A Pilot Study to Assess Visual Vertigo in People with Persistent Postural–Perceptual Dizziness with a New Computer-Based Tool
Abstract
1. Introduction
2. Material and Methods
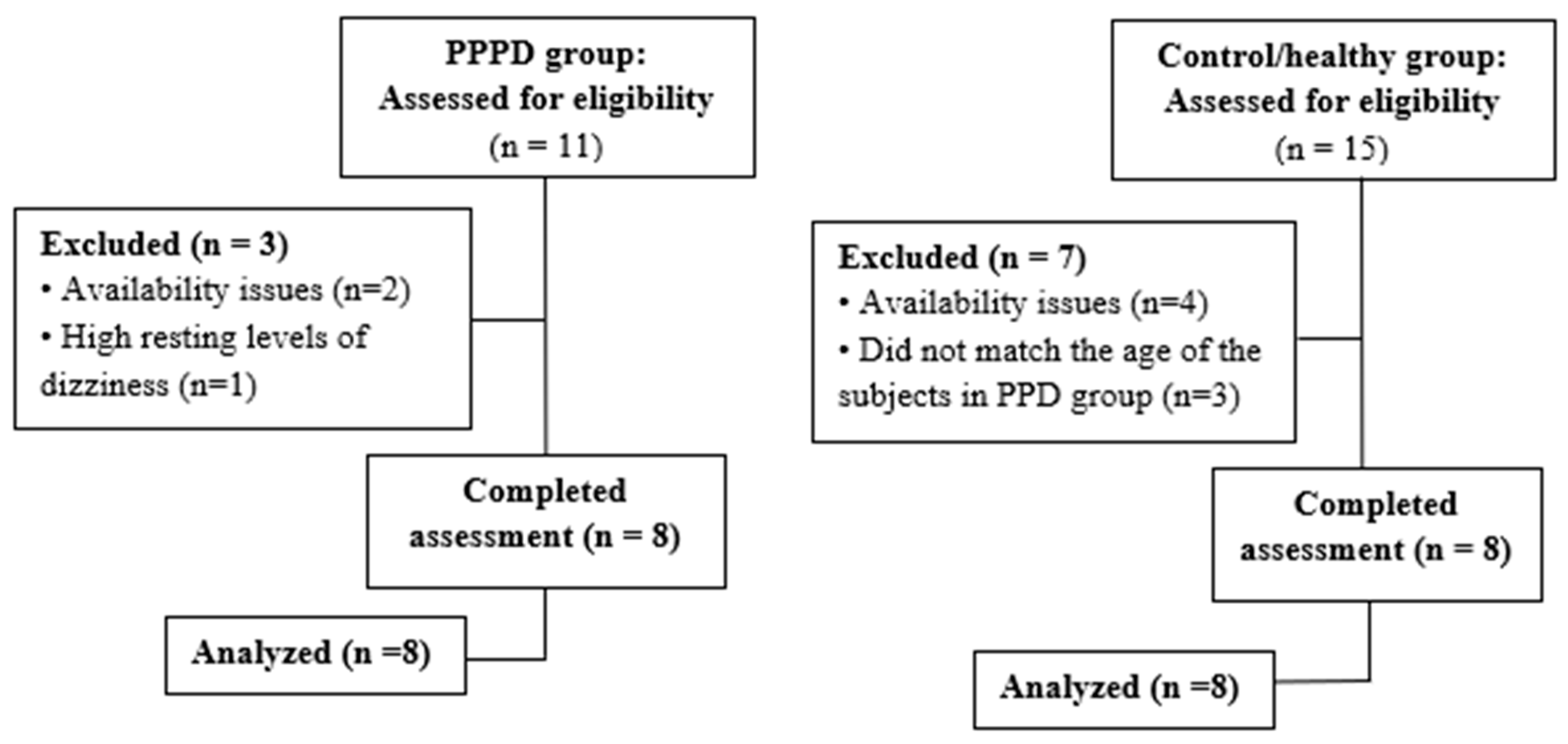
2.1. Participants
2.2. Development of a Non-immersive Virtual Reality Program
2.3. Assessments
2.4. Data Analysis
3. Results
3.1. Participant Characteristics
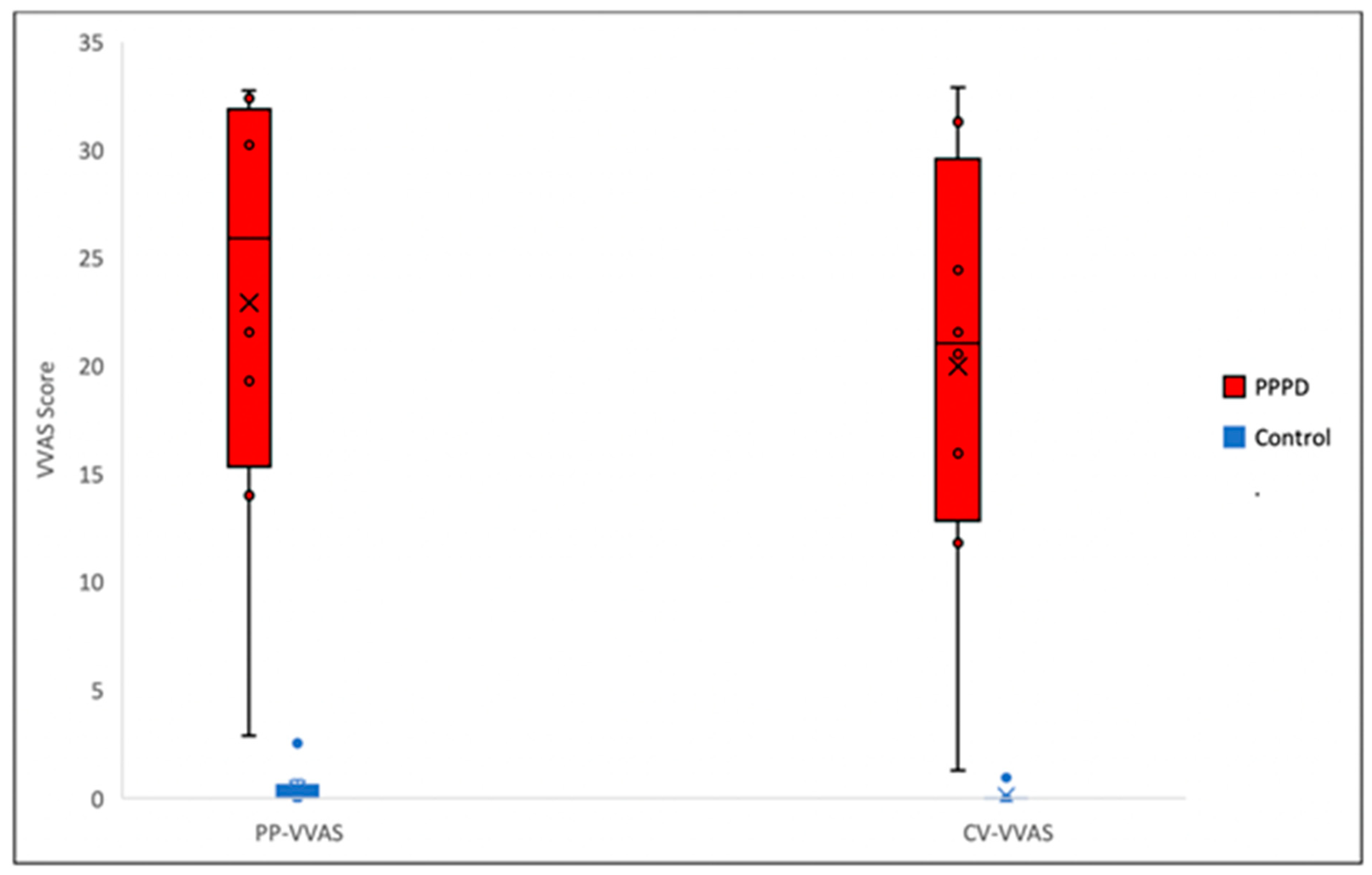
3.2. Specificity of the c-VVAS
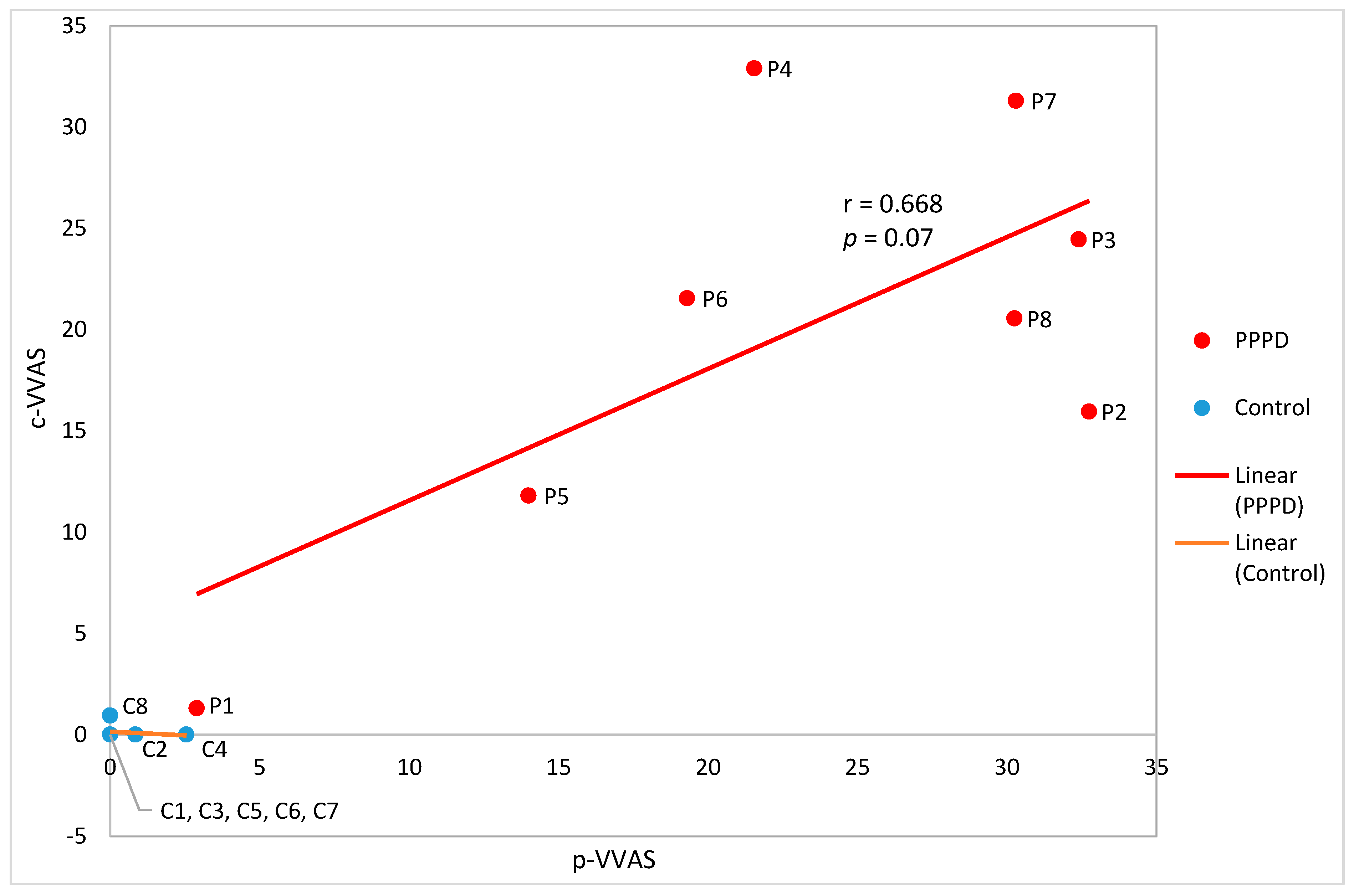
3.3. Concurrent Validity of the c-VVAS
3.4. Acceptance of the c-VVAS
4. Discussion
4.1. Specificity of the c-VVAS
4.2. Correlation of the c-VVAS with p-VVAS
4.3. c-VVAS Acceptance and Usability
4.4. Limitations and Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staab, J.P.; Eckhardt-Henn, A.; Horii, A.; Jacob, R.; Strupp, M.; Brandt, T.; Bronstein, A. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): Consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society. J. Vestib. Res. 2017, 27, 191–208. [Google Scholar] [CrossRef]
- Babu, S.; Schutt, C.A.; Bojrab, D.I. (Eds.) Diagnosis and Treatment of Vestibular Disorders; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Bronstein, A.M. Vision and vertigo. J. Neurol. Z. Neurol. 2004, 251, 381. [Google Scholar] [CrossRef] [PubMed]
- Popkirov, S.; Staab, J.P.; Stone, J. Persistent postural-perceptual dizziness (pppd): A common, characteristic and treatable cause of chronic dizziness. Pract. Neurol. 2018, 18, 5–13. [Google Scholar] [CrossRef]
