Sonographic Features of Onychopapilloma: A Single Center Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
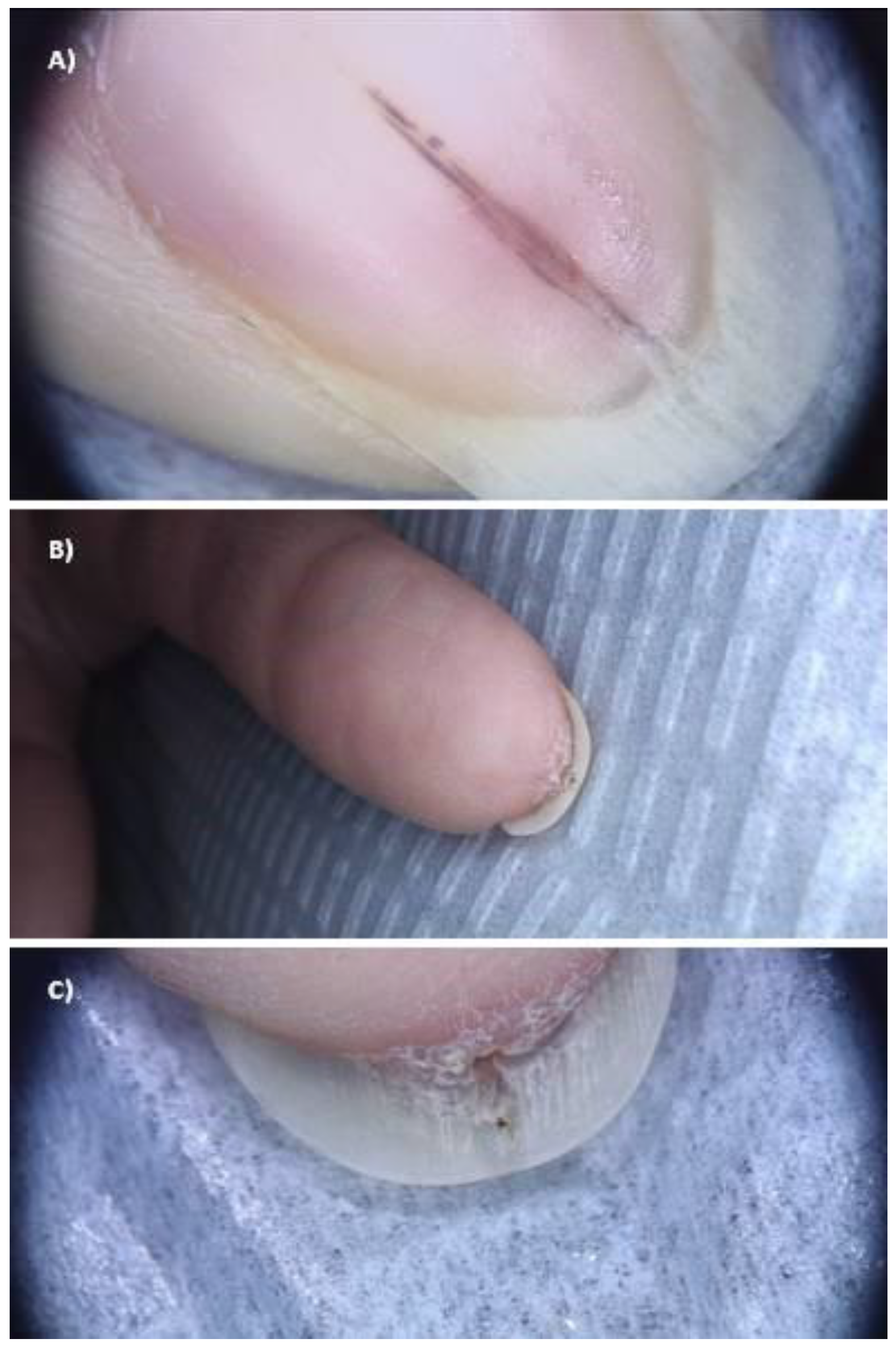
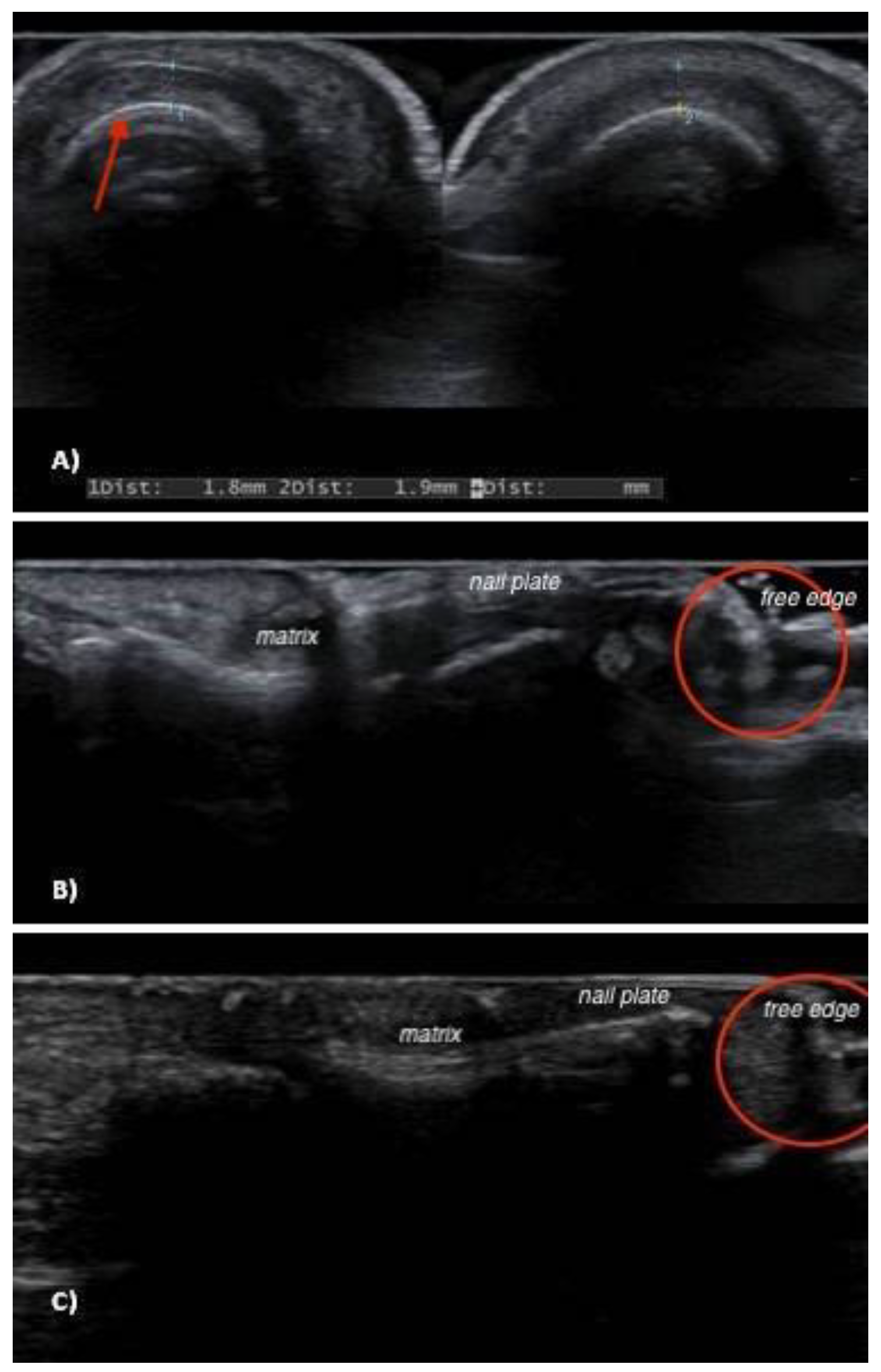
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baran, R.; Perrin, C. Localized multinucleate distal subungual keratosis. Br. J. Dermatol. 1995, 133, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Perrin, C. Linear melanonychia due to subungual keratosis of the nail bed: A report of two cases. Br. J. Dermatol. 1999, 140, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Perrin, C. Longitudinal erythronychia with distal subungual keratosis: Onychopapilloma of the nail bed and Bowen’s disease. Br. J. Dermatol. 2000, 143, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Gee, B.C.; Millard, P.R.; Dawber, R.P. Onychopapilloma is not a distinct clinicopathological entity. Br. J. Dermatol. 2002, 146, 156–157. [Google Scholar] [CrossRef]
- Richert, B.; Iorizzo, M.; Tosti, A.; André, J. Nail bed lichen planus associated with onychopapilloma. Br. J. Dermatol. 2007, 156, 1071–1072. [Google Scholar] [CrossRef]
- Criscione, V.; Telang, G.; Jellinek, N.J. Onychopapilloma presenting as longitudinal leukonychia. J. Am. Acad. Dermatol. 2010, 63, 541–542. [Google Scholar] [CrossRef]
- Miteva, M.; Fanti, P.A.; Romanelli, P.; Zaiac, M.; Tosti, A. Onychopapilloma presenting as longitudinal melanonychia. J. Am. Acad. Dermatol. 2012, 66, e242–e243. [Google Scholar] [CrossRef]
- Beggs, S.; Butala, N.; Heymann, W.R.; Rubin, A.I. Onychopapilloma Presenting as Longitudinal Erythronychia in an Adolescent. Pediatr. Dermatol. 2015, 32, e173–e174. [Google Scholar] [CrossRef]
- Ito, T.; Uchi, H.; Yamada, Y.; Oda, Y.; Furue, M. Onychopapilloma manifesting longitudinal melanonychia: A mimic of subungual malignancy. J. Dermatol. 2015, 42, 1199–1201. [Google Scholar] [CrossRef]
- Kim, M.; Sun, E.Y.; Jung, H.Y.; Cho, B.K.; Park, H.J. Onychopapilloma: A Report of Three Cases Presenting with Various Longitudinal Chromonychia. Ann. Dermatol. 2016, 28, 655–657. [Google Scholar] [CrossRef]
- Jellinek, N.J.; Lipner, S.R. Longitudinal Erythronychia: Retrospective Single-Center Study Evaluating Differential Diagnosis and the Likelihood of Malignancy. Dermatol. Surg. 2016, 42, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Schneider, S.L.; Ramirez-Quizon, M.N.; Zaiac, M.; Miteva, M. Clinical, dermoscopic, and pathologic features of onychopapilloma: A review of 47 cases. J. Am. Acad. Dermatol. 