Clinical Outcome of Ultrasound-Detected Perforated Necrotizing Enterocolitis without Radiographic Pneumoperitoneum in Very Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Data Collection and Outcome Measures
2.3. Image Acquisition and Analysis
2.4. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
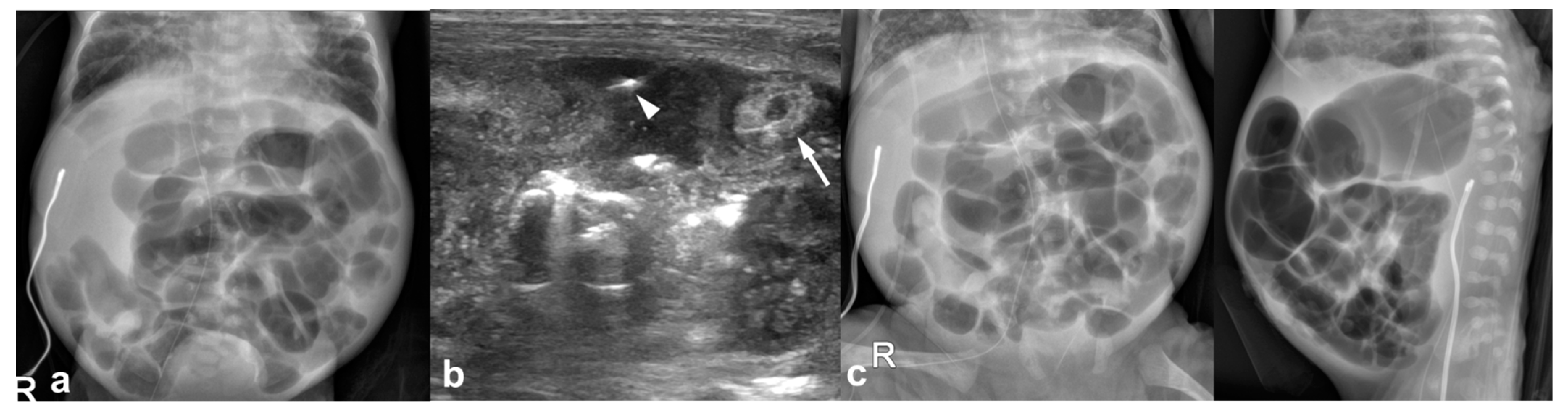
3.2. Imaging Findings
3.2.1. Abdominal Radiographs
3.2.2. Temporal Relation between Abdominal Radiographs and Bowel US
3.3. Clinical Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cuna, A.; Chan, S.; Jones, J.; Sien, M.; Robinson, A.; Rao, K.; Opfer, E. Feasibility and acceptability of a diagnostic randomized clinical trial of bowel ultrasound in infants with suspected necrotizing enterocolitis. Eur. J. Pediatr. 2022, 181, 3211–3215. [Google Scholar] [CrossRef]
- Patel, R.M.; Kandefer, S.; Walsh, M.C.; Bell, E.F.; Carlo, W.A.; Laptook, A.R.; Sanchez, P.J.; Shankaran, S.; Van Meurs, K.P.; Ball, M.B.; et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N. Engl. J. Med. 2015, 372, 331–340. [Google Scholar] [CrossRef]
- Jones, I.H.; Hall, N.J. Contemporary Outcomes for Infants with Necrotizing Enterocolitis—A Systematic Review. J. Pediatr. 2020, 220, 86–92.e3. [Google Scholar] [CrossRef]
- Janssen Lok, M.; Miyake, H.; Hock, A.; Daneman, A.; Pierro, A.; Offringa, M. Value of abdominal ultrasound in management of necrotizing enterocolitis: A systematic review and meta-analysis. Pediatr. Surg. Int. 2018, 34, 589–612. [Google Scholar] [CrossRef]
- Robinson, J.R.; Rellinger, E.J.; Hatch, L.D.; Weitkamp, J.H.; Speck, K.E.; Danko, M.; Blakely, M.L. Surgical necrotizing enterocolitis. Semin. Perinatol. 2017, 41, 70–79. [Google Scholar] [CrossRef]
- Munaco, A.J.; Veenstra, M.A.; Brownie, E.; Danielson, L.A.; Nagappala, K.B.; Klein, M.D. Timing of optimal surgical intervention for neonates with necrotizing enterocolitis. Am. Surg. 2015, 81, 438–443. [Google Scholar] [CrossRef]
- Alexander, K.M.; Chan, S.S.; Opfer, E.; Cuna, A.; Fraser, J.D.; Sharif, S.; Khashu, M. Implementation of bowel ultrasound practice for the diagnosis and management of necrotising enterocolitis. Arch. Dis. Child Fetal Neonatal Ed. 2021, 106, 96–103. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Liu, Q.; Li, X.; Wang, H.; Wang, K. Comparison of abdominal radiographs and sonography in prognostic prediction of infants with necrotizing enterocolitis. Pediatr. Surg. Int. 2018, 34, 535–541. [Google Scholar] [CrossRef]
- Faingold, R.; Daneman, A.; Tomlinson, G.; Babyn, P.S.; Manson, D.E.; Mohanta, A.; Moore, A.M.; Hellmann, J.; Smith, C.; Gerstle, T.; et al. Necrotizing enterocolitis: Assessment of bowel viability with color doppler US. Radiology 2005, 235, 587–594. [Google Scholar] [CrossRef]
- Gerdes, J.S. Diagnosis and management of bacterial infections in the neonate. Pediatr. Clin. N. Am. 2004, 51, 939–959. [Google Scholar] [CrossRef]