- Dannenbaum, E.; Chilingaryan, G.; Fung, J. Visual vertigo analogue scale: An assessment questionnaire for visual vertigo. J. Vestib. Res. 2011, 21, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Dannenbaum, E.; Chilingarian, G.; Fung, J. Validity and responsiveness of the visual vertigo analogue scale. J. Neurol. Phys. Ther. 2019, 43, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.P.; Newman, C.W. The development of the Dizziness Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 424–427. [Google Scholar] [CrossRef]
- Jacob, R.G.; Woody, S.R.; Clark, D.B.; Lilienfeld, S.O.; Hirsch, B.E.; Kucera, G.D.; Furman, J.M.; Durrant, J.D. Discomfort with space and motion: A possible marker of vestibular dysfunction assessed by the situational characteristics questionnaire. J. Psychopathol. Behav. Assess. 1993, 15, 299–324. [Google Scholar] [CrossRef]
- Yagi, C.; Morita, Y.; Kitazawa, M.; Nonomura, Y.; Yamagishi, T.; Ohshima, S.; Izumi, S.; Takahashi, K.; Horii, A. A Validated Questionnaire to Assess the Severity of Persistent Postural-Perceptual Dizziness (PPPD): The Niigata PPPD Questionnaire (NPQ). Otol. Neurotol. 2019, 40, e747–e752. [Google Scholar] [CrossRef]
- Powell, G.; Derry-Sumner, H.; Shelton, K.; Rushton, S.; Hedge, C.; Rajenderkumar, D.; Sumner, P. Visually-induced dizziness is associated with sensitivity and avoidance across all senses. J. Neurol. 2020, 267, 2260–2271. [Google Scholar] [CrossRef]
- Shahrbanian, S.; Ma, X.; Aghaei, N.; Korner-Bitensky, N.; Moshiri, K.; Simmonds, M.J. Use of virtual reality (immersive vs. non immersive) for pain management in children and adults: A systematic review of evidence from randomized controlled trials. Eur. J. Exp. Biol. 2012, 2, 1408–1422. [Google Scholar]
