin, C.-Y. Loss of skeletal muscle mass can be predicted by sarcopenia and reflects poor functional recovery at one year after surgery for geriatric hip fractures. Injury 2021, 52, 3446–3452. [Google Scholar] [CrossRef]
- Groff, H.; Kheir, M.M.; George, J.; Azboy, I.; Higuera, C.A.; Parvizi, J. Causes of in-hospital mortality after hip fractures in the elderly. HIP Int. 2020, 30, 204–209. [Google Scholar] [CrossRef]
- Chang, W.; Lv, H.; Feng, C.; Yuwen, P.; Wei, N.; Chen, W.; Zhang, Y. Preventable risk factors of mortality after hip fracture surgery: Systematic review and meta-analysis. Int. J. Surg. 2018, 52, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, Z.; Huo, H.; Zhao, P. The Impact of Frailty on Adverse Outcomes in Geriatric Hip Fracture Patients: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 890652. [Google Scholar] [CrossRef]
- Mondal, H.; Lotfollahzadeh, S. StatPearls; StatPearls Publishing LLC: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Kiya, G.T.; Zewudie, F.M. Comparison of three-fold converted hematocrit and micro-hematocrit in pregnant women. PLoS ONE 2019, 14, e0220740. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, D.; Parker, M.J. Haematological indices as surrogate markers of factors affecting mortality after hip fracture. Injury 2011, 42, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Izaks, G.J.; Westendorp, R.G.; Knook, D.L. The definition of anemia in older persons. JAMA 1999, 281, 1714–1717. [Google Scholar] [CrossRef] [PubMed]
- Halm, E.A.; Wang, J.J.; Boockvar, K.; Penrod, J.; Silberzweig, S.B.; Magaziner, J.; Koval, K.J.; Siu, A.L. The effect of perioperative anemia on clinical and functional outcomes in patients with hip fracture. J. Orthop. Trauma 2004, 18, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.J.; Doleman, B.; Moppett, I.K. A systematic review of pre-operative anaemia and blood transfusion in patients with fractured hips. Anaesthesia 2015, 70, 483–500. [Google Scholar] [CrossRef]
- Grosso, M.J.; Boddapati, V.; Cooper, H.J.; Geller, J.A.; Shah, R.P.; Neuwirth, A.L. The Effect of Preoperative Anemia on Complications After Total Hip Arthroplasty. J. Arthroplast. 2020, 35, S214–S218. [Google Scholar] [CrossRef] [PubMed]
- Viola, J.; Gomez, M.M.; Restrepo, C.; Maltenfort, M.G.; Parvizi, J. Preoperative anemia increases postoperative complications and mortality following total joint arthroplasty. J. Arthroplast. 2015, 30, 846–848. [Google Scholar] [CrossRef]
- Ryan, G.; Nowak, L.; Melo, L.; Ward, S.; Atrey, A.; Schemitsch, E.H.; Nauth, A.; Khoshbin, A. Anemia at Presentation Predicts Acute Mortality and Need for Readmission Following Geriatric Hip Fracture. JBJS Open Access 2020, 5, e20.00048. [Google Scholar] [CrossRef]
- Du, P.; Zhu, Y.; Guo, J.; Qi, S.; Qin, J.; Zheng, C.; Hou, Z.; Zhang, Y.; Tian, Q.-B.; Feng, Z. Incidence and risk factors associated with surgical site infection after surgically treated hip fractures in older adults: A retrospective cohort study. Aging Clin. Exp. Res. 2022, 34, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Nissenholtz, A.; Levy, Y.; Cooper, L.; Bugaevsky, Y.; Weiss, A.; Beloosesky, Y. Anemia in Patients after Hip Fracture Repair Surgery. Harefuah 2020, 159, 689–693. [Google Scholar] [PubMed]
