Niacin Skin Flush Backs—From the Roots of the Test to Nowadays Hope
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Niacin Skin Flush Test
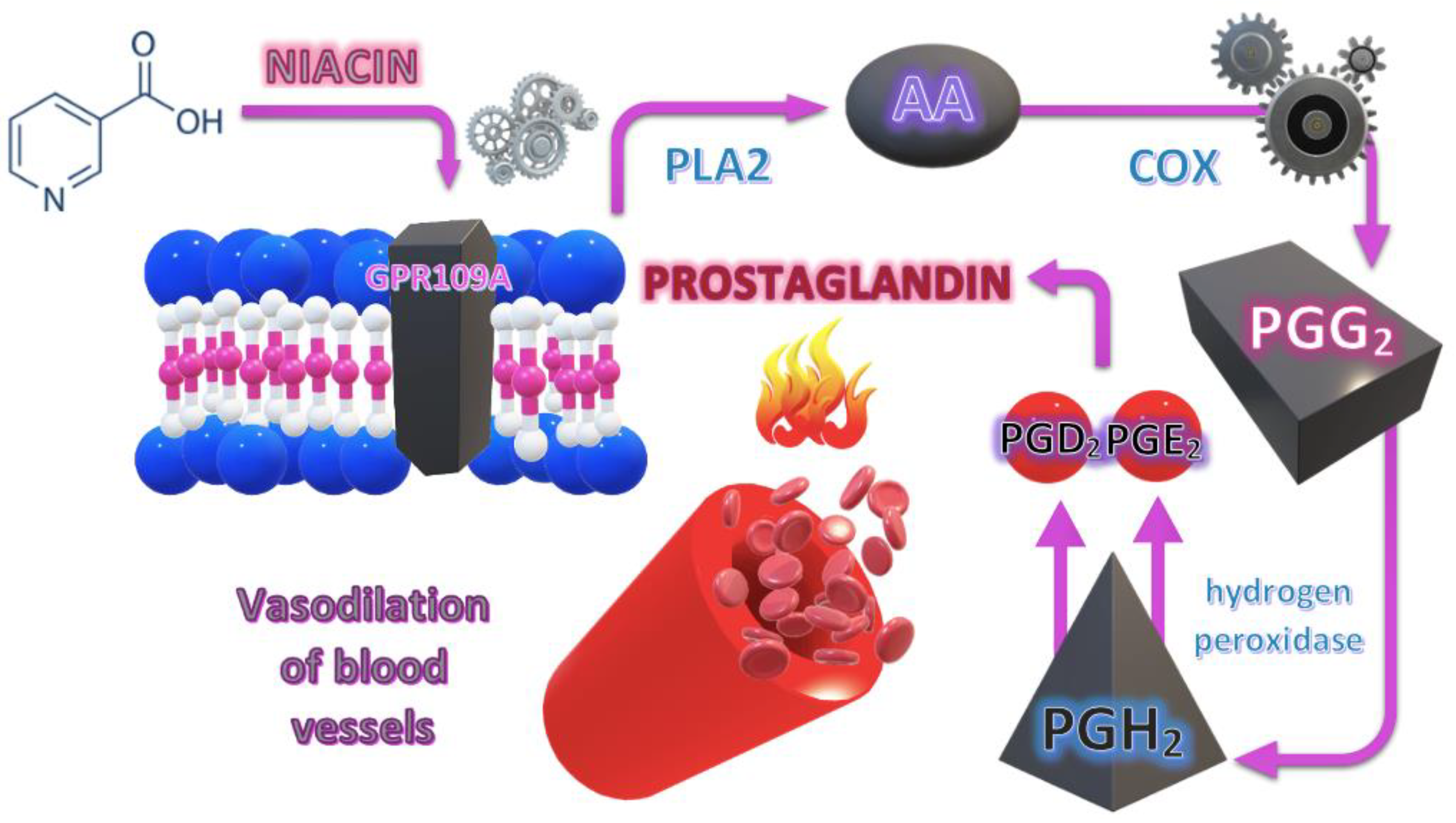
3.1.1. Pathophysiological Background of the NSFT
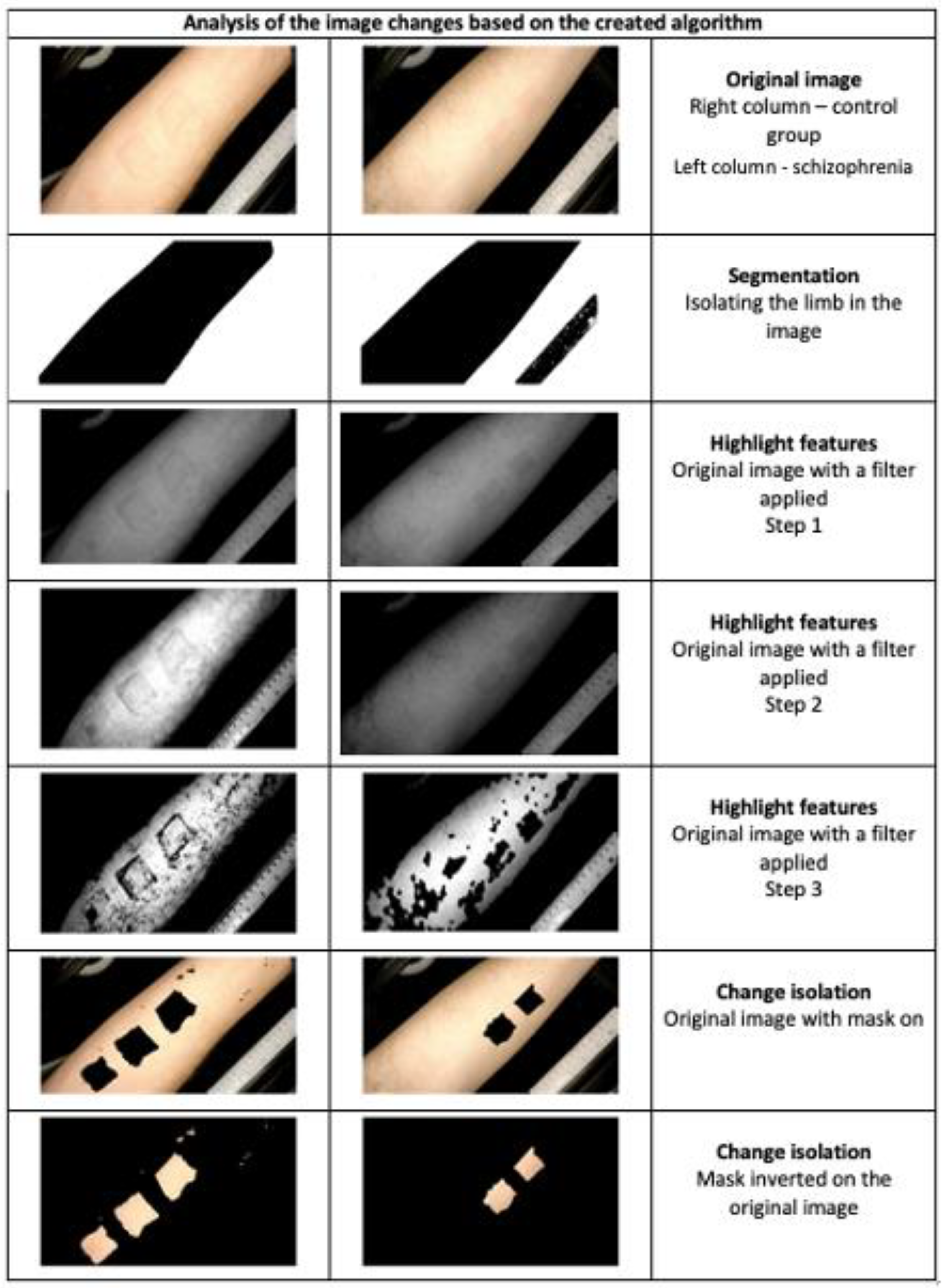
3.1.2. Measurement Methods Used in NSFT
SKINREMS—An Innovative Measurement Device for NSFT Assessment
3.1.3. Diagnostic Accuracy of the NSFT
3.1.4. Applicability of NSFT for High-Risk Psychosis
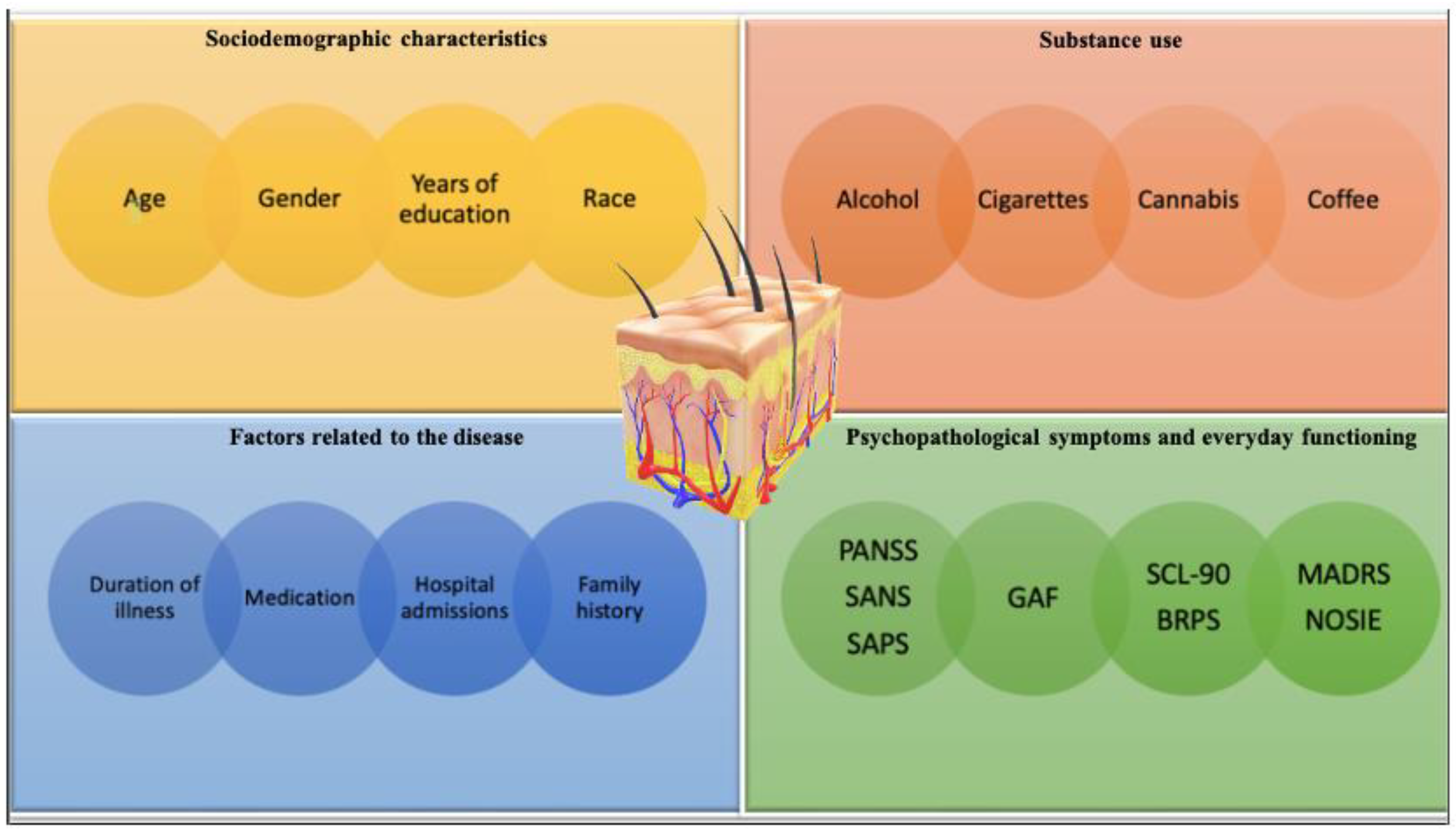
3.1.5. Factors That Can Affect the NSFT Results
Sociodemographic Characteristics
Using Psychoactive Substances
Factors Related to the Course and Treatment of Psychosis
The Intensity of Psychopathological Symptoms and Difficulties in Everyday Functioning
Genetic Pathways and the Response to NSFT
3.2. From Horrobin’s Theory to the Future
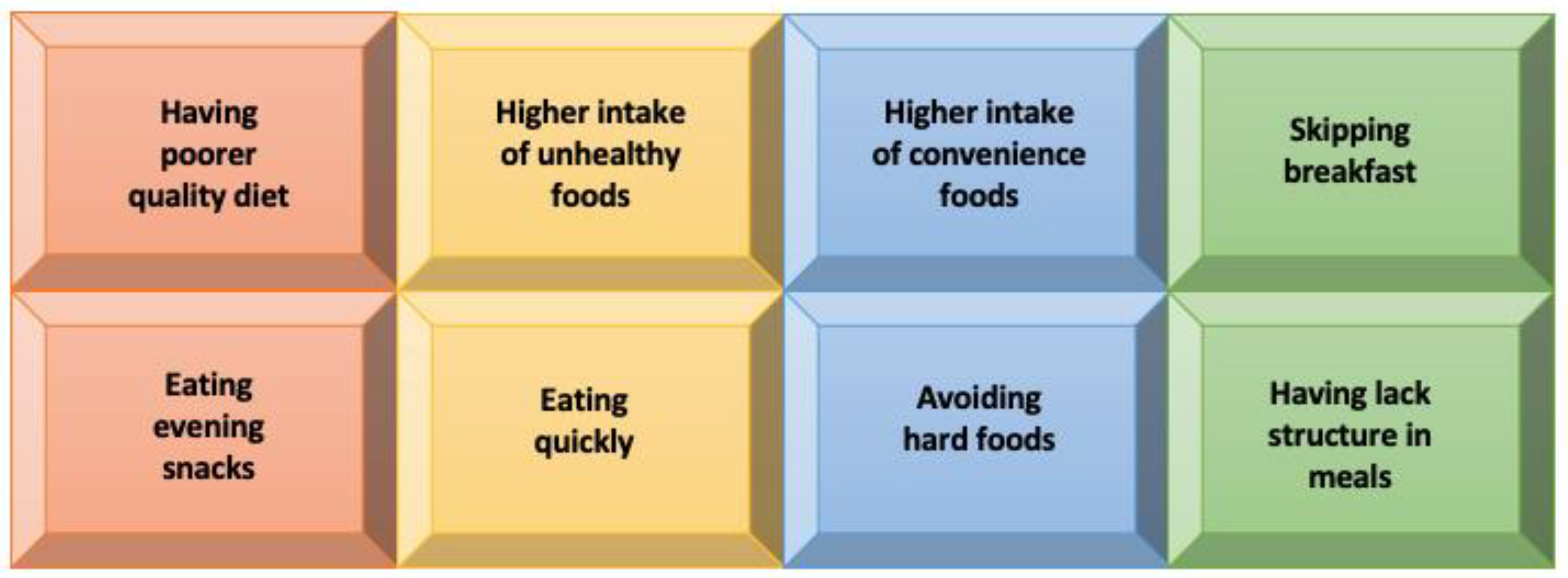
3.3. The Hope Hidden in Diet
4. Conclusions
- Creating a new classification and diagnosis of mental disorders, especially psychosis, based on pathophysiological premises.
- Searching for new therapeutic options and drugs based on the mechanisms of NSFT action.
- Early intervention and staging in psychiatry.
- Defining an individualized diet for patients, which may be a factor in alleviating symptoms and perhaps even leading to remission and its maintenance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Littlewood, R. The madness of adam and eve: How schizophrenia shaped humanity. J. R. Soc. Med. 2001, 94, 601–602. [Google Scholar] [CrossRef]
- Horrobin, D.F. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr. Res. 1998, 30, 193–208. [Google Scholar] [CrossRef]
- Goff, D.C.; Romero, K.; Paul, J.; Perez-Rodriguez, M.M.; Crandall, D.; Potkin, S.G. Biomarkers for drug development in early psychosis: Current issues and promising directions. Eur. Neuropsychopharmacol. 2016, 26, 923–937. [Google Scholar] [CrossRef]
- Buretić-Tomljanović, A.; Giacometti, J.; Nadalin, S.; Rubeša, G.; Vulin, M.; Tomljanović, D. Phospholipid membrane abnormalities and reduced niacin skin flush response in schizophrenia. Psychiatr. Danub. 2008, 20, 372–383. [Google Scholar]
- Messamore, E. The niacin response biomarker as a schizophrenia endophenotype: A status update. Prostaglandins Leukot. Essent. Fat. Acids 2017, 136, 95–97. [Google Scholar] [CrossRef]
- Kendler, K.S. Kraepelin’s Final Views on Dementia Praecox. Schizophr. Bull. 2020, 47, 635–643. [Google Scholar] [CrossRef]
- Javitt, D.C. Biotypes in Psychosis: Has the RDoC Era Arrived? Am. J. Psychiatry 2016, 173, 313–314. [Google Scholar] [CrossRef]
- Krukow, P.; Jonak, K.; Karpiński, R.; Karakuła-Juchnowicz, H. Abnormalities in hubs location and nodes centrality predict cognitive slowing and increased performance variability in first-episode schizophrenia patients. Sci. Rep. 2019, 9, 9594. [Google Scholar] [CrossRef]
- Jonak, K.; Marchewka, M.; Podkowiński, A.; Siejka, A.; Plechawska-Wójcik, M.; Karpiński, R.; Krukow, P. How Functional Connectivity Measures Affect the Outcomes of Global Neuronal Network Characteristics in Patients with Schizophrenia Compared to Healthy Controls. Brain Sci. 2023, 13, 138. [Google Scholar] [CrossRef]
- Thaker, G.K. Neurophysiological Endophenotypes Across Bipolar and Schizophrenia Psychosis. Schizophr. Bull. 2007, 34, 760–773. [Google Scholar] [CrossRef]