- Weech, S.; Kenny, S.; Barnett-Cowan, M. Presence and Cybersickness in Virtual Reality Are Negatively Related: A Review. Front. Psychol. 2019, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, R.; Maranesi, E.; Riccardi, G.R.; Di Donna, V.; Pelliccioni, P.; Luzi, R.; Lattanzio, F.; Pelliccioni, G. Non-immersive virtual reality for rehabilitation of the older people: A systematic review into efficacy and effectiveness. J. Clin. Med. 2019, 8, 1882. [Google Scholar] [CrossRef] [PubMed]
- An, C.M.; Park, Y.H. The effects of semi-immersive virtual reality therapy on standing balance and upright mobility function in individuals with chronic incomplete spinal cord injury: A preliminary study. J. Spinal Cord Med. 2018, 41, 223–229. [Google Scholar] [CrossRef]
- Larsson, P.; Vastfjall, D.; Kleiner, M. Better presence and performance in virtual environments by improved binaural sound rendering. In Proceedings of the 22nd International Conference of Virtual, Synthetic, and Entertainment Audio, Espoo, Finland, 15–17 June 2002. [Google Scholar]
- Davis, F.D. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Q. 1989, 13, 319–340. [Google Scholar] [CrossRef]
- Sezier, A.E.I.; Saywell, N.; Terry, G.; Taylor, D.; Kayes, N. Working-age adults’ perspectives on living with persistent postural-perceptual dizziness: A qualitative exploratory study. BMJ Open 2019, 9, e024326. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.G.; Brandt, T.; Furman, J.M. Psychiatric aspects of dizziness and imbalance. In Clinical Disorders of Balance Posture and Gait, 2nd ed.; Bronstein, A.M., Brandt, T., Woollacott, M.H., Nutt, J.G., Eds.; Arnold: London, UK, 2004; pp. 245–285. [Google Scholar]
