Severity and Longitudinal Course of Depression, Anxiety and Post-Traumatic Stress in Paediatric and Young Adult Cancer Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Guidance
2.2. Data Sources and Search Strategy
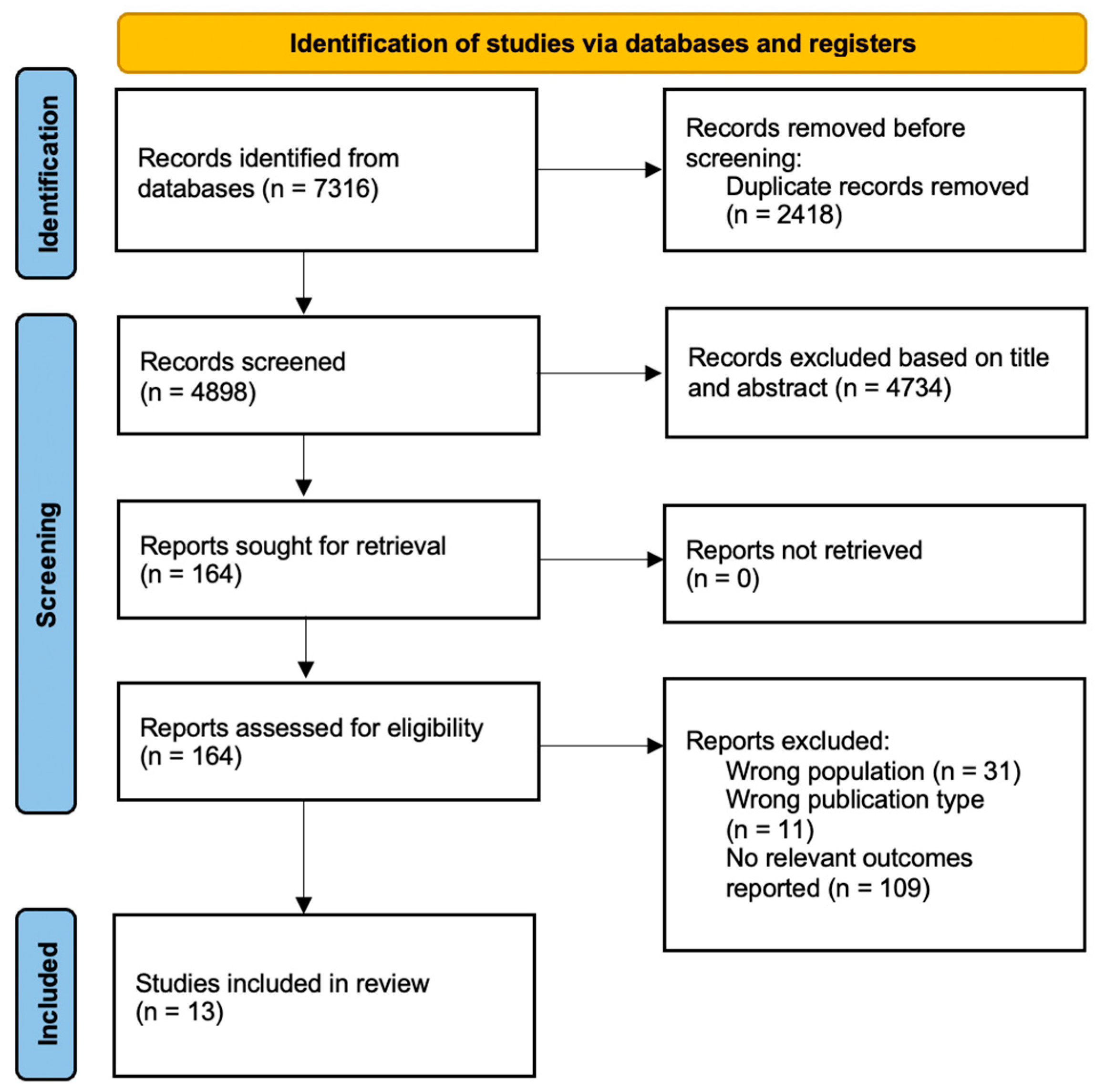
2.3. Study Selection: Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Data Analysis
3. Results
3.1. Longitudinal Course of Depressive Symptoms after Diagnosis of Cancer
3.2. Risk, Protective and Exacerbating of Depressive Symptoms
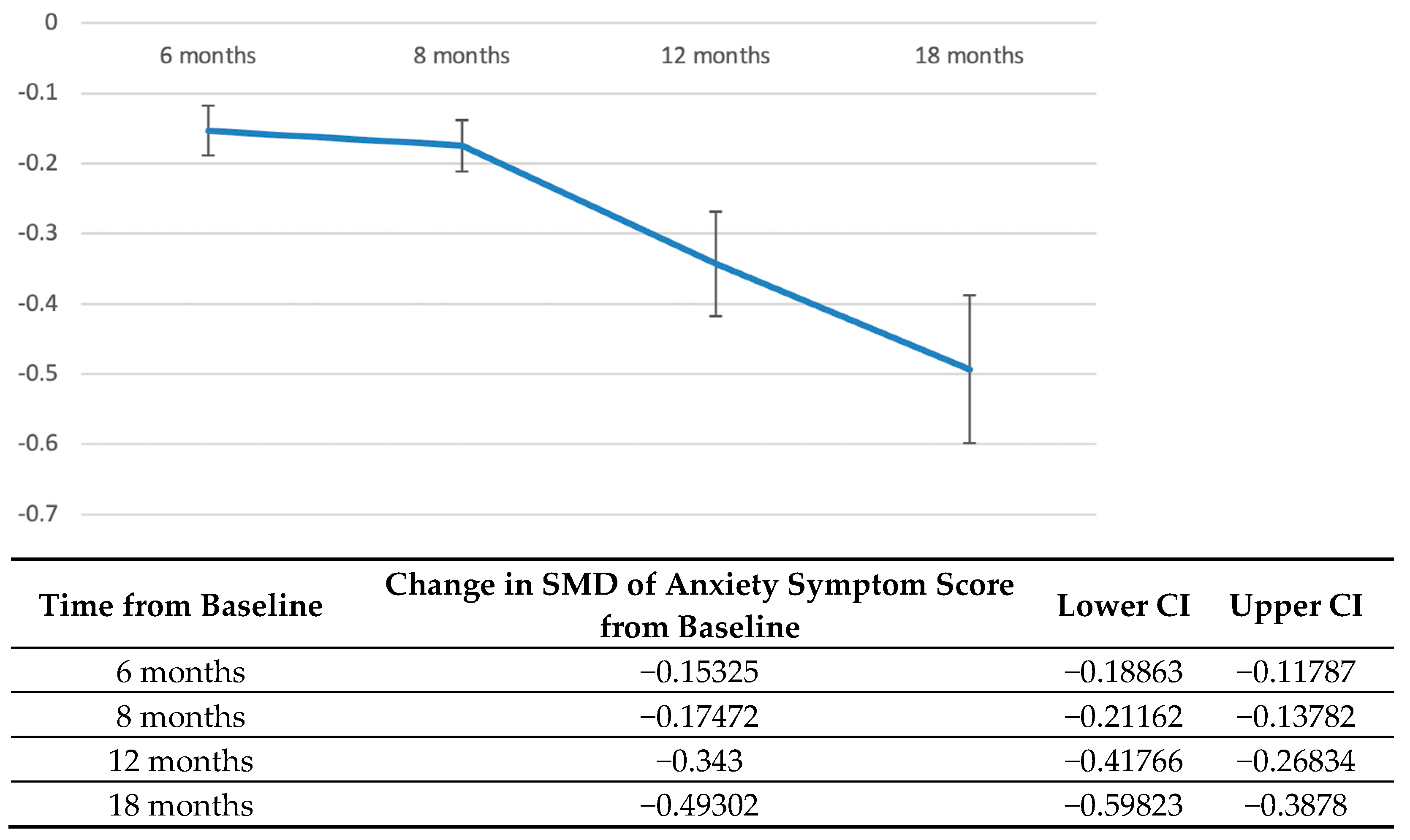
3.3. Longitudinal Course of Anxiety Symptoms after Diagnosis of Cancer
3.4. Risk, Protective and Exacerbating of Anxiety Symptoms
3.5. PTSS after Diagnosis of Cancer
3.6. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnston, W.T.; Erdmann, F.; Newton, R.; Steliarova-Foucher, E.; Schüz, J.; Roman, E. Childhood cancer: Estimating regional and global incidence. Cancer Epidemiol. 2021, 71 Pt B, 101662. [Google Scholar] [CrossRef] [PubMed]
- Kaatsch, P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010, 36, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, F.; Frederiksen, L.E.; Bonaventure, A.; Mader, L.; Hasle, H.; Robison, L.L.; Winther, J.F. Childhood cancer: Survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2021, 71 Pt B, 101733. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Yeh, J.M.; Bhakta, N.; Frazier, A.L.; Girardi, F.; Atun, R. Global childhood cancer survival estimates and priority-setting: A simulation-based analysis. Lancet Oncol. 2019, 20, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Li, H.C.; Chung, O.K.; Chiu, S.Y. The impact of cancer on children’s physical, emotional, and psychosocial well-being. Cancer Nurs. 2010, 33, 47–54. [Google Scholar] [CrossRef]
- Phillips, S.M.; Padgett, L.S.; Leisenring, W.M.; Stratton, K.K.; Bishop, K.; Krull, K.R.; Alfano, C.M.; Gibson, T.M.; de Moor, J.S.; Hartigan, D.B.; et al. Survivors of childhood cancer in the United States: Prevalence and burden of morbidity. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Dudley, W.N.; Bhakta, N.; Horan, M.R.; Wang, Z.; Bartlett, T.R.; Srivastava, D.; Yasui, Y.; Baker, J.N.; Robison, L.L.; et al. Associations of Symptom Clusters and Health Outcomes in Adult Survivors of Childhood Cancer: A Report From the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2022, 41, 497–507. [Google Scholar] [CrossRef]