2016, 74, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Halteh, P.; Magro, C.; Scher, R.K.; Lipner, S.R. Onychopapilloma Presenting as Leukonychia: Case Report and Review of the Literature. Ski. Appendage Disord. 2017, 2, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Sarkissian, L.; Fattouh, K.; Kanitakis, J.; Villani, A.P. DeRmpath & Clinic: Onychopapilloma. Eur. J. Dermatol. 2017, 27, 336–337. [Google Scholar] [PubMed]
- Delvaux, C.; Richert, B.; Lecerf, P.; André, J. Onychopapillomas: A 68-case series to determine best surgical procedure and histologic sectioning. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2025–2030. [Google Scholar] [CrossRef]
- Kim, M.; Jo, G.; Lee, C.; Mun, J.H. Onychopapilloma Presenting as Erythronychia and Leukonychia: Dermoscopic Features of Two Cases in Korea. Ann. Dermatol. 2018, 30, 742–744. [Google Scholar] [CrossRef]
- Ramos Pinheiro, R.; Cunha, N.; Lencastre, A. And next… Adnexa: Onychopapilloma. Eur. J. Dermatol. 2018, 28, 137–138. [Google Scholar] [CrossRef]
- Baltz, J.O.; Telang, G.H.; Jellinek, N.J. Subungual Caliber Persistent Artery of the Nail Unit Coincident with Onychopapilloma. Dermatol. Surg. 2020, 46, 1748–1749. [Google Scholar] [CrossRef]
- Park, H.J.; Park, H.J. A Rare Case of Onychopapilloma Presenting as a Longitudinal Erythronychia. Ann. Dermatol. 2020, 32, 74–76. [Google Scholar] [CrossRef]
- Hashimoto, H.; Ito, T.; Yamada, Y.; Oda, Y.; Furue, M. Onychopapilloma presenting as longitudinal melanonychia: A case report and literature review. Australas. J. Dermatol. 2021, 62, 244–246. [Google Scholar] [CrossRef]
- Starace, M.; Ferrari, T.; Pezzetta, S.; Savoia, F.; Zengarini, C.; Piraccini, B.M.; Alessandrini, A. Pigmented onychopapilloma in Caucasians: A case series of six patients. Int. J. Dermatol. 2021, 60, e192–e194. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.R.; Bae, K.N.; Son, J.H.; Shin, K.; Kim, H.S.; Ko, H.C.; Kim, B.S.; Kim, M.B. Onychopapilloma: Its clinical, dermoscopic and pathologic features. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, F.; Wang, X.; Liu, Z.; Wong, H.S.; Zhou, Y.; Wang, D. Expression patterns of hair-related keratins and epithelial keratins in onychopapilloma: The significance of clarifying the origin of onychopapilloma. Front. Med. 2022, 9, 1059624. [Google Scholar] [CrossRef] [PubMed]
- Starace, M.; Alessandrini, A.; Ferrari, T.; Wong, V.; Baraldi, C.; Piraccini, B.M. Clinical and onychoscopic features of histopathologically proven onychopapillomas and literature update. J. Cutan. Pathol. 2022, 49, 147–152. [Google Scholar] [CrossRef]
