Three-Dimensional Evaluation Effects of Microimplant-Assisted Rapid Palatal Expansion on the Upper Airway Volume: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
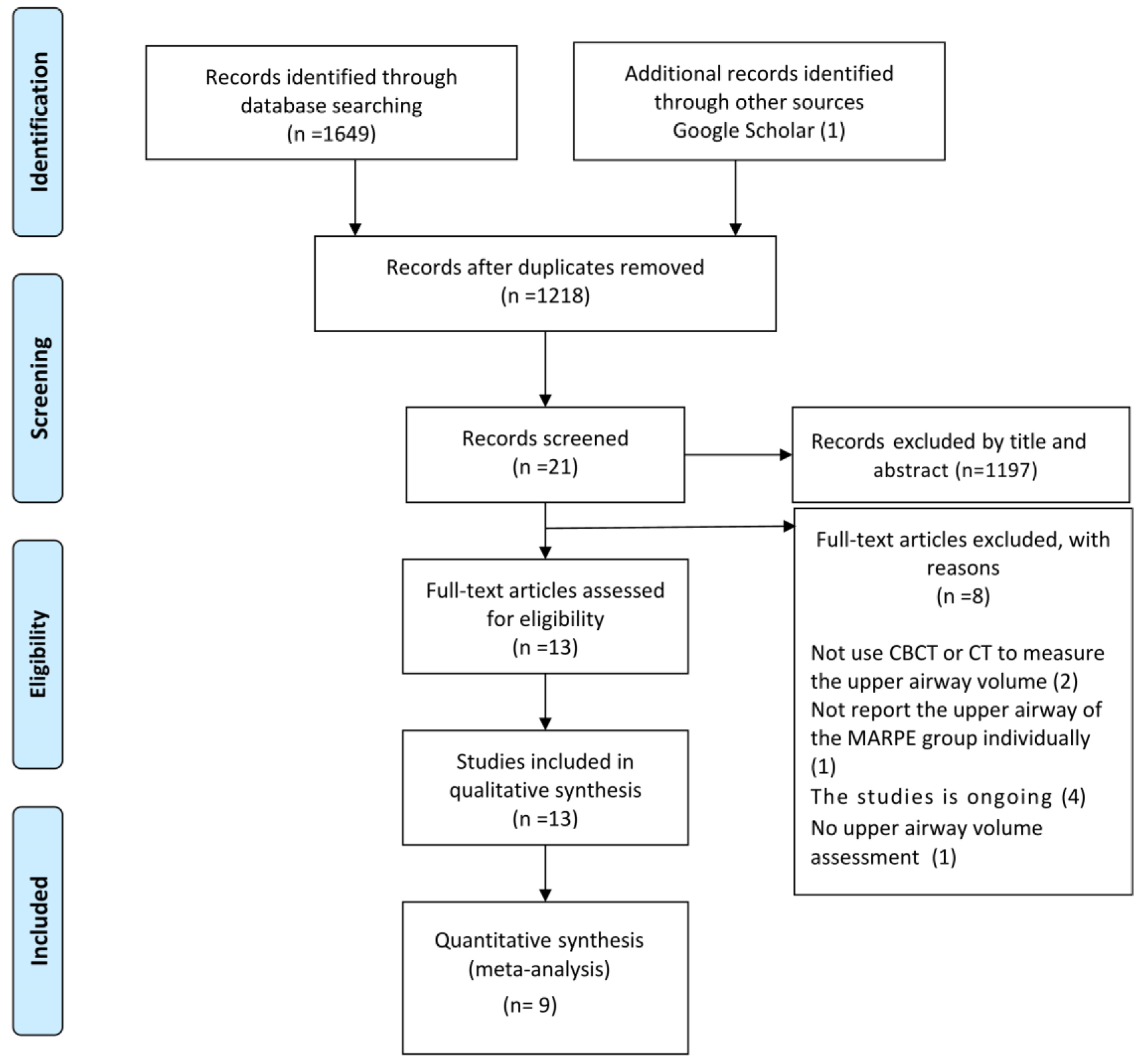
2.4. Study Selection
2.5. Data Items and Collection
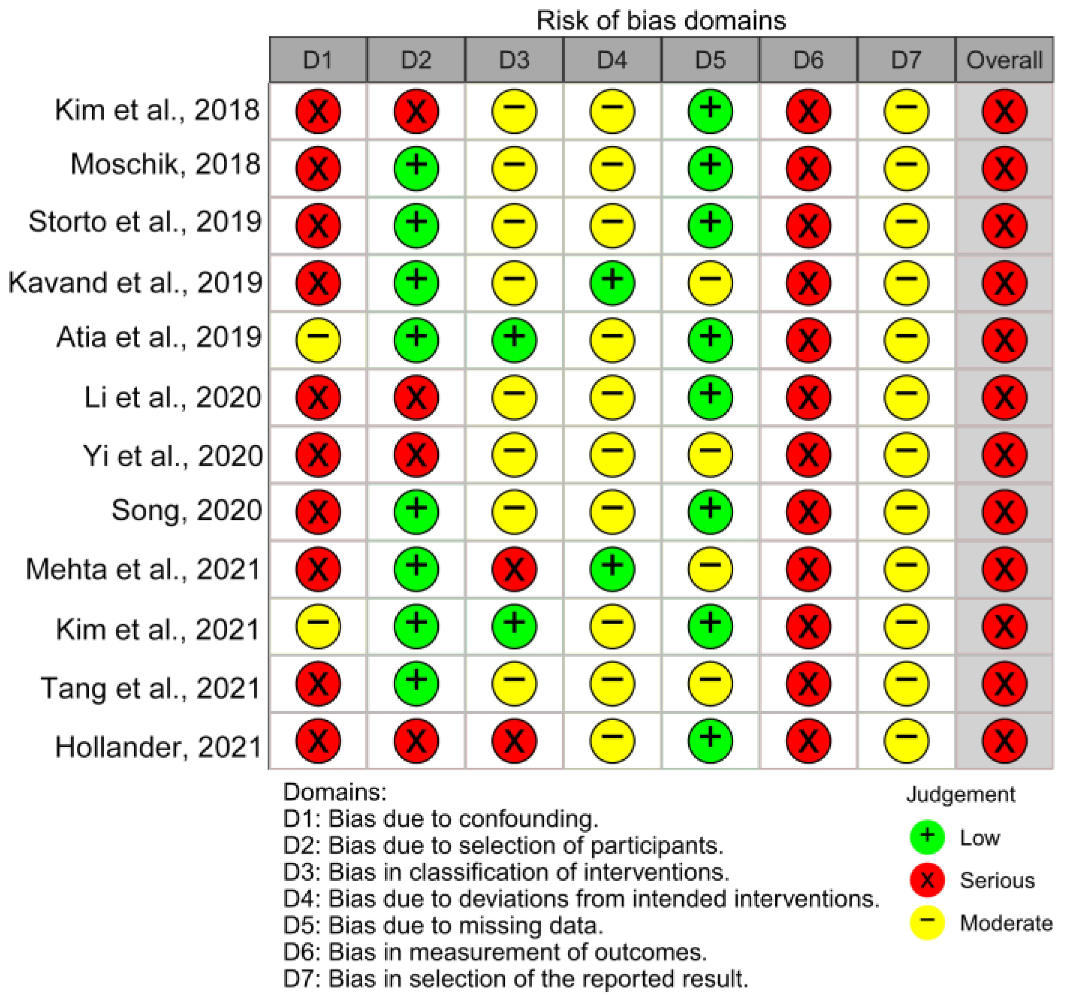
2.6. Risk of Bias in Individual Studies
2.7. Summary Measures and Approach to Synthesis
2.8. Risk of Bias Assessment across Studies
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Individual Studies and across the Studies
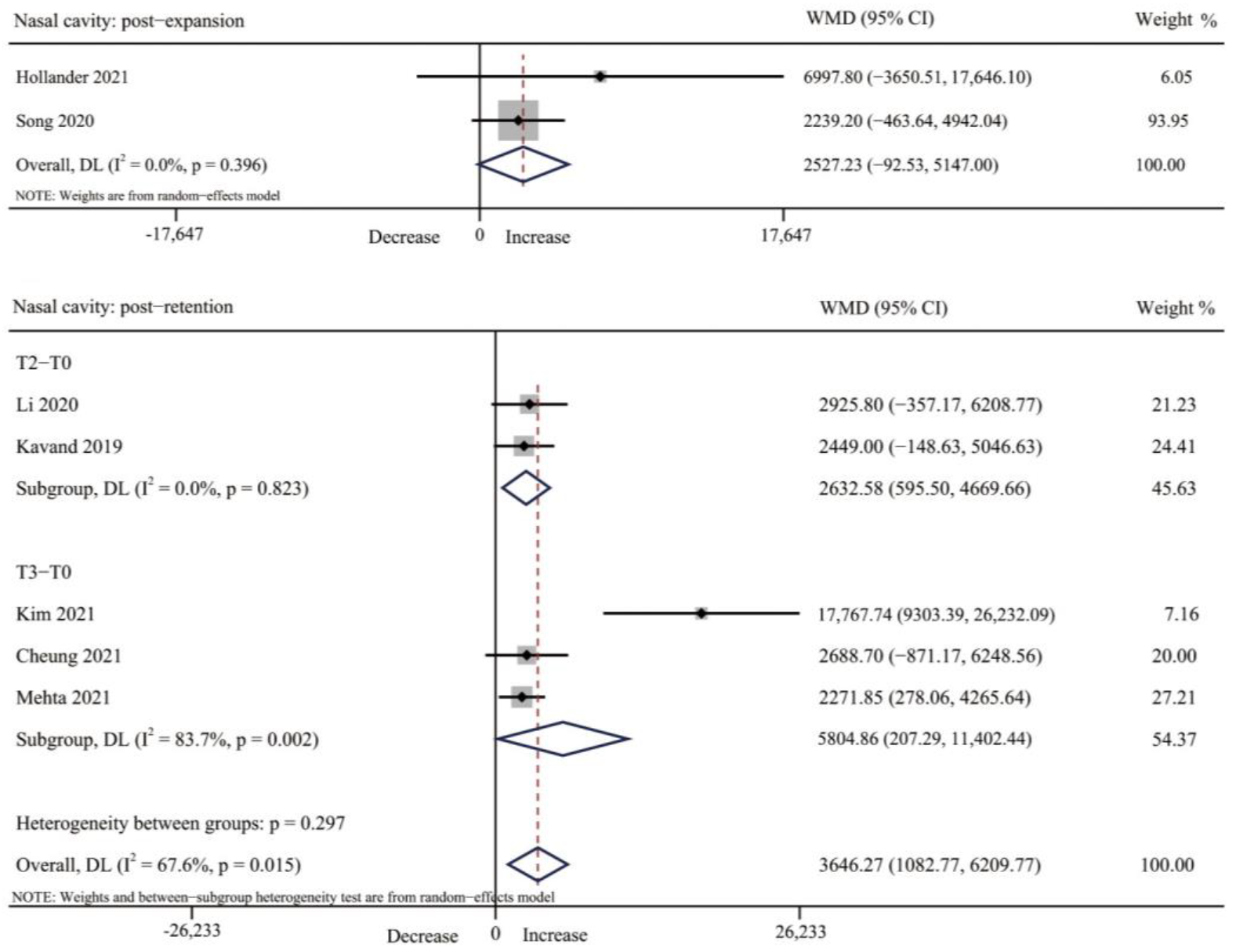
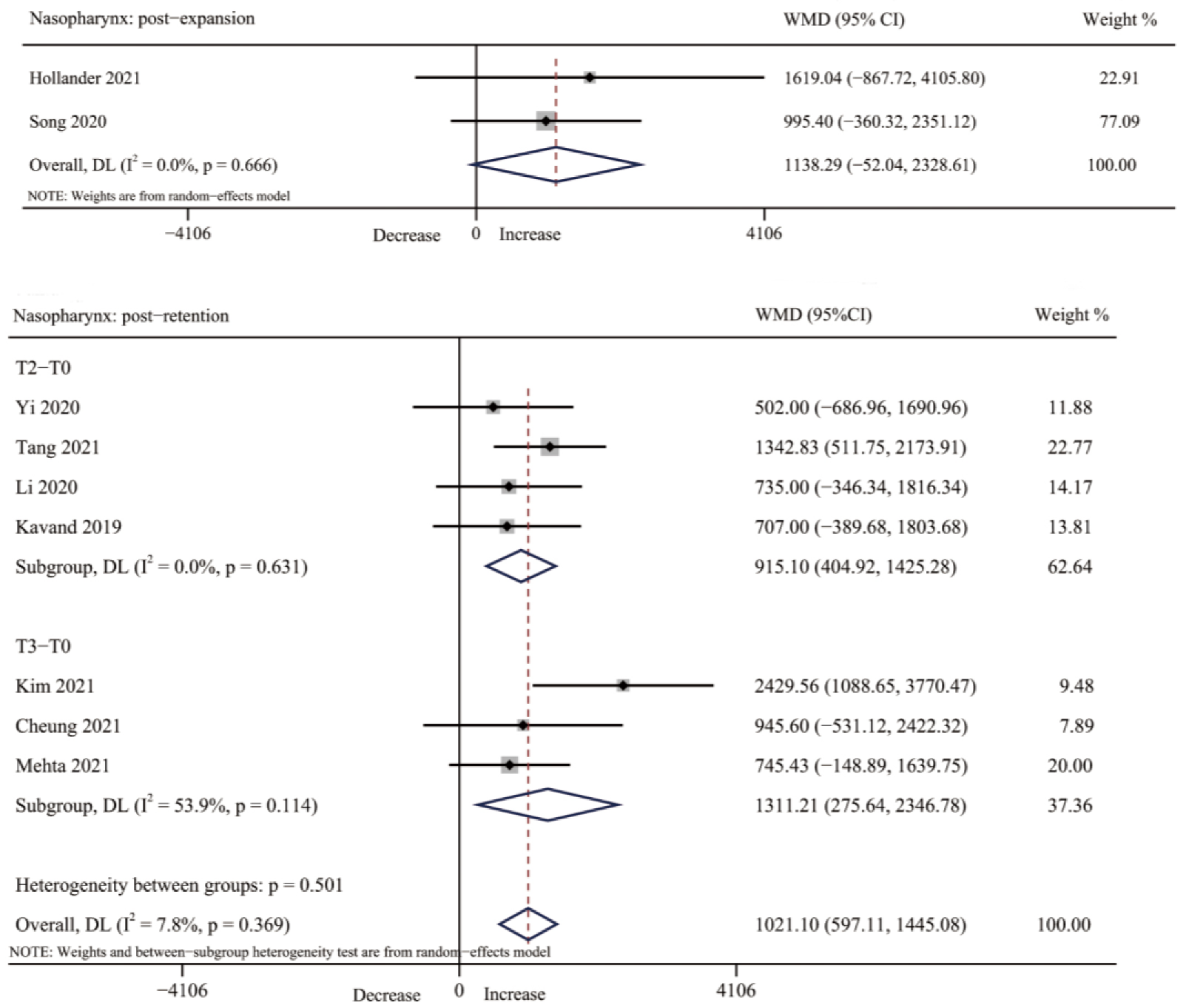
3.4. Results of Individual Studies, Meta-Analyses, and Subgroup Analyses
3.4.1. Nasal Cavity Volume
3.4.2. Nasopharynx Volume
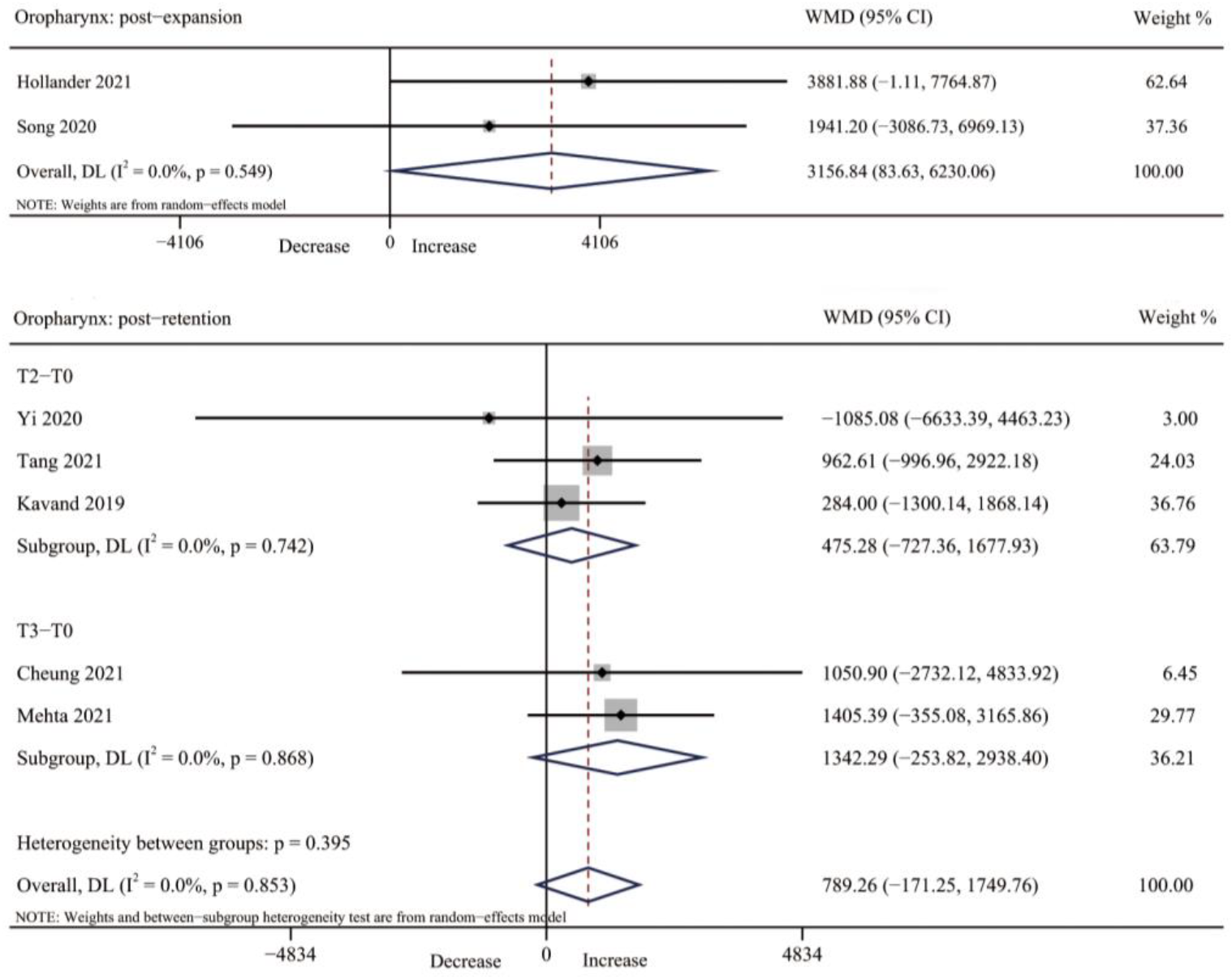
3.4.3. Oropharynx Volume
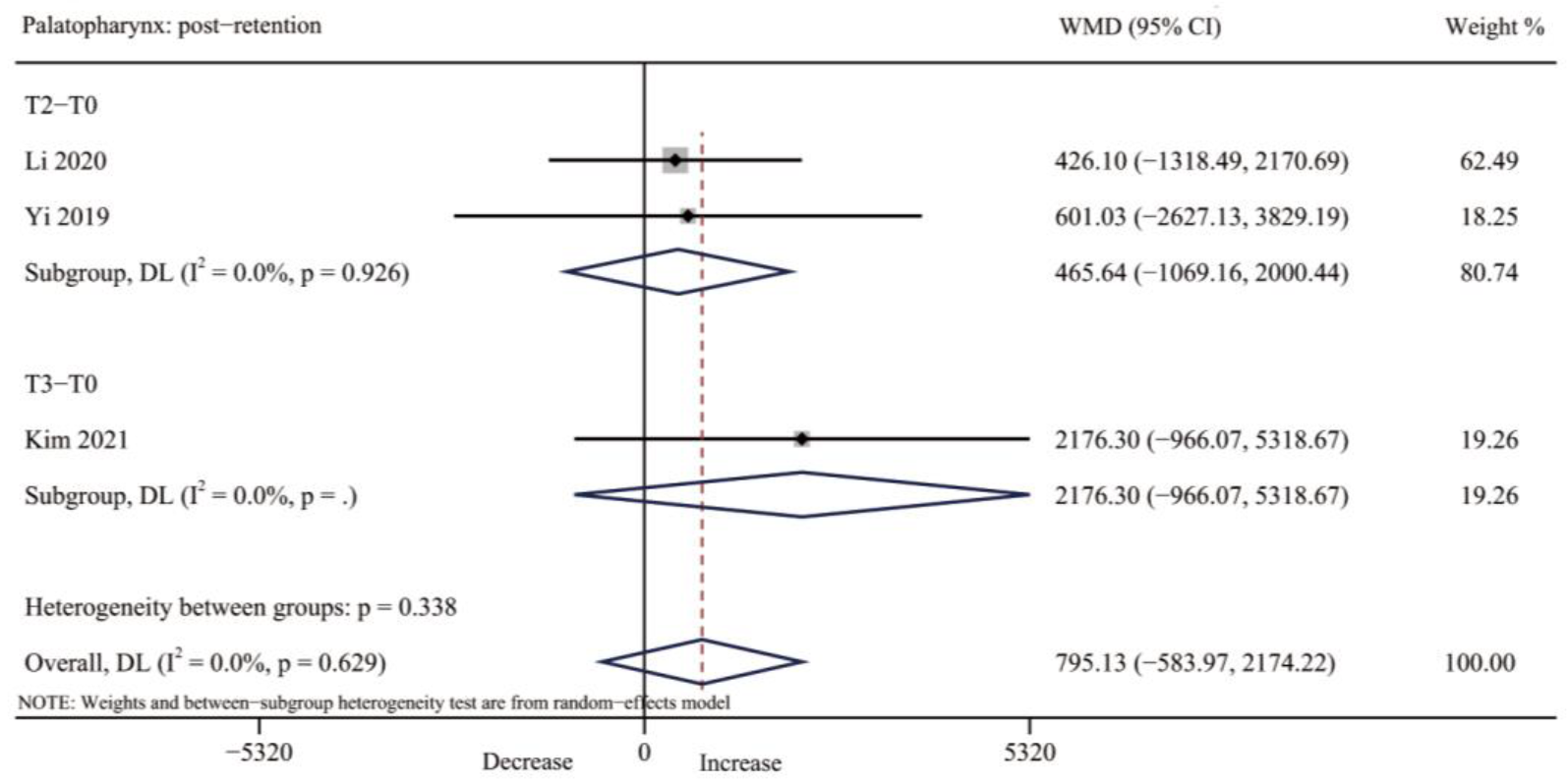
3.4.4. Palatopharynx Volume
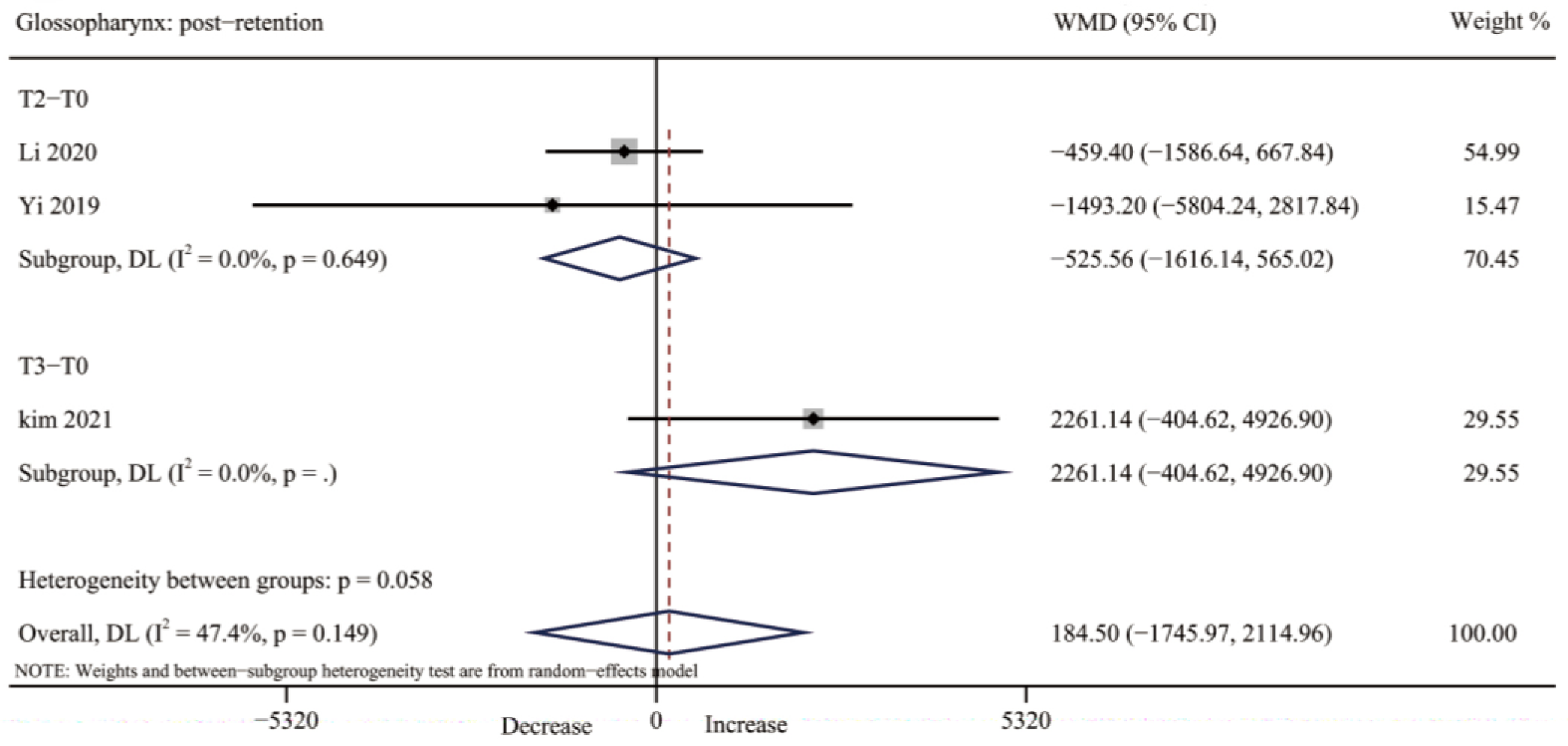
3.4.5. Glossopharynx Volume
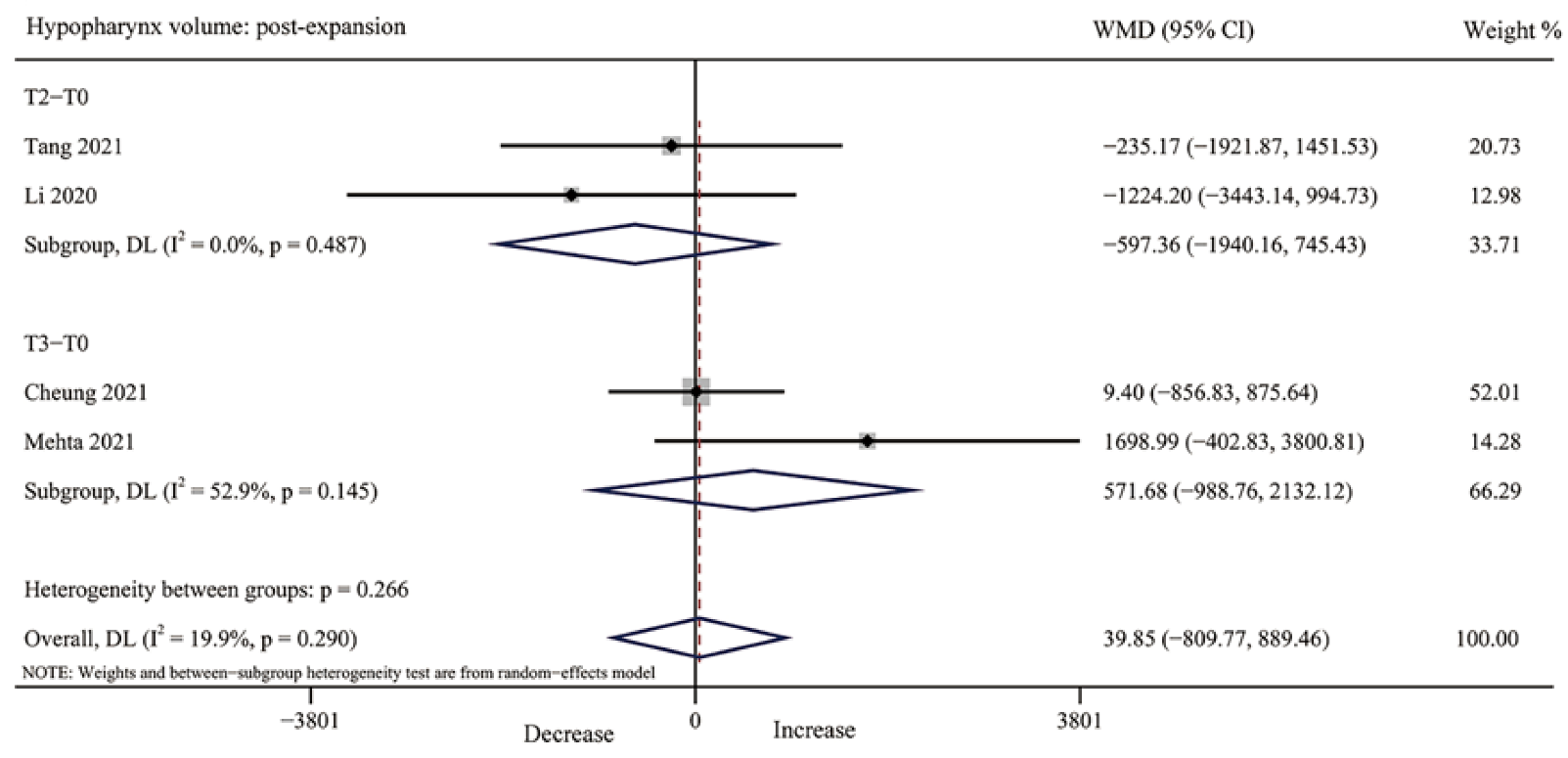
3.4.6. Hypopharynx Volume
3.4.7. Maxillary Sinus Volume
3.4.8. Additional Analyses
4. Discussion
4.1. Summary of Evidence
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timms, D.J. A study of basal movement with rapid maxillary expansion. Am. J. Orthod. 1980, 77, 500–507. [Google Scholar] [CrossRef]
- Ramires, T.; Maia, R.A.; Barone, J.R. Nasal cavity changes and the respiratory standard after maxillary expansion. Braz. J. Otorhinolaryngol. 2008, 74, 763–769. [Google Scholar] [CrossRef]
- Aloufi, F.; Preston, C.B.; Zawawi, K.H. Changes in the upper and lower pharyngeal airway spaces associated with rapid maxillary expansion. ISRN Dent. 2012, 2012, 290964. [Google Scholar] [CrossRef]
- McDonald, J.P. Airway problems in children—Can the orthodontist help? Ann. Acad. Med. Singap. 1995, 24, 158–162. [Google Scholar]
- Redline, S.; Tishler, P.V.; Tosteson, T.D. The familial aggregation of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995, 151, 682–687. [Google Scholar] [CrossRef]
- Seto, B.H.; Gotsopoulos, H.; Sims, M.R.; Cistulli, P.A. Maxillary morphology in obstructive sleep apnoea syndrome. Eur. J. Orthod. 2001, 23, 703–714. [Google Scholar] [CrossRef]
- Persson, M.; Thilander, B. Palatal suture closure in man from 15 to 35 years of age. Am. J. Orthod. 1977, 72, 42–52. [Google Scholar] [CrossRef]
- Baysal, A.; Karadede, I.; Hekimoglu, S.; Ucar, F.; Ozer, T.; Veli, I.; Uysal, T. Evaluation of root resorption following rapid maxillary expansion using cone-beam computed tomography. Angle Orthod. 2012, 82, 488–494. [Google Scholar] [CrossRef]
- Baysal, A.; Uysal, T.; Veli, I.; Ozer, T.; Karadede, I.; Hekimoglu, S. Evaluation of alveolar bone loss following rapid maxillary expansion using cone-beam computed tomography. Korean J. Orthod. 2013, 43, 83–95. [Google Scholar] [CrossRef]
- Koudstaal, M.J.; Poort, L.J.; van der Wal, K.G.; Wolvius, E.B.; Prahl-Andersen, B.; Schulten, A.J. Surgically assisted rapid maxillary expansion (SARME): A review of the literature. Int. J. Oral. Maxillofac. Surg. 2005, 34, 709–714. [Google Scholar] [CrossRef]
- Williams, B.J.; Currimbhoy, S.; Silva, A.; O’Ryan, F.S. Complications following surgically assisted rapid palatal expansion: A retrospective cohort study. J. Oral. Maxillofac. Surg. 2012, 70, 2394–2402. [Google Scholar] [CrossRef]
- Carlson, C.; Sung, J.; McComb, R.W.; Machado, A.W.; Moon, W. Microimplant-assisted rapid palatal expansion appliance to orthopedically correct transverse maxillary deficiency in an adult. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 716–728. [Google Scholar] [CrossRef]