- Zur, O.; Schoen, G.; Dickstein, R.; Feldman, J.; Berner, Y.; Dannenbaum, E.; Fung, J. Anxiety among individuals with visual vertigo and vestibulopathy. Disabil. Rehabil. 2015, 37, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Held, J.P.; Ferrer, B.; Mainetti, R.; Steblin, A.; Hertler, B.; Moreno-Conde, A.; Dueñas, A.; Pajaro, M.; Vargiu, E.; Zarco, M.J.; et al. Autonomous rehabilitation at stroke patients home for balance and gait: Safety, usability and compliance of a virtual reality system. Eur. J. Phys. Rehabil. Med. 2018, 54, 545–553. [Google Scholar] [CrossRef]
- Lloréns, R.; Noé, E.; Colomer, C.; Alcañiz, M. Effectiveness, usability, and cost-benefit of a virtual reality-based telerehabilitation program for balance recovery after stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 418–425.e2. [Google Scholar] [CrossRef]
- Syed-Abdul, S.; Malwade, S.; Nursetyo, A.A.; Sood, M.; Bhatia, M.; Barsasella, D.; Liu, M.F.; Chang, C.C.; Srinivasan, K.; Li, Y.C.J. Virtual reality among the elderly: A usefulness and acceptance study from Taiwan. BMC Geriatr. 2019, 19, 223. [Google Scholar] [CrossRef]
- Ng, A.K.T.; Chan, L.K.Y.; Lau, H.Y.K. A study of cybersickness and sensory conflict theory using a motion-coupled virtual reality system. Displays 2019, 61, 101922. [Google Scholar] [CrossRef]

| Demographic Data | Participants | p-Value | ||
|---|---|---|---|---|
| PPPD (n= 8) | Control (n = 8) | Total (n = 16) | ||
| Age (year) | 0.851 1 | |||
| Mean (SD) | 53.9 ± 13.7 | 53.0 ± 13.4 | 53.2 ± 13.5 | |
| Biological sex, n (%) | 1.000 2 | |||
| Female | 7 (87.5%) | 7 (87.5%) | 14 (87.5%) | |
| Time of onset of PPPD, n (%) | ||||
| 1–10 years ago | 6 (75.0%) | n/a | 6 (75.5%) | |
| 40–50 years ago | 2 (25.0%) | n/a | 2 (25.0%) | |
| Baseline dizziness level/10, n (%) | ||||
| 0 | 4 (50.0%) | 8 (100.0%) | 12 (75.0%) | |