- Yun, J.S.W.; Howard, A.; Prakash, S.; Kern, J.S. Clinical and histopathological features of onychopapilloma in an Australian setting: A case series of 50 patients. Australas. J. Dermatol. 2022, 63, e350–e355. [Google Scholar] [CrossRef] [PubMed]
- Jellinek, N.J. Longitudinal erythronychia: Suggestions for evaluation and management. J. Am. Acad. Dermatol. 2011, 64, 167.e1–11. [Google Scholar] [CrossRef]
- Cohen, P.R. Longitudinal erythronychia: Individual or multiple linear red bands of the nail plate: A review of clinical features and associated conditions. Am. J. Clin. Dermatol. 2011, 12, 217–231. [Google Scholar] [CrossRef]
- Cogrel, O.; Beylot-Barry, M.; Doutre, M.S. Maladie de Bowen unguéale revelée par une érythronychie longitudinale [Subungual squamous cell carcinoma revealed by longitudinal erythronychia]. Ann. Dermatol. Venereol. 2008, 135, 883–885. (In French) [Google Scholar] [CrossRef]
- Nazzaro, G. High-frequency ultrasound is a reliable diagnostic tool in dermatology. J. Clin. Ultrasound 2022, 50, 1443–1444. [Google Scholar] [CrossRef]
- Marina, M.E.; Botar Jid, C.; Roman, I.I.; Mihu, C.M.; Tătaru, A.D. Ultrasonography in psoriatic disease. Med. Ultrason. 2015, 17, 377–382. [Google Scholar] [CrossRef]
- De Berker, D.A.; Perrin, C.; Baran, R. Localized longitudinal erythronychia: Diagnostic significance and physical explanation. Arch. Dermatol. 2004, 140, 1253–1257. [Google Scholar] [CrossRef]
- Wortsman, X. Concepts, Role, and Advances on Nail Imaging. Dermatol. Clin. 2021, 39, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Sechi, A.; Alessandrini, A.; Patrizi, A.; Starace, M.; Caro, R.D.C.; Vara, G.; Brandi, N.; Golfieri, R.; Piraccini, B.M. Ultrasound features of the subungual glomus tumor and squamous cell carcinomas. Ski. Res. Technol. 2020, 26, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.J.; Lee, S.J.; Cho, K.H.; Choo, H.J.; Lee, S.M.; Lee, Y.H.; Suh, K.J.; Moon, T.Y.; Cha, J.G.; Yi, J.H.; et al. Subungual tumors: Clinicopathologic correlation with US and MR imaging findings. Radiographics 2010, 30, 1621–1636. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.; Roldán, F.A.; Varelli, C.; Bard, R.; Corvino, A.; Wortsman, X. Skin cancer: Findings and role of high-resolution ultrasound. J. Ultrasound 2019, 22, 423–431. [Google Scholar] [CrossRef]
- Jaramillo, F.A.; Mejía, D.C.Q.; Ordúz, H.M.M.; Ardila, C.G. Nail unit ultrasound: A complete guide of the nail diseases. J. Ultrasound 2017, 20, 181–192. [Google Scholar] [CrossRef]