- Park, J.J.; Park, Y.C.; Lee, K.J.; Cha, J.Y.; Tahk, J.H.; Choi, Y.J. Skeletal and dentoalveolar changes after miniscrew-assisted rapid palatal expansion in young adults: A cone-beam computed tomography study. Korean J. Orthod. 2017, 47, 77–86. [Google Scholar] [CrossRef]
- Buck, L.M.; Dalci, O.; Darendeliler, M.A.; Papageorgiou, S.N.; Papadopoulou, A.K. Volumetric upper airway changes after rapid maxillary expansion: A systematic review and meta-analysis. Eur. J. Orthod. 2017, 39, 463–473. [Google Scholar] [CrossRef]
- Niu, X.; Di Carlo, G.; Cornelis, M.A.; Cattaneo, P.M. Three-dimensional analyses of short- and long-term effects of rapid maxillary expansion on nasal cavity and upper airway: A systematic review and meta-analysis. Orthod. Craniofac. Res. 2020, 23, 250–276. [Google Scholar] [CrossRef]
- Alyessary, A.S.; Othman, S.A.; Yap, A.U.J.; Radzi, Z.; Rahman, M.T. Effects of non-surgical rapid maxillary expansion on nasal structures and breathing: A systematic review. Int. Orthod. 2019, 17, 12–19. [Google Scholar] [CrossRef]
- Giudice, A.L.; Spinuzza, P.; Rustico, L.; Messina, G.; Nucera, R. Short-term treatment effects produced by rapid maxillary expansion evaluated with computed tomography: A systematic review with meta-analysis. Korean J. Orthod. 2020, 50, 314–323. [Google Scholar] [CrossRef]
- Di Carlo, G.; Saccucci, M.; Ierardo, G.; Luzzi, V.; Occasi, F.; Zicari, A.M.; Duse, M.; Polimeni, A. Rapid Maxillary Expansion and Upper Airway Morphology: A Systematic Review on the Role of Cone Beam Computed Tomography. Biomed. Res. Int. 2017, 2017, 5460429. [Google Scholar] [CrossRef]
- Lee, W.C.; Tu, Y.K.; Huang, C.S.; Chen, R.; Fu, M.W.; Fu, E. Pharyngeal airway changes following maxillary expansion or protraction: A meta-analysis. Orthod. Craniofac. Res. 2018, 21, 4–11. [Google Scholar] [CrossRef]
- Krüsi, M.; Eliades, T.; Papageorgiou, S.N. Are there benefits from using bone-borne maxillary expansion instead of tooth-borne maxillary expansion? A systematic review with meta-analysis. Prog. Orthod. 2019, 20, 9. [Google Scholar] [CrossRef]
- Abu Arqub, S.; Mehta, S.; Iverson, M.G.; Yadav, S.; Upadhyay, M.; Almuzian, M. Does Mini Screw Assisted Rapid Palatal Expansion (MARPE) have an influence on airway and breathing in middle-aged children and adolescents? A systematic review. Int. Orthod. 2021, 19, 37–50. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Tricco, A.; Welch, V.A.; Moher, D.; Chou, R.; Lalu, M.M.; Li, T.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Morris, I.R. Functional anatomy of the upper airway. Emerg. Med. Clin. N. Am. 1988, 6, 639–669. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Interpretation of tests of heterogeneity and bias in meta-analysis. J. Eval. Clin. Pract. 2008, 14, 951–957. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Schunemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Storto, C.J.; Garcez, A.S.; Suzuki, H.; Cusmanich, K.G.; Elkenawy, I.; Moon, W.; Suzuki, S.S. Assessment of respiratory muscle strength and airflow before and after microimplant-assisted rapid palatal expansion. Angle Orthod. 2019, 89, 713–720. [Google Scholar] [CrossRef]
- Yousif, A.A.E.A.E.; Elshenawy, M.E.A.; Elmehy, G.A.E. Velopharyngeal and Glossopharyngeal Volume Changes after Implant Anchored Maxillary Expansion. Egypt. Dent. J. 2019, 65, 21–29. [Google Scholar] [CrossRef]
- Kim, J.E.; Hwang, K.J.; Kim, S.W.; Liu, S.Y.C.; Kim, S.J. Correlation between craniofacial changes and respiratory improvement after nasomaxillary skeletal expansion in pediatric obstructive sleep apnea patients. Sleep Breath. 2022, 26, 585–594. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Park, Y.-C.; Lee, K.-J.; Lintermann, A.; Han, S.-S.; Yu, H.-S.; Choi, Y.J. Assessment of changes in the nasal airway after nonsurgical miniscrew-assisted rapid maxillary expansion in young adults. Angle Orthod. 2018, 88, 435–441. [Google Scholar] [CrossRef]