- Lee, A.R.Y.B.; Yau, C.E.; Low, C.E.; Li, J.; Tyebally, S.M.; Lin, W.; Tan, L.-L.; Liao, C.-T.; Chang, W.-T.; Lee, M.X.; et al. Natural Progression of Left Ventricular Function following Anthracyclines without Cardioprotective Therapy: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 512. [Google Scholar] [CrossRef]
- Frederiksen, L.E.; Erdmann, F.; Mader, L.; Mogensen, H.; Pedersen, C.; Kenborg, L.; Bautz, A.; Talbäck, M.; Hirvonen, E.; Nielsen, T.T.; et al. Psychiatric disorders in childhood cancer survivors in Denmark, Finland, and Sweden: A register-based cohort study from the SALiCCS research programme. Lancet Psychiatry 2022, 9, 35–45. [Google Scholar] [CrossRef]
- Ljungman, L.; Remes, T.; Westin, E.; Huittinen, A.; Lonnqvist, T.; Sirkia, K.; Rantala, H.; Ojaniemi, M.; Harila, M.; Lahteenmaki, P.; et al. Health-related quality of life in long-term survivors of childhood brain tumors: A population-based cohort study. Support Care Cancer 2022, 30, 5157–5166. [Google Scholar] [CrossRef]
- Crochet, E.; Tyc, V.L.; Wang, M.; Srivastava, D.K.; Van Sickle, K.; Nathan, P.C.; Leisenring, W.; Gibson, T.M.; Armstrong, G.T.; Krull, K. Posttraumatic stress as a contributor to behavioral health outcomes and healthcare utilization in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J. Cancer Surviv. 2019, 13, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.G.; Krull, K.R.; Kadan-Lottick, N.; Nicholson, H.S.; Nathan, P.C.; Zebrack, B.; Tersak, J.M.; Ness, K.K. Social outcomes in the Childhood Cancer Survivor Study cohort. J. Clin. Oncol. 2009, 27, 2390–2395. [Google Scholar] [CrossRef] [PubMed]
- Arnett, J.J. Emerging Adulthood: A Theory of Development from the Late Teens through the Twenties; American Psychological Association: Worcester, MA, USA, 2000; pp. 469–480. [Google Scholar]
- Hobbie, W.L.; Stuber, M.; Meeske, K.; Wissler, K.; Rourke, M.T.; Ruccione, K.; Hinkle, A.; Kazak, A.E. Symptoms of posttraumatic stress in young adult survivors of childhood cancer. J. Clin. Oncol. 2000, 18, 4060–4066. [Google Scholar] [CrossRef] [PubMed]
- Maercker, A.; Michael, T.; Fehm, L.; Becker, E.S.; Margraf, J. Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. Br. J. Psychiatry 2004, 184, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Landolt, M.A.; Vollrath, M.; Ribi, K.; Gnehm, H.E.; Sennhauser, F.H. Incidence and associations of parental and child posttraumatic stress symptoms in pediatric patients. J. Child Psychol. Psychiatry 2003, 44, 1199–1207. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Riitano, D.; Lisy, K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int. J. Health Policy Manag. 