| Year of Publication | Authors | Patients (n) | Age, Gender (n) | Clinical Features (n) | Histopathological Features | Surgery/Follow-Up (n) | Type of Study |
|---|---|---|---|---|---|---|---|
| 1995 | Baran and Perrin [1] | 4 | Not reported | Distal onycholysis (3) Melanonychia (1) Splinter hemorrhages (3) Subungual hyperkeratosis (4) | Nail bed acanthosis, papillomatosis, and matrix metaplasia; multinucleated KC | Surgery | Case reports |
| 1999 | Baran and Perrin [2] | 2 | 71, F (1), 55, M (1) | Melanonychia (2) Subungual hyperkeratosis (1) | Homogeneous corneal mass with parakeratosis | Surgery | Case reports |
| 2000 | Baran and Perrin [3] | 14 | Age Range 18–66 Gender not reported | Distal onycholysis (14) Erythronychia (14) Splinter hemorrhages (14) Subungual hyperkeratosis (14) | Nail bed acanthosis, papillomatosis, and metaplasia; multinucleated KC | Surgery | Case series |
| 2002 | Gee et al. [4] | 1 | Not reported | Erythronychia (1) Subungual hyperkeratosis (1) | Nail bed acanthosis, papillomatosis, and matrix metaplasia. Nail matrix basaloid cells with palisading | Surgery | Case report |
| 2007 | Richert et al. [5] | 1 | 19, F (1) | Distal onycholysis (1) Subungual hyperkeratosis (1) | Nail bed acanthosis and hypergranulosis + lichenoid dermal infiltrate | Surgery | Case report |
| 2010 | Criscione et al. [6] | 1 | 50, F (1) | Distal V-shaped notch and split (1) Leukonychia (1) Subungual hyperkeratosis (1) | Nail bed acanthosis, papillomatosis, and matrix metaplasia | Surgery | Case report |
| 2012 | Miteva et al. [7] | 1 | 58, F (1) | Melanonychia (1) Subungual hyperkeratosis (1) | Nail bed acanthosis, papillomatosis, and matrix metaplasia | Surgery | Case report |
| 2015 | Beggs et al. [8] | 1 | 15, M (1) | Erythronychia (1) Subungual hyperkeratosis (1) | Nail bed acanthosis, papillomatosis, and matrix metaplasia | Surgery | Case report |
| 2015 | Ito et al. [9] | 1 | 37, F (1) | Distal onycholysis (1) Melanonychia (1) | Nail bed acanthosis and matrix metaplasia. Elongated hyperplastic rete ridges | Surgery | Case report |
| 2016 | Kim et al. [10] | 3 | 47.8, F (1), M (2) | Erythronychia (1) Multiple yellow chromonychia (1) Reddish-yellow chromonychia (1) | Digitation of the epithelium with abundant eosinophilic cytoplasm | Surgery | Case reports |
| 2016 | Jellinek et al. [11] | 41 | 58, F (24), M (17) | Erythronychia (41) | Not reported | Surgery | Retrospective single-center study |