- Yi, F.; Liu, S.; Lei, L.; Liu, O.; Zhang, L.; Peng, Q.; Lu, Y. Changes of the upper airway and bone in microimplant-assisted rapid palatal expansion: A cone-beam computed tomography (CBCT) study. J. X-ray Sci. Technol. 2020, 28, 271–283. [Google Scholar] [CrossRef]
- Li, Q.; Tang, H.; Liu, X.; Luo, Q.; Jiang, Z.; Martin, D.; Guo, J. Comparison of dimensions and volume of upper airway before and after mini-implant assisted rapid maxillary expansion. Angle Orthod. 2020, 90, 432–441. [Google Scholar] [CrossRef]
- Moschik, C.E. Morphometric Analysis of Maxillary Skeletal Expansion Effects on the Nasal Cavity. Master’s Thesis, University of California, Los Angeles, CA, USA, 2018. [Google Scholar]
- Kavand, G.; Lagravère, M.; Kula, K.; Stewart, K.; Ghoneima, A. Retrospective CBCT analysis of airway volume changes after bone-borne vs tooth-borne rapid maxillary expansion. Angle Orthod. 2019, 89, 566–574. [Google Scholar] [CrossRef]
- Mehta, S.; Wang, D.; Kuo, C.-L.; Mu, J.; Vich, M.L.; Allareddy, V.; Tadinada, A.; Yadav, S. Long-term effects of mini-screw-assisted rapid palatal expansion on airway. Angle Orthod. 2021, 91, 195–205. [Google Scholar] [CrossRef]
- Song, J. Retrospective Evaluation of the Changes in the Nasal and Pharyngeal Airway Volume after Miniscrew Assisted Rapid Palatal Expansion (MARPE) Appliance. Master’s Thesis, West Virginia University, Morgantown, WV, USA, 2020. [Google Scholar]
- Tang, H.Y.; Liu, P.P.; Xu, Q.P.; Hou, Y.Y.; Guo, J. A comparative analysis of aerodynamic and anatomic characteristics of upper airway before and after mini-implant-assisted rapid maxillary expansion. Am. J. Orthod. Dentofac. 2021, 159, E301–E310. [Google Scholar] [CrossRef]
- Hollander, Z.P. Measuring Airway Changes After Treatment with the Maxillary Skeletal Expander Using Three Dimension Cone Beam Computed Tomography and Computational Fluid Dynamic Analysis. Master’s Thesis, University of California, Los Angeles, CA, USA, 2021. [Google Scholar]
- Cheung, G.C.; Dalci, O.; Mustac, S.; Papageorgiou, S.N.; Hammond, S.; Darendeliler, M.A.; Papadopoulou, A.K. The upper airway volume effects produced by Hyrax, Hybrid-Hyrax, and Keles keyless expanders: A single-centre randomized controlled trial. Eur. J. Orthod. 2021, 43, 254–264. [Google Scholar] [CrossRef]
- Cistulli, P.A. Craniofacial abnormalities in obstructive sleep apnoea: Implications for treatment. Respirology 1996, 1, 167–174. [Google Scholar] [CrossRef]
- Cistulli, P.A.; Richards, G.N.; Palmisano, R.G.; Unger, G.; Berthon-Jones, M.; Sullivan, C.E. Influence of maxillary constriction on nasal resistance and sleep apnea severity in patients with Marfan’s syndrome. Chest 1996, 110, 1184–1188. [Google Scholar] [CrossRef]
- Brunetto, D.P.; Sant’Anna, E.F.; Machado, A.W.; Moon, W. Non-surgical treatment of transverse deficiency in adults using Microimplant-assisted Rapid Palatal Expansion (MARPE). Dent. Press J. Orthod. 2017, 22, 110–125. [Google Scholar] [CrossRef]
- Brunetto, D.P.; Moschik, C.E.; Dominguez-Mompell, R.; Jaria, E.; Sant’Anna, E.F.; Moon, W. Mini-implant assisted rapid palatal expansion (MARPE) effects on adult obstructive sleep apnea (OSA) and quality of life: A multi-center prospective controlled trial. Prog. Orthod. 2022, 23, 3. [Google Scholar] [CrossRef]
- Lenza, M.G.; Lenza, M.M.; Dalstra, M.; Melsen, B.; Cattaneo, P.M. An analysis of different approaches to the assessment of upper airway morphology: A CBCT study. Orthod. Craniofac. Res. 2010, 13, 96–105. [Google Scholar] [CrossRef]