- Nadalin, S.; Buretić-Tomljanović, A.; Rubesa, G.; Tomljanović, D.; Gudelj, L. Niacin skin flush test: A research tool for studying schizophrenia. Psychiatr. Danub. 2010, 22, 14–27. [Google Scholar]
- Clark, L.A.; Cuthbert, B.; Lewis-Fernández, R.; Narrow, W.E.; Reed, G.M. Three Approaches to Understanding and Classifying Mental Disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol. Sci. Public Interest 2017, 18, 72–145. [Google Scholar] [CrossRef]
- Clementz, B.; Trotti, R.; Pearlson, G.D.; Keshavan, M.; Gershon, E.; Keedy, S.; Ivleva, E.; Mcdowell, J.E.; Tamminga, C. O3.4. Psychosis Phenotypes From B-Snip For Clinical Advances: Biotype Characteristics And Targets. Schizophr. Bull. 2020, 46 (Suppl. 1), S7. [Google Scholar] [CrossRef]
- Goodby, E. The Maudsley Family Study of Psychosis—A Quest For Intermediate Phenotypes (Maudsley Monograph no. 50). Edited by C. McDonald. (Pp. 248; £24.95; ISBN 978-1-84169-734-5.). Psycho-Log. Med. 2010, 40, 1225–1226. [Google Scholar] [CrossRef]
- Ivleva, E.I.; Morris, D.W.; Moates, A.F.; Suppes, T.; Thaker, G.K.; Tamminga, C.A. Genetics and intermediate phenotypes of the schizophrenia—Bipolar disorder boundary. Neurosci. Biobehav. Rev. 2010, 34, 897–921. [Google Scholar]
- Lichtenstein, P.; Yip, B.H.; Björk, C.; Pawitan, Y.; Cannon, T.D.; Sullivan, P.F.; Hultman, C.M. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: A population-based study. Lancet 2009, 373, 234–239. [Google Scholar] [CrossRef]
- Cheniaux, E.; Landeira-Fernandez, J.; Telles, L.L.; Lessa, J.L.M.; Dias, A.; Duncan, T.; Versiani, M. Does schizoaffective disorder really exist? A systematic review of the studies that compared schizoaffective disorder with schizophrenia or mood disorders. J. Affect. Disord. 2008, 106, 209–217. [Google Scholar] [CrossRef]
- Ward, P.; Sutherland, J.; Glen, E.; Glen, A. Niacin skin flush in schizophrenia: A preliminary report. Schizophr. Res. 1998, 29, 269–274. [Google Scholar] [CrossRef]
- Puri, B.K.; Easton, T.; Das, I.; Kidane, L.; Richardson, A.J. The niacin skin flush test in schizophrenia: A replication study. Int. J. Clin. Pract. 2001, 55, 368–370. [Google Scholar]
- Messamore, E. The niacin skin flush abnormality in schizophrenia: A quantitative dose–response study. Schizophr. Res. 2003, 62, 251–258. [Google Scholar] [CrossRef]
- Nilsson, B.; Hultman, C.; Wiesel, F.-A. Niacin skin-flush response and electrodermal activity in patients with schizophrenia and healthy controls. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 339–346. [Google Scholar] [CrossRef]
- Hudson, C.J.; Lin, A.; Cogan, S.; Cashman, F.; Warsh, J.J. The niacin challenge test: Clinical manifestation of altered transmembrane signal transduction in schizophrenia? Biol. Psychiatry 1997, 41, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Rybakowski, J.; Weterle, R. Niacin test in schizophrenia and affective illness. Biol. Psychiatry 1991, 29, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Keshavan, M.S.; Nasrallah, H.A.; Tandon, R. Schizophrenia, “Just the Facts” 6. Moving ahead with the schizophrenia concept: From the elephant to the mouse. Schizophr. Res. 2011, 127, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Bosveld-van Haandel, L.; Knegtering, R.; Kluiter, H.; van den Bosch, R.J. Niacin skin flushing in schizophrenic and depressed patients and healthy controls. Psychiatry Res. 2006, 143, 303–306. [Google Scholar] [CrossRef]