| 2016 | Tosti et al. [12] | 47 | Age not reported F (33), M (14) | Distal fissures (11) Erythronychia (25) Leukonychia (7) Melanonychia (8) Splinter hemorrhages (11) Subungual mass (47) | Nail bed acanthosis, papillomatosis. Subungual hyperkeratosis and focal parakeratosis | Surgery | Retrospective single-center study |
| 2017 | Halteh et al. [13] | 1 | 71, F (1) | Leukonychia (1) Subungual hyperkeratosis (1) | Nail bed and matrix metaplasia | Surgery | Case report |
| 2017 | Sarkissian et al. [14] | 1 | 38, M (1) | Erythronychia (1) Splinter hemorrhages (1) Subungual hyperkeratosis (1) | Nail bed acanthosis, papillomatosis, and parakeratosis | Surgery | Case report |
| 2018 | Delvaux et al. [15] | 68 | 46.1 ± 2.2, F (42), M (26) | Distal fissures (33) Distal onycholysis (34) Erythronychia (53) Notch in the lunula (39) Subungual hyperkeratosis (56) | Nail bed papillomatosis and acanthosis. Onychogenisis zone | Surgery (63) Follow-up (4) Lost at follow-up (1) | Retrospective single-center study |
| 2018 | Kim et al. [16] | 2 | 62, 59, F (2) | Distal hyperkeratosis (2) Erythronychia (1) Splinter hemorrhages (2) White- yellow chromonychia (2) | Nail acanthosis, papillomatosis, and matrix metaplasia | Surgery | Case reports |
| 2018 | Ramos Pinheiro et al. [17] | 1 | 63, F (1) | Erythronychia (1) Hyperkeratotic mass (1) | Nail bed and distal matrix papillomatosis and acanthosis. Hyperkeratotic horn | Surgery | Case report |
| 2019 | Baltz et al. [18] | 1 | 60, F (1) | Distal onycholysis (1) Erythronychia (1) | Nail bed acanthosis and matrix metaplasia | Surgery | Case report |
| 2020 | Park et al. [19] | 1 | 50, F (1) | Distal onycholysis (1) Erythronychia (1) Splinter hemorrhages (1) Subungual hyperkeratosis (1) | Nail bed acanthosis and papillomatosis | Surgery | Case report |
| 2021 | Hashimoto et al. [20] | 1 | 54, M (1) | Melanonychia (1) | Nail bed hyperplasia and matrix metaplasia | Surgery | Case report |
| 2021 | Starace et al. [21] | 6 | 59, F (3), M (3) | Melanonychia (6) | Distal matrix acanthosis and nail bed papillomatosis | Surgery | Case series |
| 2022 | Kim et al. [22] | 39 | Age range 16–77, F (16), M (23) | Erythronychia (22) | Nail bed papillomatosis | Surgery | Retrospective single-center study |
| 2022 | Liu et al. [23] | 11 | Age range 13–54, F (6), M (5) | Distal V-shaped notch and split (2) Erythronychia (6) Leukonychia (2) Melanonychia (3) Splinter hemorrhages (2) Subungual hyperkeratosis (11) | Nail bed papillomatosis with or without acanthosis and matrix metaplasia | Surgery | Retrospective single-center study |