- Aboudara, C.; Nielsen, I.; Huang, J.C.; Maki, K.; Miller, A.J.; Hatcher, D. Comparison of airway space with conventional lateral headfilms and 3-dimensional reconstruction from cone-beam computed tomography. Am. J. Orthod. Dentofacial. Orthop. 2009, 135, 468–479. [Google Scholar] [CrossRef]
- Ghoneima, A.; Kula, K. Accuracy and reliability of cone-beam computed tomography for airway volume analysis. Eur. J. Orthod. 2013, 35, 256–261. [Google Scholar] [CrossRef]
- Buck, L.M.; Dalci, O.; Darendeliler, M.A.; Papadopoulou, A.K. Effect of Surgically Assisted Rapid Maxillary Expansion on Upper Airway Volume: A Systematic Review. J. Oral. Maxillofac. Surg. 2016, 74, 1025–1043. [Google Scholar] [CrossRef]
- Sutthiprapaporn, P.; Tanimoto, K.; Ohtsuka, M.; Nagasaki, T.; Iida, Y.; Katsumata, A. Positional changes of oropharyngeal structures due to gravity in the upright and supine positions. Dentomaxillofac. Rad. 2008, 37, 130–136. [Google Scholar] [CrossRef]
- Yildirim, N.; Fitzpatrick, M.F.; Whyte, K.F.; Jalleh, R.; Wightman, A.J.; Douglas, N.J. The effect of posture on upper airway dimensions in normal subjects and in patients with the sleep apnea/hypopnea syndrome. Am. Rev. Respir. Dis. 1991, 144, 845–847. [Google Scholar] [CrossRef]
- Jan, M.A.; Marshall, I.; Douglas, N.J. Effect of posture on upper airway dimensions in normal human. Am. J. Respir. Crit. Care Med. 1994, 149, 145–148. [Google Scholar] [CrossRef]
- Van Holsbeke, C.S.; Verhulst, S.L.; Vos, W.; De Backer, J.W.; Vinchurkar, S.C.; Verdonck, P.R.; Van Doorn, J.W.; Nadjmi, N.; De Backer, W.A. Change in upper airway geometry between upright and supine position during tidal nasal breathing. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 51–57. [Google Scholar] [CrossRef]
- Muto, T.; Takeda, S.; Kanazawa, M.; Yamazaki, A.; Fujiwara, Y.; Mizoguchi, I. The effect of head posture on the pharyngeal airway space (PAS). Int. J. Oral. Maxillofac. Surg. 2002, 31, 579–583. [Google Scholar] [CrossRef]
- Hellsing, E. Changes in the pharyngeal airway in relation to extension of the head. Eur. J. Orthod. 1989, 11, 359–365. [Google Scholar] [CrossRef]
- Lopatienė, K.; Dabkutė, A.; Juškevičiūtė, V. Vertical and sagittal morphology of the facial skeleton and the pharyngeal airway. Stomatologija 2016, 18, 21–25. [Google Scholar]
- Warren, D.W.; Hairfield, W.M.; Dalston, E.T. Effect of age on nasal cross-sectional area and respiratory mode in children. Laryngoscope 1990, 100, 89–93. [Google Scholar] [CrossRef]

| Study | Study Design | Participants | Control | Inclusion Criteria | Intervention | Main Outcome |
|---|---|---|---|---|---|---|
| Storto et al., 2019 [30] | Prospective clinical study | 20 pts (13 f, 7 m) mean age: 17.1 yrs | NO | Patients with maxillary transverse deficiency; permanent dentition; CS6 skeletal maturation stage; mouth breathers | Maxillary skeletal expander | Nasopharynx volume Oropharynx volume |
| Kim et al., 2018 [33] | Retrospective clinical study | 14 pts (10 f, 4 m) mean age: 22.76 ± 3.3 yrs range: 18.3–26.5 yrs | NO | Young adults (>18 years of age) with a transverse discrepancy; successful opening of the mid-palatal suture; non-extraction treatment; availability of CBCT images obtained before and after expansion. | Modified conventional four-banded hyrax expander | Nasal cavity volume Nasopharynx volume |
| Yi et al., 2020 [34] | Retrospective clinical study | 13 pts (10 f, 3 m) mean age: 19.95 ± 4.39 yrs range: 15–29 yrs | NO | Maxillary constriction; good oral hygiene and periodontal condition; no history of orthodontics; maxillofacial trauma or respiratory tract therapy; no systemic diseases; no other maxillofacial deformity; did not take long-term drugs; the mid-palatal suture stage was C, D, E; successful maxillary expansion; had follow-up imaging data. | The palatal bracket implant anchorage arch expander | Nasopharynx volume Oropharynx volume Palatopharynx volume Glossopharynx volume |