- Berger, G.E.; Smesny, S.; Schäfer, M.R.; Milleit, B.; Langbein, K.; Hipler, U.-C.; Milleit, C.; Klier, C.; Schlögelhofer, M.; Holub, M.; et al. Niacin Skin Sensitivity Is Increased in Adolescents at Ultra-High Risk for Psychosis. PLoS ONE 2016, 11, e0148429. [Google Scholar] [CrossRef]
- Tavares, H.; Yacubian, J.; Talib, L.L.; Barbosa, N.R.; Gattaz, W.F. Increased phospholipase A2 activity in schizophrenia with absent response to niacin. Schizophr. Res. 2002, 61, 1–6. [Google Scholar] [CrossRef]
- Fillman, S.G.; Sinclair, D.; Fung, S.J.; Webster, M.J.; Shannon Weickert, C. Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl. Psychiatry 2014, 4, e365. [Google Scholar] [CrossRef]
- Niwa, K.; Araki, E.; Morham, S.G.; Ross, M.E.; Iadecola, C. Cyclooxygenase-2 Contributes to Functional Hyperemia in Whisker-Barrel Cortex. J. Neurosci. 2000, 20, 763–770. [Google Scholar] [CrossRef]
- Nilsson, B.; Holm, G.; Hultman, C.; Ekselius, L. Cognition and autonomic function in schizophrenia: Inferior cognitive test performance in electrodermal and niacin skin flush non-responders. Eur. Psychiatry 2015, 30, 8–13. [Google Scholar] [CrossRef]
- Jacobson, T.A. A “Hot” Topic in Dyslipidemia Management—“How to Beat a Flush”: Optimizing Niacin Tolerability to Promote Long-term Treatment Adherence and Coronary Disease Prevention. Mayo Clin. Proc. 2010, 85, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Messamore, E. Niacin subsensitivity is associated with functional impairment in schizophrenia. Schizophr. Res. 2012, 137, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Karakula-Juchnowicz, H.; Rog, J.; Wolszczak, P.; Jonak, K.; Stelmach, E.; Krukow, P. SKINREMS—A New Method for Assessment of the Niacin Skin Flush Test Response in Schizophrenia. J. Clin. Med. 2020, 9, 1848. [Google Scholar] [CrossRef]
- Glen, A.I.M.; Cooper, S.J.; Rybakowski, J.; Vaddadi, K.; Brayshaw, N.; Horrobin, D. Membrane fatty acids, niacin flushing and clinical parameters. Prostaglandins Leukot. Essent. Fat. Acids 1996, 55, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Horrobin, D.F. Schizophrenia as a prostaglandin deficiency disease. Lancet 1977, 309, 936–937. [Google Scholar] [CrossRef]
- Horrobin, D.F.; Glen, A.M.; Vaddadi, K. The membrane hypothesis of schizophrenia. Schizophr. Res. 1994, 13, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Horrobin, D.F. Schizophrenia: A biochemical disorder? Biomedicine 1980, 32, 54–55. [Google Scholar]
- Puri, B.K.; Hirsch, S.R.; Easton, T.; Richardson, A.J. A volumetric biochemical niacin flush-based index that noninvasively detects fatty acid deficiency in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 49–52. [Google Scholar] [CrossRef]
- Lin, S.-H.; Liu, C.-M.; Chang, S.-S.; Hwu, H.-G.; Liu, S.K.; Hwang, T.-J.; Hsieh, M.H.; Guo, S.-C.; Chen, W.J. Familial Aggregation in Skin Flush Response to Niacin Patch Among Schizophrenic Patients and Their Nonpsychotic Relatives. Schizophr. Bull. 2006, 33, 174–182. [Google Scholar] [CrossRef]
- Ross, B. Reduced vasodilatory response to methylnicotinate in schizophrenia as assessed by laser Doppler flowmetry. Eur. Neuropsychopharmacol. 2004, 14, 191–197. [Google Scholar] [CrossRef]