- Gruson, K.I.; Aharonoff, G.B.; Egol, K.A.; Zuckerman, J.D.; Koval, K.J. The relationship between admission hemoglobin level and outcome after hip fracture. J. Orthop. Trauma 2002, 16, 39–44. [Google Scholar] [CrossRef]
- Bolton, D.; Bush, C.; Wallace, M.T. Nonagenarian hip fractures: Morbidity and mortality at a single institution. J. Clin. Orthop. Trauma 2021, 14, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Tamim, H.M.; Richards, T.; Spahn, D.R.; Rosendaal, F.R.; Habbal, A.; Khreiss, M.; Dahdaleh, F.S.; Khavandi, K.; Sfeir, P.M.; et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: A retrospective cohort study. Lancet 2011, 378, 1396–1407. [Google Scholar] [CrossRef]
- Simunovic, N.; Devereaux, P.J.; Sprague, S.; Guyatt, G.H.; Schemitsch, E.; DeBeer, J.; Bhandari, M. Effect of early surgery after hip fracture on mortality and complications: Systematic review and meta-analysis. CMAJ 2010, 182, 1609–1616. [Google Scholar] [CrossRef]
- Xu, B.Y.; Yan, S.; Low, L.L.; Vasanwala, F.F.; Low, S.G. Predictors of poor functional outcomes and mortality in patients with hip fracture: A systematic review. BMC Musculoskelet. Disord. 2019, 20, 568. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Zhang, Y.; Chen, A.C.; Liu, T.; Yang, H.; Zhu, X.; He, F. The effects of dementia on the prognosis and mortality of hip fracture surgery: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2021, 33, 3161–3172. [Google Scholar] [CrossRef]
- Musallam, K.M.; Porter, J.; Sfeir, P.M.; Tamim, H.M.; Richards, T.; Lotta, L.A.; Peyvandi, F.; Jamali, F.R. Raised haematocrit concentration and the risk of death and vascular complications after major surgery. Br. J. Surg. 2013, 100, 1030–1036. [Google Scholar] [CrossRef]
- Gupta, P.K.; Sundaram, A.; MacTaggart, J.N.; Johanning, J.M.; Gupta, H.; Fang, X.; Forse, R.A.; Balters, M.; Longo, G.M.; Sugimoto, J.T.; et al. Preoperative anemia is an independent predictor of postoperative mortality and adverse cardiac events in elderly patients undergoing elective vascular operations. Ann. Surg. 2013, 258, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Papadopoulos, D.V.; Trikoupis, I.G.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Vaiopoulos, A.G.; Piovani, D.; Nikolopoulos, G.K.; Kokoris, S.I.; et al. The Prognostic Performance of Rotational Thromboelastometry for Excessive Bleeding and Increased Transfusion Requirements in Hip Fracture Surgeries. Thromb. Haemost. 2022, 122, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Papadopoulos, D.V.; Roustemis, A.G.; Trikoupis, I.G.; Piovani, D.; Tsante, K.A.; Mantzios, P.G.; Mavrogenis, A.F.; Sokou, R.; Kokoris, S.I.; et al. Rotational Thromboelastometry Predicts Transfusion Requirements in Total Joint Arthroplasties. Semin. Thromb. Hemost. 2023, 49, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, H.R.; Sim, Y.E.; Hao, Y.; Lin, G.Y.; Liew, G.H.C.; Lamoureux, E.L.; Tan, M.H. Association between preoperative anaemia with length of hospital stay among patients undergoing primary total knee arthroplasty in Singapore: A single-centre retrospective study. BMJ Open 2017, 7, e016403. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, K.J.; Williamson, L.; Alexander, J.; Filliter, C.; Sobolev, B.; Guy, P.; Bearne, L.; Sackley, C. Prognostic factors of functional outcome after hip fracture surgery: A systematic review. Age Ageing 2018, 47, 661–670. [Google Scholar] [CrossRef]