| Li et al., 2020 [35] | Retrospective clinical study | 22 pts (18 f, 4 m) mean age: 22.6 ± 4.5 yrs range: 18–35 yrs | NO | Young adults (18–35 years old) with transverse maxillary discrepancy; successful opening of the mid-palatal suture; availability of CBCT images obtained before and after expansion. | Maxillary skeletal expander | Nasal cavity volume Nasopharynx volume, Palatopharynx volume Glossopharynx volume Hypopharynx volume |
| Moschik, 2018 [36] | Retrospective clinical study | 16 pts (10 f, 6 m) mean age: 20.7 yrs range: 17–26 yrs | Tooth-borne group: 6 pts (3 f, 3 m) mean age: 12.2 yrs range: 9–15 yrs | Non-growing (CVMS IV) maxillary transverse deficiency; had CBCT imaging done before and after expansion; visible split of mid-palatal suture on CBCT, received no previous orthodontic treatment; had no craniofacial abnormalities | Maxillary skeletal expander | Nasal cavity volume |
| Kavand et al., 2019 [37] | Retrospective clinical study | 18 pts (12 f, 6 m) mean age: 14.7 ± 1.4 yrs range: 11–15 yrs | Tooth-borne group: 18 pts (10 f, 8 m); mean age: 14.4 ± 1.3 yrs range: 11–15 yrs | Individuals between 11 and 15 years of age with no history of orthodontic treatment; temporomandibular joint disorder; adenoidectomy or tonsillectomy; periodontal diseases; systemic diseases; craniofacial anomalies; and no active caries; bilateral maxillary crossbite | Bone-borne rapid maxillary expander | Nasal cavity volume Nasopharynx volume Oropharynx volume Maxillary sinus volume |
| Atia et al., 2019 [31] | Prospective clinical study | 10 pts (all man) range: 12–14 yrs | Conventional hyrax group: 10 pts (all man); range: 12–14 yrs | All patients were males; aged 12 to 14 years old; all patients were free from any syndrome or congenital defects that may affect the craniofacial structures; no previous orthodontic treatment; no previous history of facial or cranial trauma; absence of any breathing disorders; maxillary constriction |
Hybrid hyrax expander | Oropharynx volume |
| Cheung et al., 2021 [42] | Randomized controlled trial | 19 pts (11 f, 8 m) mean age: 14.3 ± 1.7 yrs range: 10–16 yrs | Hyrax group and Keles group (random allocation from the total sample as 1:1:1 ratio) | Unilateral or bilateral posterior crossbite; maxillary transverse deficiency of more than 5 mm; erupted first permanent molars and premolars; adequate oral hygiene; and no history of previous orthodontic treatment and no history of craniofacial defects, syndromes, or surgery |
Hybrid hyrax expander | Nasal cavity volume Nasopharynx volume Oropharynx volume Hypopharynx volume Maxillary sinus volume |
| Kim et al., 2021 [32] | Prospective clinical study | 26 pts mean age: 13.6 ± 2.9 yrs range: 9–18 yrs | NO | Diagnosed with OSA based on the AHI criteria and maxillary transverse constriction; The patients with syndromic craniofacial deformity, history of orthodontic treatment or adenotonsillectomy, obesity with body mass index (BMI) greater than 23 kg/m2, and ATH with Friedman’s classes 3 and 4 were excluded | Maxillary skeletal expander | Nasal cavity volume Nasopharynx volume Palatopharynx volume Glossopharynx volume |
| Mehta et al., 2021 [38] | Retrospective clinical study | 20 pts mean age: 13.69 ± 1.74 years range: 11–15 yrs | Rapid palatal expansion (RPE) group 21 pts, mean age: 13.9 ± 1.14 yrs, and control group 19 pts, mean age: 13.3 ± 1.49 yrs | Patients aged 11 to 15 years, with no history of prior orthodontics, temporomandibular joint disorder, adenoidectomy or tonsillectomy, and the presence of a bilateral maxillary crossbite | Bone-borne rapid maxillary expander | Nasal cavity volume Nasopharynx volume Oropharynx volume Hypopharynx volume |