- Yao, J.K.; Dougherty, G.G.; Gautier, C.H.; Haas, G.L.; Condray, R.; Kasckow, J.W.; Kisslinger, B.L.; Gurklis, J.A.; Messamore, E. Prevalence and Specificity of the Abnormal Niacin Response: A Potential Endophenotype Marker in Schizophrenia. Schizophr. Bull. 2015, 42, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Smesny, S.; Berger, G.; Rosburg, T.; Riemann, S.; Riehemann, S.; McGorry, P.; Sauer, H. Potential use of the topical niacin skin test in early psychosis—A combined approach using optical reflection spectroscopy and a descriptive rating scale. J. Psychiatr. Res. 2003, 37, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, X.; Jiang, J.; Hu, X.; Qing, Y.; Wang, D.; Yang, T.; Yang, C.; Zhang, J.; Yang, P.; et al. Identification of the Niacin-Blunted Subgroup of Schizophrenia Patients from Mood Disorders and Healthy Individuals in Chinese Population. Schizophr. Bull. 2017, 44, 896–907. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, L.; Gan, R.; Wu, G.; Tang, X.; Wei, Y.; Cui, H.; Hui, L.; Tang, Y.; Li, C.; et al. A potential objective marker in first-episode schizophrenia based on abnormal niacin response. Schizophr. Res. 2021, 243, 405–412. [Google Scholar] [CrossRef]
- Wang, D.-D.; Hu, X.-W.; Jiang, J.; Sun, L.-Y.; Qing, Y.; Yang, X.-H.; Gao, Y.; Cui, G.-P.; Li, M.-H.; Wang, P.-K.; et al. Attenuated and delayed niacin skin flushing in schizophrenia and affective disorders: A potential clinical auxiliary diagnostic marker. Schizophr. Res. 2021, 230, 53–60. [Google Scholar] [CrossRef]
- Liu, C.-M.; Chang, S.-S.; Liao, S.-C.; Hwang, T.-J.; Shieh, M.-H.; Liu, S.-K.; Chen, W.J.; Hwu, H.-G. Absent response to niacin skin patch is specific to schizophrenia and independent of smoking. Psychiatry Res. 2007, 152, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Hudson, C.; Gotowiec, A.; Seeman, M.; Warsh, J.; Ross, B.M. Clinical subtyping reveals significant differences in calcium-dependent phospholipase A2 activity in schizophrenia. Biol. Psychiatry 1999, 46, 401–405. [Google Scholar] [CrossRef]
- McGorry, P.D.; Goldstone, S.D.; Parker, A.G.; Rickwood, D.J.; Hickie, I.B. Cultures for mental health care of young people: An Australian blueprint for reform. Lancet Psychiatry 2014, 1, 559–568. [Google Scholar] [CrossRef]
- Yung, A.R.; Fusar-Poli, P.; Nelson, B. The Ultra High Risk Approach to Define Psychosis Risk. Curr. Pharm. Des. 2012, 18, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Schultze-Lutter, F.; Ruhrmann, S.; Picker, H. Basic symptoms and UHR criteria in the prediction of first-episode psychosis. Schizophr. Res. 2008, 102, 155–156. [Google Scholar] [CrossRef]
- Amminger, G.P.; Schäfer, M.R.; Papageorgiou, K.; Klier, C.M.; Cotton, S.M.; Harrigan, S.M.; Mackinnon, A.; McGorry, P.D.; Berger, G.E. Long-Chain ω-3 Fatty Acids for Indicated Prevention of Psychotic Disorders. Arch. Gen. Psychiatry 2010, 67, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.; Wei, Y.; Wu, G.; Zeng, J.; Hu, Y.; Xu, L.; Tang, X.; Liu, X.; Liu, H.; Chen, T.; et al. Attenuated niacin-induced skin flush response in individuals with clinical high risk for psychosis. Gen. Psychiatry 2022, 35, e100748. [Google Scholar] [CrossRef]
- Zhang, T.; Gan, R.; Zeng, J.; Ye, J.; Hu, Y.; Xu, L.; Wei, Y.; Tang, X.; Li, C.; Liu, H.; et al. Attenuated niacin response is associated with a subtype of first-episode drug-naïve psychosis characterized as serious negative symptoms. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Langbein, K.; Schmidt, U.; Schack, S.; Biesel, N.J.; Rudzok, M.; Amminger, G.P.; Berger, M.; Sauer, H.; Smesny, S. State marker properties of niacin skin sensitivity in ultra-high risk groups for psychosis—An optical reflection spectroscopy study. Schizophr. Res. 2017, 192, 377–384. [Google Scholar] [CrossRef]