- Almeida, N.D.; Lee, R.; Bestourous, D.; Klein, A.L.; Parekh, N.R.; Sack, K.; Sherman, J.H. Perioperative Complications Associated with Severity of Anemia in Geriatric Patients Undergoing Spinal Procedures. World Neurosurg. 2022, 135, e307–e320. [Google Scholar] [CrossRef]
- Bodewes, T.C.; Pothof, A.B.; Darling, J.D.; Deery, S.E.; Jones, D.W.; Soden, P.A.; Moll, F.L.; Schermerhorn, M.L. Preoperative anemia associated with adverse outcomes after infrainguinal bypass surgery in patients with chronic limb-threatening ischemia. J. Vasc. Surg. 2017, 66, 1775–1785.e2. [Google Scholar] [CrossRef]
- Kavak, M.; Oğuz, S.; Akkoyun, Z.; İnan, U. Predictive factors associated with thirty-day mortality in geriatric patients with hip fractures. Acta Orthop. Traumatol. Turc. 2022, 56, 240–244. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Yuan, Y.; Li, X.; Liu, X.; Fan, B.; Yang, M.; Wu, X. Risk factor analysis for in-hospital death of geriatric hip fracture patients. Saudi Med. J. 2022, 43, 197–201. [Google Scholar] [CrossRef]
- Stone, A.V.; Jinnah, A.; Wells, B.; Atkinson, H.; Miller, A.N.; Futrell, W.M.; Lenoir, K.; Emory, C.L. Nutritional markers may identify patients with greater risk of re-admission after geriatric hip fractures. Int. Orthop. 2018, 42, 231–238. [Google Scholar] [CrossRef]
- Provenzano, G.; Jenkins, S.; Higginbotham, W.; Markel, D.C. Factors That Influence Time to Operating Room for Geriatric Hip Fractures: A Quality Improvement Initiative. Arthroplast. Today 2022, 15, 115–119. [Google Scholar] [CrossRef]
- Lai, Y.C.; Tang, P.L.; Kuo, T.J.; Hsu, C.J. Different impacts of dementia on two-year mortality after osteosynthesis and hemiarthroplasty in treating geriatric hip fractures. Arch. Gerontol. Geriatr. 2018, 79, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Ercin, E.; Bilgili, M.G.; Sari, C.; Basaran, S.H.; Tanriverdi, B.; Edipoglu, E.; Celen, K.M.; Cetingok, H.; Kural, C. Risk factors for mortality in geriatric hip fractures: A compressional study of different surgical procedures in 785 consecutive patients. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Guzon-Illescas, O.; Fernandez, E.P.; Villarias, N.C.; Donate, F.J.Q.; Peña, M.; Alonso-Blas, C.; Vadillo, A.G.; Mazzucchelli, R. Mortality after osteoporotic hip fracture: Incidence, trends, and associated factors. J. Orthop. Surg. Res. 2019, 14, 203. [Google Scholar] [CrossRef]
- Gauci, R.; Hunter, M.; Bruce, D.G.; Davis, W.A.; Davis, T.M.E. Anemia complicating type 2 diabetes: Prevalence, risk factors and prognosis. J. Diabetes Complicat. 2017, 31, 1169–1174. [Google Scholar] [CrossRef]
- Divo, M.; Celli, B.R. Multimorbidity in Patients with Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2020, 41, 405–419. [Google Scholar] [CrossRef]
- Cherabuddi, M.R.; Kurra, N.; Doosetty, S.; Gandrakota, N. Atypical Presentation of Interval Colorectal Cancer/Post-Colonoscopy Colorectal Cancer in a Nursing Home Patient. Cureus 2022, 14, e24849. [Google Scholar] [CrossRef] [PubMed]
- Bach, V.; Schruckmayer, G.; Sam, I.; Kemmler, G.; Stauder, R. Prevalence and possible causes of anemia in the elderly: A cross-sectional analysis of a large European university hospital cohort. Clin. Interv. Aging 2014, 9, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).