| Song, 2020 [39] | Retrospective clinical study | 20 pts range: 8–22 yrs | NO | Any age for patients; Using MARPE treatment to correct maxillary transverse discrepancy; No history of previous orthodontic or orthopedic treatment; No history of craniofacial syndrome or deformities | Maxillary skeletal expander | Nasal cavity volume Nasopharynx volume Oropharynx volume |
| Tang et al., 2021 [40] | Retrospective clinical study | 30 pts (21 f, 9 m) mean age: 23.82 ± 3.90 yrs range: 18–33 yrs | NO | Aged >18 years; maxillomandibular skeletal transverse discrepancy 3 mm or greater; no history of expansion treatment or orthognathic surgery; and no severe dentofacial anomalies such as a cleft lip or palate | Maxillary skeletal expander | Nasopharynx volume Oropharynx volume Hypopharynx volume |
| Hollander, 2021 [41] | Retrospective clinical study | 16 pts (12 f, 4 m) | Non-expansion group 8 pts (5 f, 3 m) | Adult patients; maxillary transverse deficiency; successful opening of the mid-palatal suture; non-extraction treatment; and availability of CBCT images; a history of orthodontic treatment and presence of craniofacial syndromes or systemic diseases were excluded | Maxillary skeletal expander | Nasal cavity volume Nasopharynx volume Oropharynx volume |
| Study | Expansion Device | Expansion Protocol | Duration | Retention |
|---|---|---|---|---|
| Storto et al., 2019 [30] | Maxillary skeletal expander (supported on U6s, additional skeletal anchorage with four micro-implants) | Twice a day (0.25 mm/turn) until the necessary expansion was achieved | Activated until the complete maxillary expansion | Not reported |
| Kim et al., 2018 [33] | Modified conventional four-banded hyrax RME appliance (supported on U4s & U6s and additional skeletal anchorage with four micro-implants) | Once a day (0.2 mm/turn) until the required expansion was achieved | The mean duration of expansion was 28 days (range: 18–35 days) | The MARPE appliance was maintained for mean of 15.1 weeks after the completion of the expansion |
| Yi et al., 2020 [34] | The palatal bracket implant anchorage arch expander (skeletal anchorage with four micro-implants) | Twice a day (0.25 mm/turn) for 14 days until the required expansion was achieved 7 mm | Activated 14 days (expansion was achieved 7mm) | Not reported |
| Li et al., 2020 [35] | Maxillary skeletal expander (supported on U6s, additional skeletal anchorage with four micro-implants) | Two turns every other day (0.13 mm/ turn) until maxillary skeletal width was no longer less than that of the mandible | The mean duration of expansion was 38 days (range: 30–43 days) | No description of the retention protocol The retention time was at least 3 months |
| Moschik, 2018 [36] | Maxillary skeletal expander (supported on U6s, additional skeletal anchorage with four micro-implants) | Four times per day, resulting in 0.6 mm activation (0.16mm/turn) | Not reported | Not reported |
| Kavand et al., 2019 [37] | Bone-borne rapid maxillary expander (skeletal anchorage with two micro-implants) | Twice a day (0.25 mm/turn) until mesio-palatal cusps of the maxillary first molars were in contact with the buccal cusps of mandibular first molars | Activated until the mesio-palatal cusps of the maxillary first permanent molars were in contact with the buccal cusps of mandibular first permanent molars | Not reported |
| Atia et al., 2019 [31] | Hybrid hyrax (supported on U4s & U6s and additional skeletal anchorage with two micro-implants) | Twice per day for ten days at a constant rate. | Ten consecutive days | Not reported |