- Smesny, S.; Milleit, B.; Hipler, U.-C.; Milleit, C.; Schäfer, M.R.; Klier, C.M.; Holub, M.; Holzer, I.; Berger, G.E.; Otto, M.; et al. Omega-3 fatty acid supplementation changes intracellular phospholipase A2 activity and membrane fatty acid profiles in individuals at ultra-high risk for psychosis. Mol. Psychiatry 2013, 19, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Lien, Y.-J.; Huang, S.-S.; Liu, C.-M.; Hwu, H.-G.; Faraone, S.V.; Tsuang, M.T.; Chen, W.J. A Genome-wide Quantitative Linkage Scan of Niacin Skin Flush Response in Families With Schizophrenia. Schizophr. Bull. 2011, 39, 68–76. [Google Scholar] [CrossRef]
- Smesny, S.; Rosburg, T.; Klemm, S.; Riemann, S.; Baur, K.; Rudolph, N.; Grunwald, S.; Sauer, H. The influence of age and gender on niacin skin test results—Implications for the use as a biochemical marker in schizophrenia. J. Psychiatr. Res. 2004, 38, 537–543. [Google Scholar] [CrossRef]
- Hafner, H.; an der Heiden, W.; Behrens, S.; Gattaz, W.F.; Hambrecht, M.; Löffler, W.; Maurer, K.; Munk-Jørgensen, P.; Nowotny, B.; Riecher-Rössler, A.; et al. Causes and Consequences of the Gender Difference in Age at Onset of Schizophrenia. Schizophr. Bull. 1998, 24, 99–113. [Google Scholar] [CrossRef]
- Könnecke, R.; Häfner, H.; Maurer, K.; Löffler, W.; der Heiden, W.A. Main risk factors for schizophrenia: Increased familial loading and pre- and peri-natal complications antagonize the protective effect of oestrogen in women. Schizophr. Res. 2000, 44, 81–93. [Google Scholar] [CrossRef]
- Oades, R.D.; Schepker, R. Serum gonadal steroid hormones in young schizophrenic patients. Psychoneuroendocrinology 1994, 19, 373–385. [Google Scholar] [CrossRef]
- Horrobin, D.F.; Ally, A.I.; Karmali, R.A.; Karmazyn, M.; Manku, M.S.; Morgan, R.O. Prostaglandins and schizophrenia: Further discussion of the evidence. Psychol. Med. 1978, 8, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, P.; Wolkin, A.; Rotrosen, J. Niacin-induced flush as a measure of prostaglandin activity in alcoholics and schizophrenics. Biol. Psychiatry 1986, 21, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Smesny, S.; Rosburg, T.; Riemann, S.; Baur, K.; Rudolph, N.; Berger, G.; Sauer, H. Impaired niacin sensitivity in acute first-episode but not in multi-episode schizophrenia. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 393–402. [Google Scholar] [CrossRef]
- Smesny, S.; Rosburg, T.; Baur, K.; Rudolph, N.; Sauer, H. Cannabinoids Influence Lipid–Arachidonic Acid Pathways in Schizophrenia. Neuropsychopharmacology 2007, 32, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-S.; Liu, C.-M.; Lin, S.-H.; Hwu, H.-G.; Hwang, T.-J.; Liu, S.K.; Hsieh, M.H.; Guo, S.-C.; Chen, W.J. Impaired Flush Response to Niacin Skin Patch Among Schizophrenia Patients and Their Nonpsychotic Relatives: The Effect of Genetic Loading. Schizophr. Bull. 2008, 35, 213–221. [Google Scholar] [CrossRef]
- Yang, X.; Li, M.; Jiang, J.; Hu, X.; Qing, Y.; Sun, L.; Yang, T.; Wang, D.; Cui, G.; Gao, Y.; et al. Dysregulation of phospholipase and cyclooxygenase expression is involved in Schizophrenia. Ebiomedicine 2021, 64, 103239. [Google Scholar] [CrossRef]