2014, 3, 123–128. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat. Med. 2007, 26, 4544–4562. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Kunin-Batson, A.S.; Lu, X.; Balsamo, L.; Graber, K.; Devidas, M.; Hunger, S.P.; Carroll, W.L.; Winick, N.J.; Mattano, L.A., Jr.; Maloney, K.W.; et al. Prevalence and predictors of anxiety and depression after completion of chemotherapy for childhood acute lymphoblastic leukemia: A prospective longitudinal study. Cancer 2016, 122, 1608–1617. [Google Scholar] [CrossRef]
- Myers, R.M.; Balsamo, L.; Lu, X.; Devidas, M.; Hunger, S.P.; Carroll, W.L.; Winick, N.J.; Maloney, K.W.; Kadan-Lottick, N.S. A prospective study of anxiety, depression, and behavioral changes in the first year after a diagnosis of childhood acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Cancer 2014, 120, 1417–1425. [Google Scholar] [CrossRef]
- Sargin Yildirim, N.; Demirkaya, M.; Sevinir, B.B.; Guler, S.; Vural, A.P.; Demiroz, C.; Cirpan Kantarcioglu, A. A prospective follow-up of quality of life, depression, and anxiety in children with lymphoma and solid tumors. Turk. J. Med. Sci. 2017, 47, 1078–1088. [Google Scholar] [CrossRef]
- Jorngarden, A.; Mattsson, E.; von Essen, L. Health-related quality of life, anxiety and depression among adolescents and young adults with cancer: A prospective longitudinal study. Eur. J. Cancer 2007, 43, 1952–1958. [Google Scholar] [CrossRef]
- Larsson, G.; Mattsson, E.; von Essen, L. Aspects of quality of life, anxiety, and depression among persons diagnosed with cancer during adolescence: A long-term follow-up study. Eur. J. Cancer 2010, 46, 1062–1068. [Google Scholar] [CrossRef]
- Yardeni, M.; Abebe Campino, G.; Hasson-Ohayon, I.; Basel, D.; Hertz-Palmor, N.; Bursztyn, S.; Weisman, H.; Pessach, I.M.; Toren, A.; Gothelf, D. Trajectories and risk factors for anxiety and depression in children and adolescents with cancer: A 1-year follow-up. Cancer Med. 2021, 10, 5653–5660. [Google Scholar] [CrossRef]
- Werk, R.S.; Koyama, T.; Sun, L.; Wolden, S.; Kelly, K.M.; Constine, L.S.; Schwartz, C.L.; Friedman, D.L. Post-Traumatic Stress Symptoms in Adolescent Hodgkin Lymphoma Survivors: A Report from Children’s Oncology Group AHOD0031. J Adolesc. Young Adult Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, L.; Barrera, M.; Schulte, F.; Chung, J.; Cataudella, D.; Janzen, L.; Bartels, U.; Downie, A. Predicting social withdrawal, anxiety and depression symptoms in pediatric brain tumor survivors. J. Psychosoc. Oncol. 