- May, L.A.; Epelman, M.; Daneman, A. Ultrasound for necrotizing enterocolitis: How can we optimize imaging and what are the most critical findings? Pediatr. Radiol. 2022. [Google Scholar] [CrossRef]
- Silva, C.T.; Daneman, A.; Navarro, O.M.; Moineddin, R.; Levine, D.; Moore, A.M. A prospective comparison of intestinal sonography and abdominal radiographs in a neonatal intensive care unit. Pediatr. Radiol. 2013, 43, 1453–1463. [Google Scholar] [CrossRef]
- McBride, W.J.; Roy, S.; Brudnicki, A.; Stringel, G. Correlation of complex ascites with intestinal gangrene and perforation in neonates with necrotizing enterocolitis. J. Pediatr. Surg. 2010, 45, 887–889. [Google Scholar] [CrossRef]
- Miller, S.F.; Seibert, J.J.; Kinder, D.L.; Wilson, A.R. Use of ultrasound in the detection of occult bowel perforation in neonates. J. Ultrasound Med. 1993, 12, 531–535. [Google Scholar] [CrossRef]
- Muchantef, K.; Epelman, M.; Darge, K.; Kirpalani, H.; Laje, P.; Anupindi, S.A. Sonographic and radiographic imaging features of the neonate with necrotizing enterocolitis: Correlating findings with outcomes. Pediatr. Radiol. 2013, 43, 1444–1452. [Google Scholar] [CrossRef]
- Silva, C.T.; Daneman, A.; Navarro, O.M.; Moore, A.M.; Moineddin, R.; Gerstle, J.T.; Mittal, A.; Brindle, M.; Epelman, M. Correlation of sonographic findings and outcome in necrotizing enterocolitis. Pediatr. Radiol. 2007, 37, 274–282. [Google Scholar] [CrossRef]
- Yikilmaz, A.; Hall, N.J.; Daneman, A.; Gerstle, J.T.; Navarro, O.M.; Moineddin, R.; Pleasants, H.; Pierro, A. Prospective evaluation of the impact of sonography on the management and surgical intervention of neonates with necrotizing enterocolitis. Pediatr. Surg. Int. 2014, 30, 1231–1240. [Google Scholar] [CrossRef]
- Ballance, W.A.; Dahms, B.B.; Shenker, N.; Kliegman, R.M. Pathology of neonatal necrotizing enterocolitis: A ten-year experience. J. Pediatr. 1990, 117, S6–S13. [Google Scholar] [CrossRef]
- Epelman, M.; Daneman, A.; Navarro, O.M.; Morag, I.; Moore, A.M.; Kim, J.H.; Faingold, R.; Taylor, G.; Gerstle, J.T. Necrotizing enterocolitis: Review of state-of-the-art imaging findings with pathologic correlation. Radiographics 2007, 27, 285–305. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef]
- Sharma, R.; Hudak, M.L.; Tepas, J.J., 3rd; Wludyka, P.S.; Marvin, W.J.; Bradshaw, J.A.; Pieper, P. Impact of gestational age on the clinical presentation and surgical outcome of necrotizing enterocolitis. J. Perinatol. 2006, 26, 342–347. [Google Scholar] [CrossRef]
- Kodroff, M.B.; Hartenberg, M.A.; Goldschmidt, R.A. Ultrasonographic diagnosis of gangrenous bowel in neonatal necrotizing enterocolitis. Pediatr. Radiol. 1984, 14, 168–170. [Google Scholar] [CrossRef]