- Covault, J.; Pettinati, H.; Moak, D.; Mueller, T.; Kranzler, H.R. Association of a long-chain fatty acid-CoA ligase 4 gene polymorphism with depression and with enhanced niacin-induced dermal erythema. Am. J. Med. Genet. 2004, 127B, 42–47. [Google Scholar] [CrossRef]
- Nadalin, S.; Giacometti, J.; Jonovska, S.; Tomljanović, D.; Buretić-Tomljanović, A. The impact of PLA2G4A and PTGS2 gene polymorphisms, and red blood cell PUFAs deficit on niacin skin flush response in schizophrenia patients. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 185–190. [Google Scholar] [CrossRef]
- Nadalin, S.; Radović, I.; Buretić-Tomljanović, A. Polymorphisms in PLA2G6 and PLA2G4C genes for calcium-independent phospholipase A2 do not contribute to attenuated niacin skin flush response in schizophrenia patients. Prostaglandins Leukot. Essent. Fat. Acids 2015, 100, 29–32. [Google Scholar] [CrossRef]
- Wood, S.J.; Yung, A.R.; McGorry, P.D.; Pantelis, C. Neuroimaging and Treatment Evidence for Clinical Staging in Psychotic Disorders: From the At-Risk Mental State to Chronic Schizophrenia. Biol. Psychiatry 2011, 70, 619–625. [Google Scholar] [CrossRef]
- Wyatt, R.J. Early intervention for schizophrenia: Can the course of the illness be altered? Biol. Psychiatry 1995, 38, 1–3. [Google Scholar] [CrossRef]
- Fava, G.A.; Kellner, R. Staging: A neglected dimension in psychiatric classification. Acta Psychiatr. Scand. 1993, 87, 225–230. [Google Scholar] [CrossRef]
- Agius, M.; Goh, C.; Ulhaq, S.; McGorry, P. The staging model in schizophrenia, and its clinical implications. Psychiatr. Danub. 2010, 22, 211–220. [Google Scholar] [PubMed]
- McFarlane, W.R. Prevention of and early intervention for psychosis. Audio-Dig. Psychiatry 2009, 38, 95–107. [Google Scholar]
- McGorry, P.D.; Nelson, B.; Goldstone, S.; Yung, A. Clinical Staging: A Heuristic and Practical Strategy for New Research and Better Health and Social Outcomes for Psychotic and Related Mood Disorders. Can. J. Psychiatry 2010, 55, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, H.; Tian, R.; Gan, R.; Xu, W.; Zhang, T.; Wang, J. Artificial intelligence-assisted niacin skin flush screening in early psychosis identification and prediction. Gen. Psychiatry 2022, 35, e100753. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
- Sarris, J.; Ravindran, A.; Yatham, L.N.; Marx, W.; Rucklidge, J.J.; McIntyre, R.S.; Akhondzadeh, S.; Benedetti, F.; Caneo, C.; Cramer, H.; et al. Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J. Biol. Psychiatry 2022, 23, 424–455. [Google Scholar] [CrossRef]
- Bradbury, J. Docosahexaenoic Acid (DHA): An Ancient Nutrient for the Modern Human Brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef]
- Mukhopadhyay, R. Essential Fatty Acids: The Work of George and Mildred Burr. J. Biol. Chem. 2012, 287, 35439–35441. [Google Scholar] [CrossRef]
- Brenna, J.T.; Salem, N.; Sinclair, A.J.; Cunnane, S.C. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary Aspects of the Dietary Omega–6:Omega–3 Fatty Acid Ratio: Medical Implications. World Rev. Nutr. Diet. 2009, 100, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Trans fatty acids. In Handbook of Lipids in Human Nutrition; Spiller, G.A., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 91–99. [Google Scholar]