| 1–4 | 2 (25.0%) | 0 (0.0%) | 2 (12.5%) | |
| 5–7 | 1 (12.5%) | 0 (0.0%) | 1 (6.25%) | |
| 8–10 | 1 (12.5%) | 0 (0.0%) | 1 (6.25%) | |
| PT Treatment at the JRH, n (%) | ||||
| Currently under treatment | 2 (25.0%) | n/a | 2 (25.0%) | |
| Discharged | 6 (75.0%) | n/a | 6 (75.0%) | |
| Other treatment received, n (%) | ||||
| None | 2 (25.0%) | n/a | 2 (25.0%) | |
| Medication | 3 (37.5%) | n/a | 3 (37.5%) | |
| Osteopathy | 2 (25.0%) | n/a | 2 (25.0%) | |
| Multiple modalities (meds+ acupuncture + osteopathy + psychotherapy) | 1 (12.5%) | n/a | 1 (12.5%) | |
| Anxiety, n (%) | ||||
| None | 1 (12.5%) | 8 (100.0%) | 9 (56.25%) | |
| Diagnosed | 4 (50.0%) | 0 (0.0%) | 4 (25.0%) | |
| Undiagnosed | 3 (37.5%) | 0 (0.0%) | 3 (18.75%) | |
| PPPD vs. Control | Mean Score of PPPD (n = 8) | Mean Score of Control (n = 8) | Mann–Whitney U Coefficient | p-Value |
|---|---|---|---|---|
| p-VVAS | 22.931 | 0.425 | 0.000 | 0.001 |
| c-VVAS | 19.975 | 0.119 | 0.000 | 0.000 |
| Scenario | Mean Score of PPPD (n = 8) | Mean Score of Control (n = 8) | Mann–Whitney U Coefficient | p-Value |
|---|---|---|---|---|
| Supermarket | 4.356 | 0.000 | 8.000 | 0.007 |
| Passenger in a car | 5.144 | 0.119 | 1.000 | 0.001 |
| Busy intersection | 2.938 | 0.000 | 4.000 | 0.001 |
| Shopping mall | 3.500 | 0.000 | 4.000 | 0.001 |
| Escalator | 4.038 | 0.000 | 8.000 | 0.004 |
| Total | 19.975 | 0.119 | 0.000 | 0.000 |
| Scenario | Pearson Correlation Coefficient (r) | p-Value |
|---|---|---|
| Supermarket | 0.663 | 0.073 |
| Passenger in a car | 0.444 | 0.270 |
| Busy intersection | 0.593 | 0.121 |
| Shopping mall | 0.032 | 0.941 |
| Escalator | 0.907 | 0.005 |