2019, 37, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Prikken, S.; Raymaekers, K.; Lemiere, J.; Vercruysse, T.; Uyttebroeck, A.; Luyckx, K. Worries and Benefit Finding in Cancer Survivors and Parents: A Longitudinal Study. J. Pediatr. Psychol. 2022, 47, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.; Zebrack, B.J.; Meeske, K.A.; Embry, L.; Aguilar, C.; Block, R.; Hayes-Lattin, B.; Li, Y.; Butler, M.; Cole, S. Prevalence and predictors of post-traumatic stress symptoms in adolescent and young adult cancer survivors: A 1-year follow-up study. Psychooncology 2013, 22, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.L.; Busner, J.; Weinhold, C.; Lenon, P. Depressive symptoms in children and adolescents with cancer: A longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry 1987, 26, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.; Torres, A.; Morgadinho, R.; Pereira, A. Psychosocial outcomes in young adults with cancer: Emotional distress, quality of life and personal growth. Arch. Psychiatr. Nurs. 2013, 27, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Singh, A.; Singh, T.B.; Upadhyay, S. Psychological morbidity in children undergoing chemotherapy for acute lymphoblastic leukemia. Indian J. Pediatr. 2014, 81, 699–701. [Google Scholar] [CrossRef]
- Yaffe Ornstein, M.; Friedlander, E.; Katz, S.; Elhasid, R. Prospective assessment of anxiety among pediatric oncology patients and their caregivers during the COVID-19 pandemic a cohort study. J. Psychosoc. Oncol. 2022, 1–14. [Google Scholar] [CrossRef]
- Rajandram, R.K.; Jenewein, J.; McGrath, C.; Zwahlen, R.A. Coping processes relevant to posttraumatic growth: An evidence-based review. Support. Care Cancer 2011, 19, 583–589. [Google Scholar] [CrossRef]
- Gori, A.; Topino, E.; Sette, A.; Cramer, H. Pathways to post-traumatic growth in cancer patients: Moderated mediation and single mediation analyses with resilience, personality, and coping strategies. J. Affect Disord. 2021, 279, 692–700. [Google Scholar] [CrossRef]
- Menger, F.; Mohammed Halim, N.A.; Rimmer, B.; Sharp, L. Post-traumatic growth after cancer: A scoping review of qualitative research. Support Care Cancer 2021, 29, 7013–7027. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D. Traumatic stress: Effects on the brain. Dialogues Clin. Neurosci. 2006, 8, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kaminga, A.C.; Dai, W.; Deng, J.; Wang, Z.; Pan, X.; Liu, A. The prevalence of moderate-to-high posttraumatic growth: A systematic review and meta-analysis. J. Affect Disord. 