- Eaton, S.B.; Konner, M. Paleolithic Nutrition. N. Engl. J. Med. 1985, 312, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. New products from the agri-food industry: The return of n-3 fatty acids into the food supply. Lipids 1999, 34, S297–S301. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Aucoin, M.; LaChance, L.; Cooley, K.; Kidd, S. Diet and Psychosis: A Scoping Review. Neuropsychobiology 2018, 79, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.G.; Gallego, J.A.; John, M.; Hanna, L.A.; Zhang, J.-P.; Birnbaum, M.L.; Greenberg, J.; Naraine, M.; Peters, B.D.; McNamara, R.K.; et al. A potential role for adjunctive omega-3 polyunsaturated fatty acids for depression and anxiety symptoms in recent onset psychosis: Results from a 16 week randomized placebo-controlled trial for participants concurrently treated with risperidone. Schizophr. Res. 2018, 204, 295–303. [Google Scholar] [CrossRef]
- Jones, H.J.; Borges, M.C.; Carnegie, R.; Mongan, D.; Rogers, P.J.; Lewis, S.J.; Thompson, A.D.; Zammit, S. Associations between plasma fatty acid concentrations and schizophrenia: A two-sample Mendelian randomisation study. Lancet Psychiatry 2021, 8, 1062–1070. [Google Scholar] [CrossRef]
- McLaverty, A.; Allott, K.A.; Berger, M.; Hester, R.; McGorry, P.D.; Nelson, B.; Markulev, C.; Yuen, H.P.; Schäfer, M.R.; Mossaheb, N.; et al. Omega-3 fatty acids and neurocognitive ability in young people at ultra-high risk for psychosis. Early Interv. Psychiatry 2020, 15, 874–881. [Google Scholar] [CrossRef]
- Hsu, M.-C.; Huang, Y.-S.; Ouyang, W.-C. Beneficial effects of omega-3 fatty acid supplementation in schizophrenia: Possible mechanisms. Lipids Health Dis. 2020, 19, 159. [Google Scholar] [CrossRef]
- Frajerman, A.; Scoriels, L.; Kebir, O.; Chaumette, B. Shared Biological Pathways between Antipsychotics and Omega-3 Fatty Acids: A Key Feature for Schizophrenia Preventive Treatment? Int. J. Mol. Sci. 2021, 22, 6881. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, K.; Kusumoto, Y.; Kanchi, N.; Kinoshita, H.; Kanegae, S.; Yamaguchi, N.; Ozawa, H. Recent trends in mental illness and omega-3 fatty acids. J. Neural Transm. 2020, 127, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-C.; Ouyang, W.-C. A Systematic Review of Effectiveness of Omega-3 Fatty Acid Supplementation on Symptoms, Social Functions, and Neurobiological Variables in Schizophrenia. Biol. Res. Nurs. 2021, 23, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Makino, K.K.; Martin, C.E.; Dickerson, F.; Boronow, J.; Fenton, W.S. Smoking, gender, and dietary influences on erythrocyte essential fatty acid composition among patients with schizophrenia or schizoaffective disorder. Biol. Psychiatry 2003, 53, 431–441. [Google Scholar] [CrossRef] [PubMed]

| Study | Year of Publication | Individuals | Country | Sensitivity | Specificity | Type of Data | |
|---|---|---|---|---|---|---|---|
| 1. | Smesny et al. [42] | 2003 | 25 SCH | Australia | 92.0% | 84% in SCH and HCs | Quantitative |
| 25 HCs | |||||||
| 2. | Karakula-Juchnowicz et al. [33] | 2020 | 56 SCH | Poland | 91% | 72% in SCH and BD | Semi-quantitative |
| 29 BD | 71% | 66% in SCH and HCs | |||||
| 45 HCs | 55% | 54% in BD and HCs | |||||
| 3. | Puri et al. [19] | 2001 | 21 SCH | UK | 90% | 75% in SCH and HCs | Semi-quantitative |
| 20 HCs | |||||||