| Cheung et al., 2021 [42] | Hybrid hyrax (supported on U6s and additional skeletal anchorage with two micro-implants) | Twice a day (0.5 mm) until palatal cusps of the upper first molars were in contact with the buccal cusps of the lower first molars | Until palatal cusps of the upper first molars were in contact with the buccal cusps of the lower first molars | The expander was locked, and the patient instructed to return in 6 months |
| Kim et al., 2021 [32] | Maxillary skeletal expander (supported on U6s and additional skeletal anchorage with four micro-implants) | One turn (0.25 mm) a day for 3–4 weeks | 24.3 days (range: 20–26 days) | The expander was removed on 6.2 ± 1.6 months after starting expansion on average |
| Mehta et al., 2021 [38] | Bone-borne rapid maxillary expander (skeletal anchorage with two micro-implants) | Two turns per day | Not reported | Not reported |
| Song, 2020 [39] | Maxillary skeletal expander (supported on U4s & U6s and additional skeletal anchorage with four micro-implants) | Depending on the amount of transverse correction needed, the number of turns varied between patients | When the lingual cusps of the maxillary first molars were in edge–edge contact with the buccal cusps of the mandibular first molars, appliance activation was terminated. | Not reported |
| Tang et al., 2021 [40] | Maxillary skeletal expander (supported on U6s, additional skeletal anchorage with four micro-implants) | Depending on the severity of each patient, ranging from 40–60 turns. | Duration of expansion ranged from 40 to 60 days | The retention after activation was 3 months |
| Hollander, 2021 [41] | Maxillary skeletal expander (supported on U6s, additional skeletal anchorage with four micro-implants) | Not reported | Not reported | Not reported |
| Study | Measurement Method | Follow-Up Points | Airway Regions | Treated Group Changes | Change Percentage % |
|---|---|---|---|---|---|
| Storto et al., 2019 [30] | CBCT | T0: before expansion T1: immediately after expansion | Nasopharynx volume | T0–T1: 16,058 (2171.98); 21,835.55 (1937.64) | 26% |
| Kim et al., 2018 [33] | CBCT | T0: before expansion T1: immediately after expansion | Nasal cavity volume Nasopharynx volume | ΔT1–T0: 1061.6 (613.9) ΔT1–T0: 513.3 (727.8) | 9.9% 6.4% |
| Yi et al., 2020 [34] | CBCT | T0: before expansion T2: three months after expansion | Nasopharynx volume Oropharynx volume Palatopharynx volume Glossopharynx volume | T0–T2: 5922.61 (1938.28); 6424.61 (1798.58) T0–T2: 21,057.11 (9371.71); 19,972.03 (8026.73) T0–T2: 11,201.39 (4071.85); 11,802.42 (4322.75) T0–T2: 10,020.89 (6403.14); 8527.69 (4679.10) | 8.48% N/A N/A N/A |
| Li et al., 2020 [35] | CBCT | T0: before expansion T2: three months after expansion | Nasal cavity volume Nasopharynx volume Palatopharynx volume Glossopharynx volume Hypopharynx volume | T0–T2: 18,110.7 (6236.8); 21,036.5 (4777.8) T0–T2: 5212.1 (1509.9); 5947.1 (2101.6) T0–T2: 7477.8 (2901.6); 7903.9 (3001.9) T0–T2: 4080.1 (1656.4); 4539.5 (2129.2) T0–T2: 10,597.7 (3925.2); 9373.5 (3576.4) | 16.2% 14.1%. 5.7% 11.26% −11.6% |
| Moschik, 2018 [36] | CBCT | T0: before expansion T1: immediately after expansion | Left nasal cavity volume Right nasal cavity volume Total nasal cavity volume | T0–T1: 10,481.00 (463.4996); 13,695.00 (477.159) T0–T1: 9938.06 (449.1738); 12,730.69 (470.5434) T0–T1: 20,419.06; 26,425.69 | N/A N/A 22.73% |
| Kavand et al., 2019 [37] | CBCT | T0: before expansion T2: three months after expansion | Nasal cavity volume Nasopharynx volume Oropharynx volume Left maxillary sinus volume Right maxillary sinus volume | T0–T2: 14,860 (3109); 16,726 (3041) T0–T2: 3760 (1630); 4580 (1819) T0–T2: 11,746 (4269); 12,297 (3660) T0–T2: 13,004 (3926); 13,739 (3759) T0–T2: 12,369 (4039); 13,184 (3821) | 16.1% 20.0% 2.6% 2.1% 5.2% |