2019, 243, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yang, X.; Zheng, W.; Wang, B.; Fu, L.; Luo, D.; Hu, Y.; Ju, N.; Xu, H.; Fang, Y.; et al. Depression, anxiety and post-traumatic growth among COVID-19 survivors six-month after discharge. Eur. J. Psychotraumatology 2022, 13, 2055294. [Google Scholar] [CrossRef] [PubMed]
- Long, L.J.; Phillips, C.A.; Glover, N.; Richardson, A.L.; D’Souza, J.M.; Cunningham-Erdogdu, P.; Gallagher, M.W. A Meta-analytic Review of the Relationship Between Posttraumatic Growth, Anxiety, and Depression. J. Happiness Stud. 2021, 22, 3703–3728. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Maccallum, F.; Chow, A.Y.M. Depression, Anxiety and Post-traumatic Growth Among Bereaved Adults: A Latent Class Analysis. Front. Psychol. 2020, 11, 575311. [Google Scholar] [CrossRef] [PubMed]
- Barskova, T.; Oesterreich, R. Post-traumatic growth in people living with a serious medical condition and its relations to physical and mental health: A systematic review. Disabil Rehabil. 2009, 31, 1709–1733. [Google Scholar] [CrossRef] [PubMed]
- Morrill, E.F.; Brewer, N.T.; O’Neill, S.C.; Lillie, S.E.; Dees, E.C.; Carey, L.A.; Rimer, B.K. The interaction of post-traumatic growth and post-traumatic stress symptoms in predicting depressive symptoms and quality of life. Psychooncology 2008, 17, 948–953. [Google Scholar] [CrossRef]
- De Bellis, M.D.; Zisk, A. The biological effects of childhood trauma. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 185–222. [Google Scholar] [CrossRef]
- Lee, A.; Leong, I.; Lau, G.; Tan, A.W.; Ho, R.C.M.; Ho, C.S.H.; Chen, M.Z. Depression and anxiety in older adults with cancer: Systematic review and meta-summary of risk, protective and exacerbating factors. Gen. Hosp. Psychiatry 2023, 81, 32–42. [Google Scholar] [CrossRef]
- Lee, A.R.Y.B.; Tariq, A.; Lau, G.; Tok, N.W.K.; Tam, W.W.S.; Ho, C.S.H.; Vitamin, E. Alpha-Tocopherol, and Its Effects on Depression and Anxiety: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 656. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.Y.B.; Yau, C.E.; Mai, A.S.; Tan, W.A.; Ong, B.S.Y.; Yam, N.E.; Ho, C.S.H. Transcranial alternating current stimulation and its effects on cognition and the treatment of psychiatric disorders: A systematic review and meta-analysis. Ther. Adv. Chronic Dis. 2022, 13, 20406223221140390. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.H.; Kozloff, N.; Sacks, D. Pediatric depression: An evidence-based update on treatment interventions. Curr. Psychiatry Rep. 2013, 15, 381. [Google Scholar] [CrossRef] [PubMed]