| 4. | Smesny et al. [42] | 2003 | 25 SCH | Australia | 84.0%, | 76% in SCH and HCs | Semi-quantitative |
| 25 HCs | |||||||
| 5. | Ward et al. [18] | 1998 | 35 SCH | UK | 83% | 77% in SCH and HCs | Semi-quantitative |
| 22 HCs | |||||||
| 6. | Horrobin [37] | 1980 | N/A | UK | 80% | N/A | Qualitative |
| 7. | Puri et al. [38] | 2002 | 27 SCH | UK | 77.8% | 65.38% in SCH and HCs | Semi-quantitative |
| 26 HCs | |||||||
| 8. | Messamore et al. [20] | 2003 | 27 SCH | USA | 74.0% | 81% in SCH and HCs | Quantitative |
| 21 HCs | |||||||
| 9. | Ross et al. [40] | 2004 | 27 SCH | Canada | 70.0% | 86% in SCH and HCs | Quantitative |
| 26 BD | 81% in SCH and BP | ||||||
| 10. | Hu et al. [44] | 2022 | 82 SCH | China | 57% | 89% | Quantitative |
| 41 BD | |||||||
| 80 HCs | |||||||
| 11. | Wang et al. [45] | 2021 | 307 SCH | China | 55.28% | 83.56% in SCH or AD and HCs | Semi-quantitative |
| 179 BD | |||||||
| 127 UD | |||||||
| 148 HCs | |||||||
| 12. | Glen et al. [34] | 1996 | 126 SCH | UK | 52% | N/A | Qualitative |
| 13. | Liu et al. [46] | 2007 | 61 SCH | Taiwan | 49.2%, | 92.5% in SCH and HCs | Semi-quantitative |
| 18 BD | 88.2% in SCH and BP | ||||||
| 14. | Hudson et al. [47] | 1999 | 23 SCH | Canada | 43.0% | 97% in SCH and HCs | Quantitative |
| 30 HCs | |||||||
| 15. | Hudson et. al. [22] | 1997 | 33 SCH | Canada | 42.9%, | 94.4% in SCH and BP | Quantitative |
| 18 BD | 100% in SCH and HCs | ||||||
| 28 HCs | 97.8% in SCH, BP, and HCs | ||||||
| 16. | Sun et al. [43] | 2017 | 163 SCH | China | 23.3–42.0% | 82.4%-88.9% | Semi-quantitative |
| 63 MDD | |||||||
| 63 HCs | |||||||
| 17. | Yao et al. [41] | 2015 | 70 SCH | USA | 30.0% | 95% in SCH and HCs | Quantitative |
| 59 BD | 97% in SCH and BP | ||||||
| 90 SCH | 32.0% | 95% in SCH and HCs | |||||
| 93 HCs | 87% in SCH and MDD | ||||||
| 18. | Rybakowski et al. [23] | 1991 | 33 SCH | Poland | 24%, | 100% in SCH and AD | Qualitative |
| 18 AD | |||||||
| 19. | Tavares et al. [27] | 2003 | 38 SCH | Brazil | 23.7% | 85.8% in SCH and HCs | Semi-quantitative |
| 28 HCs | |||||||
| 20. | Lin et al. [39] | 2006 | 153 SCH | Taiwan | 13.7% | 96.8% in SCH and HCs | Semi-quantitative |
| 94 HCs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitarz, R.; Juchnowicz, D.; Karakuła, K.; Forma, A.; Baj, J.; Rog, J.; Karpiński, R.; Machrowska, A.; Karakuła-Juchnowicz, H. Niacin Skin Flush Backs—From the Roots of the Test to Nowadays Hope. J. Clin. Med. 2023, 12, 1879. https://doi.org/10.3390/jcm12051879
Sitarz R, Juchnowicz D, Karakuła K, Forma A, Baj J, Rog J, Karpiński R, Machrowska A, Karakuła-Juchnowicz H. Niacin Skin Flush Backs—From the Roots of the Test to Nowadays Hope. Journal of Clinical Medicine. 2023; 12(5):1879. https://doi.org/10.3390/jcm12051879
Chicago/Turabian StyleSitarz, Ryszard, Dariusz Juchnowicz, Kaja Karakuła, Alicja Forma, Jacek Baj, Joanna Rog, Robert Karpiński, Anna Machrowska, and Hanna Karakuła-Juchnowicz. 2023. "Niacin Skin Flush Backs—From the Roots of the Test to Nowadays Hope" Journal of Clinical Medicine 12, no. 5: 1879. https://doi.org/10.3390/jcm12051879
APA StyleSitarz, R., Juchnowicz, D., Karakuła, K., Forma, A., Baj, J., Rog, J., Karpiński, R., Machrowska, A., & Karakuła-Juchnowicz, H. (2023). Niacin Skin Flush Backs—From the Roots of the Test to Nowadays Hope. Journal of Clinical Medicine, 12(5), 1879. https://doi.org/10.3390/jcm12051879