| 2022 | Starace et al. [24] | 17 | 56.3, F (13), M (4) | Distal fissures (8) Distal onycholysis (4) Distal subungual keratotic papule (5) Erythronychia (9) Leukonychia (3) Melanonychia (2) Splinter hemorrhages (2) Yellow-brown chromonychia (1) | Nail bed acanthosis, papillomatosis, and matrix metaplasia. Splinter hemorrhages | Surgery | Retrospective single-center study |
| 2022 | Yun et al. [25] | 50 | 54.5, F (68%), M (32%) | Distal fissures (12) Erythronychia (11) Subungual hyperkeratosis (15) | Nail bed papillomatosis and matrix metaplasia, subungual hyperkeratosis, and hemorrhage | Surgery | Retrospective single-center study |
| Tot (24) | - | 316 | F (183); M (113) | Chromonychia (5) Distal fissures (64) Distal onycholysis (59) Erythronychia (183) Leukonychia (12) Melanonychia (22) Notch in the lunula (40) Splinter hemorrhages (34) Subungual hyperkeratosis (151) | Nail bed papillomatosis and matrix metaplasia | Surgery (311), follow up (5) | - |
| Patient (Age, Sex) | Clinical Features | Ultrasonography | ||
|---|---|---|---|---|
| Nail Bed Dishomogeneity | Distal Subungual Mass | Doppler Signal | ||
| 1 (M, 66) | Melanonychia | NO | YES, hyperechoic | NO |
| 2 (M, 71) | Erythronychia | NO | NO | NO |
| 3 (F, 64) | Melanonychia | YES | YES, hyperechoic | NO |
| 4 (F, 34) | Erythronychia | YES | YES, hyperechoic | NO |
| 5 (F, 23) | Splinter hemorrhages | YES | YES, hyperechoic | NO |
| 6 (F, 75) | Erythronychia | NO | YES, hyperechoic | NO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattioli, M.A.; Aromolo, I.F.; Spigariolo, C.B.; Marzano, A.V.; Nazzaro, G. Sonographic Features of Onychopapilloma: A Single Center Retrospective Observational Study. J. Clin. Med. 2023, 12, 1795. https://doi.org/10.3390/jcm12051795
Mattioli MA, Aromolo IF, Spigariolo CB, Marzano AV, Nazzaro G. Sonographic Features of Onychopapilloma: A Single Center Retrospective Observational Study. Journal of Clinical Medicine. 2023; 12(5):1795. https://doi.org/10.3390/jcm12051795
Chicago/Turabian StyleMattioli, Maria A., Italo F. Aromolo, Cristina B. Spigariolo, Angelo V. Marzano, and Gianluca Nazzaro. 2023. "Sonographic Features of Onychopapilloma: A Single Center Retrospective Observational Study" Journal of Clinical Medicine 12, no. 5: 1795. https://doi.org/10.3390/jcm12051795
APA StyleMattioli, M. A., Aromolo, I. F., Spigariolo, C. B., Marzano, A. V., & Nazzaro, G. (2023). Sonographic Features of Onychopapilloma: A Single Center Retrospective Observational Study. Journal of Clinical Medicine, 12(5), 1795. https://doi.org/10.3390/jcm12051795