- Cuna, A.C.; Reddy, N.; Robinson, A.L.; Chan, S.S. Bowel ultrasound for predicting surgical management of necrotizing enterocolitis: A systematic review and meta-analysis. Pediatr. Radiol. 2018, 48, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, F.; Zhang, Y.; Camp, M.; Mukherjee, D.; Gabre-Kidan, A.; Colombani, P.M.; Chang, D.C. Necrotizing enterocolitis in 20,822 infants: Analysis of medical and surgical treatments. Clin. Pediatr. 2010, 49, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Faingold, R. Technical aspects of abdominal ultrasound and color Doppler assessment of bowel viability in necrotizing enterocolitis. Pediatr. Radiol. 2018, 48, 617–619. [Google Scholar] [CrossRef] [PubMed]


| Variables | Total (n = 57) | Case (n = 12) | Control (n = 45) | p |
|---|---|---|---|---|
| Gestational age, weeks a | 24.6 (23.4, 25.4) | 25.3 (24.4, 26.1) | 24.6 (23.3, 25) | 0.119 b |
| Birth weight, g a | 620 (540, 730) | 680 (620, 835) | 610 (520, 730) | 0.106 b |
| Male sex | 33 (58%) | 5 (42%) | 28 (62%) | 0.200 c |
| Apgar score a | ||||
| At 1 min | 4 (3, 5) | 4 (2.5, 5.5) | 4 (3, 5) | 0.584 b |
| At 5 min | 7 (6, 8) | 7 (5, 8) | 7 (6, 9) | 0.301 b |
| Small for gestational age | 13 (23%) | 2 (17%) | 11 (24%) | 0.713 c |
| Chorioamnionitis | 28 (49%) | 5 (42%) | 23 (51%) | 0.561 c |
| Premature rupture of membrane | 17 (30%) | 3 (25%) | 14 (31%) | 1.000 c |
| Antenatal corticosteroid use | 56 (98%) | 11 (92%) | 45 (100%) | 0.211 c |
| High-grade intraventricular hemorrhage | 21 (37%) | 3 (25%) | 18 (40%) | 0.504 c |
| Neonatal sepsis | 52 (91%) | 8 (67%) | 44 (98%) | 0.006 c |
| Acute kidney injury | 22 (39%) | 4 (33%) | 18 (40%) | 0.841 c |
| NSAIDs use | 12 (21%) | 4 (33%) | 8 (18%) | 0.254 c |
| HFOV support | 32 (56%) | 5 (42%) | 27 (60%) | 0.418 c |
| Preoperative pletelet counts, ×109/L d | 154.1 ± 130.0 | 235.3 ± 161.5 | 132.4 ± 112.7 | 0.057 b |
| Postnatal age at laparotomy, days a | 21 (14, 36) | 30 (22, 45.5) | 20 (12, 31) | 0.018 b |
| Abdominal Radiographs | Total (n = 57) | Case (n = 12) | Control (n = 45) | p |
| Free air | 45 (79%) | 0 (0%) | 45 (100%) | |
| Bowel gas pattern | 1.000 | |||
| 1. Normal | 0 | 0 (0%) | 0 (0%) | |
| 2. Decreased gas or gasless | 10 (18%) | 2 (17%) | 8 (18%) | |
| 3: Ileus without elongated loops | 7 (12%) | 1 (8%) | 6 (13%) | |
| 4. Ileus with elongated loops | 40 (70%) | 9 (75%) | 31 (69%) | |
| Pneumatosis | 19 (33%) | 3 (25%) | 16 (36%) | 0.732 |
| Portal venous gas | 1 (2%) | 0 (0%) | 1 (2%) | 1.000 |
| Bowel US | Total (n = 48) | Case (n = 12) | Control (n = 36) | p |
| Interval between preoperative US and laparotomy, days | 3.5 ± 2.4 | 2.2 ± 2.6 | 3.9 ± 2.3 | 0.029 |
| Free air | 9 (19%) | 1 (8%) | 8 (22%) | 0.416 |
| Portal venous gas | 3 (6%) | 0 (0%) | 3 (8%) | 0.563 |
| Pneumatosis | 21 (44%) | 4 (33%) | 17 (47%) | 0.510 |
| Abdominal fluid | 48 (100%) | 12 (100%) | 36 (100%) | |