| Atia et al., 2019 [31] | CT | T0: before expansion T1: immediately after expansion | Oropharynx volume 1 Oropharynx volume 2 | T0–T1: 13.86 (0.60); 16.82 (0.87) T0–T1: 11.44 (0.28); 13.96 (1.02) | N/A N/A |
| Cheung et al., 2021 [42] | CBCT | T0: before expansion T3: six months after expansion | Nasal cavity volume Nasopharynx volume Oropharynx volume Hypopharynx volume Maxillary sinus volume | T0–T3: 26,630.8 (5659.0); 29,319.5 (5536.7) T0–T3: 5416.8 (2194.0); 6362.4 (2443.8) T0–T3: 11,651.8 (6208.3); 12,702.7 (5678.1) T0–T3: 3441.9 (1430.0); 3451.3 (1290.9) T0–T3: 23,433.05 (9577.7); 23,813.4 (8131.1) | 10.1% 17.5% 9.0% 0.3% 10.0% |
| Kim et al., 2021 [32] | CBCT | T0: before expansion T3: six months after expansion | Nasal cavity volume Nasopharynx volume Palatopharynx volume Glossopharynx volume | T0–T3: 22,987.80 (9483.35); 40,755.54 (13,083.33) T0–T3: 5072.68 (1533.46); 7502.24 (2049.73) T0–T3: 9060.23 (4072.48); 11,236.53 (4404.78) T0–T3: 9861.37 (3464.25); 12,122.51 (3727.92) | 77.2% 47.9% 24.0%. N/A |
| Mehta et al., 2021 [38] | CBCT | T0: before expansion T3: six months after expansion | Nasal cavity volume Nasopharynx volume Oropharynx volume Hypopharynx volume | T0–T3: 16,204.1 (3100.53); 18,475.95 (3329.13) T0–T3: 3412.89 (1425.84); 4158.32 (1459.81) T0–T3: 6270.35 (2617.56); 7675.74 (3047.01) T0–T3: 6662.93 (3459.65); 8361.92 (3321.25) | 14.4% 21.8% 19.2% 4.4% |
| Song, 2020 [39] | CBCT | T0: before expansion T1: immediately after expansion | Nasal cavity volume Nasopharynx volume Oropharynx volume | T0–T1:15,892.7 (3025.0); 18,131 (4814.1) T0–T1: 5874.6 (2172.6); 4879.2 (1847.6) T0–T1: 17,855.4 (7806.0); 15,914.2 (7137.3) | N/A |
| Tang et al., 2021 [40] | CBCT | T0: before expansion T2: three months after expansion | Nasopharynx volume Oropharynx volume Hypopharynx volume | T0–T2: 6463.86 (1459.17); 7806.69 (1806.87) T0–T2: 10,886.67 (3382.94); 11,849.28 (4306.25) T0–T2: 8542.31 (3426.18); 8307.14 (3237.12) | N/A |
| Hollander, 2021 [41] | CBCT | T0: before expansion T1: immediately after expansion | Nasal cavity volume Nasopharynx volume Oropharynx volume | T0–T1: 80,448.93 (15,387.18); 87,446.73 (15,345.97) T0–T1: 8572.62 (3354.84); 10,191.66 (3808.14) T0–T1: 8624.04 (4758.53); 12,505.92 (6336.88) | 9.21% 19.99% 54.88% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhai, M.; Wang, M.; Cui, S.; Cheng, C.; Wang, J.; Wei, F. Three-Dimensional Evaluation Effects of Microimplant-Assisted Rapid Palatal Expansion on the Upper Airway Volume: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1790. https://doi.org/10.3390/jcm12051790
Li L, Zhai M, Wang M, Cui S, Cheng C, Wang J, Wei F. Three-Dimensional Evaluation Effects of Microimplant-Assisted Rapid Palatal Expansion on the Upper Airway Volume: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(5):1790. https://doi.org/10.3390/jcm12051790
Chicago/Turabian StyleLi, Lan, Mingrui Zhai, Mengqiao Wang, Shuyue Cui, Chen Cheng, Jixiao Wang, and Fulan Wei. 2023. "Three-Dimensional Evaluation Effects of Microimplant-Assisted Rapid Palatal Expansion on the Upper Airway Volume: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 5: 1790. https://doi.org/10.3390/jcm12051790
APA StyleLi, L., Zhai, M., Wang, M., Cui, S., Cheng, C., Wang, J., & Wei, F. (2023). Three-Dimensional Evaluation Effects of Microimplant-Assisted Rapid Palatal Expansion on the Upper Airway Volume: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(5), 1790. https://doi.org/10.3390/jcm12051790