- Phipps, S.; Long, A.; Hudson, M.; Rai, S.N. Symptoms of post-traumatic stress in children with cancer and their parents: Effects of informant and time from diagnosis. Pediatr. Blood Cancer 2005, 45, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Ozono, S.; Saeki, T.; Mantani, T.; Ogata, A.; Okamura, H.; Yamawaki, S. Factors related to posttraumatic stress in adolescent survivors of childhood cancer and their parents. Support Care Cancer 2007, 15, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Taïeb, O.; Moro, M.R.; Baubet, T.; Revah-Lévy, A.; Flament, M.F. Posttraumatic stress symptoms after childhood cancer. Eur. Child Adolesc. Psychiatry 2003, 12, 255–264. [Google Scholar] [CrossRef]
- Suthershinii, G.; Tan, W.A.; Lee, A.R.Y.B.; Chen, M.Z. Behavioral Interventions for the Patient–Caregiver Unit in Patients with Chronic Heart Failure: A Systematic Review of Caregiver Outcomes. J. Multidiscip. Healthc. 2022, 15, 921. [Google Scholar]
- Coughtrey, A.; Millington, A.; Bennett, S.; Christie, D.; Hough, R.; Su, M.T.; Constantinou, M.P.; Shafran, R. The effectiveness of psychosocial interventions for psychological outcomes in pediatric oncology: A systematic review. J. Pain. Symptom. Manag. 2018, 55, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C. The effects of stressful life events on depression. Annu. Rev. Psychol. 1997, 48, 191–214. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Ness, K.K.; Neglia, J.P.; Whitton, J.A.; Green, D.M.; Zeltzer, L.K.; Robison, L.L.; Mertens, A.C. Fatigue and sleep disturbance in adult survivors of childhood cancer: A report from the childhood cancer survivor study (CCSS). Sleep 2008, 31, 271–281. [Google Scholar] [CrossRef]


| Author | Publication Year | Region of Study | N, Cancer Type | Control Characteristics | Age at Cancer Diagnosis * | Age at the Time Data Were Collected * | Depression Scale | Anxiety Scale | Post-Traumatic Stress Scales |
|---|---|---|---|---|---|---|---|---|---|
| Kunin-Batson et al. [26] | 2016 | USA | 160, Acute lymphocytic leukaemia | Normative population data | Range: 1–9.9 | Range: 2–10.9 | BASC-2 | BASC-2 | - |
| Myers et al. [27] | 2014 | USA | 159, Acute lymphocytic leukaemia | Normative population data | 4.9 (2.2) | 5.9 (2.2) | BASC-2 | BASC-2 | - |
| Gupta et al. [38] | 2014 | India | 40, Acute lymphocytic leukaemia | Matched controls | Range: 6 to 14 | Range: 6 to 14 | CPMS | CPMS | - |