| Category | Mean Score of PPPD (%) (n = 8) | Mean Score of Control (%) (n = 8) | Mean Score of Both Groups (%) (n = 16) |
|---|---|---|---|
| Perceived EOU (statement 1, 2) | 98.44 | 100.00 | 99.22 |
| Attitude and intention to use (statement 3, 4) | 85.94 | 100.00 | 92.97 |
| Perceived usefulness (statement 5, 6, 7) | 90.63 | 81.25 | 85.94 |
| Total score (*) | 91.52 | 91.96 | 91.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-A.J.; Dion Parenteau, M.-L.; Marchand, K.; Zhang, H.Z.; Dannenbaum, E.; Lamontagne, A.; Fung, J. A Pilot Study to Assess Visual Vertigo in People with Persistent Postural–Perceptual Dizziness with a New Computer-Based Tool. J. Clin. Med. 2023, 12, 1766. https://doi.org/10.3390/jcm12051766
Chen T-AJ, Dion Parenteau M-L, Marchand K, Zhang HZ, Dannenbaum E, Lamontagne A, Fung J. A Pilot Study to Assess Visual Vertigo in People with Persistent Postural–Perceptual Dizziness with a New Computer-Based Tool. Journal of Clinical Medicine. 2023; 12(5):1766. https://doi.org/10.3390/jcm12051766
Chicago/Turabian StyleChen, Tsun-Ai Jasper, Marie-Li Dion Parenteau, Kirby Marchand, Hong Zhi Zhang, Elizabeth Dannenbaum, Anouk Lamontagne, and Joyce Fung. 2023. "A Pilot Study to Assess Visual Vertigo in People with Persistent Postural–Perceptual Dizziness with a New Computer-Based Tool" Journal of Clinical Medicine 12, no. 5: 1766. https://doi.org/10.3390/jcm12051766
APA StyleChen, T.-A. J., Dion Parenteau, M.-L., Marchand, K., Zhang, H. Z., Dannenbaum, E., Lamontagne, A., & Fung, J. (2023). A Pilot Study to Assess Visual Vertigo in People with Persistent Postural–Perceptual Dizziness with a New Computer-Based Tool. Journal of Clinical Medicine, 12(5), 1766. https://doi.org/10.3390/jcm12051766