| Complex ascites or focal fluid collections | 39 (81%) | 12 (100%) | 27 (75%) | < 0.001 |
| Simple ascites | 2 (4%) | 0 (0%) | 2 (6%) | 1.000 |
| Bowel wall thickening | 45 (94%) | 12 (100%) | 33 (92%) | 0.563 |
| Absent or decreased bowel perfusion | 7 (15%) | 3 (25%) | 4 (11%) | 0.345 |
| Interval between first detection of complex ascites or focal fluid collections on US and laparotomy, days | 6.2 ± 5.6 | 4.3 ± 4.1 | 7.1 ± 6.0 | 0.158 |
| Case (n = 12) | Control (n = 45) | p | Multivariable Analyses | ||
|---|---|---|---|---|---|
| Adjusted OR/Estimate | p f | ||||
| Primary outcome | |||||
| Death before discharge | 1 (8%) | 20 (44%) | 0.040 d | 0.02 (0.00, 0.61) a | 0.025 |
| Secondary outcomes | |||||
| Short bowel syndrome | 1 (8%) | 5 (11%) | 1.000 d | 2.25 (0.13, 38.2) a | 0.576 |
| TPN dependence for 3 months or more | 2 (17%) | 7 (16%) | 1.000 d | 1.79 (0.20, 16.15 a | 0.603 |
| Length of hospital stay, days | 135 (126, 163) b | 133 (121, 209) b | 0.797 e | −16.64 (55.92) c | 0.770 |
| Bowel stricture requiring surgery | 1 (8%) | 5 (11%) | 1.000 d | 0.10 (0.00, 5.49) a | 0.262 |
| Sepsis after laparotomy | 2 (17%) | 19 (42%) | 0.177 d | 0.31 (0.02, 5.37) a | 0.425 |
| Acute kidney injury after laparotomy | 1 (8%) | 21 (47%) | 0.019 d | 0.02 (0.00, 1.00) a | 0.050 |
| Body weight at 36 weeks PMA, g | 1690 (1460, 1970) b | 1535 (1230, 1810) b | 0.007 e | 297.64 (226.58) c | 0.210 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.K.; Jeon, T.Y.; Kim, K.; Kim, Y.J.; Yoo, S.-Y.; Kim, J.H.; Chang, Y.S.; Lee, S.; Seo, J.-M.; Moon, S.-H. Clinical Outcome of Ultrasound-Detected Perforated Necrotizing Enterocolitis without Radiographic Pneumoperitoneum in Very Preterm Infants. J. Clin. Med. 2023, 12, 1805. https://doi.org/10.3390/jcm12051805
Kim MK, Jeon TY, Kim K, Kim YJ, Yoo S-Y, Kim JH, Chang YS, Lee S, Seo J-M, Moon S-H. Clinical Outcome of Ultrasound-Detected Perforated Necrotizing Enterocolitis without Radiographic Pneumoperitoneum in Very Preterm Infants. Journal of Clinical Medicine. 2023; 12(5):1805. https://doi.org/10.3390/jcm12051805
Chicago/Turabian StyleKim, Myoung Kyoung, Tae Yeon Jeon, Kyunga Kim, Yu Jin Kim, So-Young Yoo, Ji Hye Kim, Yun Sil Chang, Sanghoon Lee, Jeong-Meen Seo, and Sung-Hoon Moon. 2023. "Clinical Outcome of Ultrasound-Detected Perforated Necrotizing Enterocolitis without Radiographic Pneumoperitoneum in Very Preterm Infants" Journal of Clinical Medicine 12, no. 5: 1805. https://doi.org/10.3390/jcm12051805
APA StyleKim, M. K., Jeon, T. Y., Kim, K., Kim, Y. J., Yoo, S.-Y., Kim, J. H., Chang, Y. S., Lee, S., Seo, J.-M., & Moon, S.-H. (2023). Clinical Outcome of Ultrasound-Detected Perforated Necrotizing Enterocolitis without Radiographic Pneumoperitoneum in Very Preterm Infants. Journal of Clinical Medicine, 12(5), 1805. https://doi.org/10.3390/jcm12051805