| Werk et al. [32] | 2022 | USA | 1721, Hodgkin’s lymphoma | No reference group | 14.6 (3.3) | 14.5 (3.4) | - | - | Novel scale |
| Kaplan et al. [36] | 1987 | USA | 21, Haematological cancers | Matched controls | 9.71 (1.52) | 15.4 (1.82) | CDI and BDI | - | - |
| Desjardins et al. [33] | 2019 | Canada | 91, Central nervous system | Caregivers | NR | 11.21 (2.8) | BASC-2 | BASC-2 | - |
| Monteiro et al. [37] | 2013 | Portugal | 11, Various | Matched controls | NR | NR | HADS | HADS | - |
| Prikken et al. [34] | 2022 | Belgium | 125, Various | Parents | 14 to 19 years | NR | CES-D | - | - |
| Kwak et al. [35] | 2013 | USA | 87, Various | No comparison group | 22.7 | 23.2 | - | - | PDS, BSI-18 and SF-36 |
| Sargin Yildirim et al. [28] | 2017 | Turkey | 50, Various | Parents | 12.14 (2.97) | 12.39 (2.97) | CDI | SCARED | - |
| Jorngarden et al. [29] | 2007 | Sweden | 56, Various | General population | 15.7 | NR | HADS | HADS | - |
| Larsson et al. [30] | 2010 | Sweden | 61, Various | Normative population data | Range: 13–19 | 4 years after diagnosis | HADS | HADS | - |
| Yardeni et al. [31] | 2021 | Israel | 99, Various | Parents | NR | 13.56 (3.63) | PROMIS and K-SADS | PROMIS and K-SADS | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.R.Y.B.; Yau, C.E.; Low, C.E.; Li, J.; Ho, R.C.M.; Ho, C.S.H. Severity and Longitudinal Course of Depression, Anxiety and Post-Traumatic Stress in Paediatric and Young Adult Cancer Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1784. https://doi.org/10.3390/jcm12051784
Lee ARYB, Yau CE, Low CE, Li J, Ho RCM, Ho CSH. Severity and Longitudinal Course of Depression, Anxiety and Post-Traumatic Stress in Paediatric and Young Adult Cancer Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(5):1784. https://doi.org/10.3390/jcm12051784
Chicago/Turabian StyleLee, Ainsley Ryan Yan Bin, Chun En Yau, Chen Ee Low, Jiaqi Li, Roger C. M. Ho, and Cyrus Su Hui Ho. 2023. "Severity and Longitudinal Course of Depression, Anxiety and Post-Traumatic Stress in Paediatric and Young Adult Cancer Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 5: 1784. https://doi.org/10.3390/jcm12051784
APA StyleLee, A. R. Y. B., Yau, C. E., Low, C. E., Li, J., Ho, R. C. M., & Ho, C. S. H. (2023). Severity and Longitudinal Course of Depression, Anxiety and Post-Traumatic Stress in Paediatric and Young Adult Cancer Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(5), 1784. https://doi.org/10.3390/jcm12051784







