Vagus Nerve Stimulation and Its Cardioprotective Abilities: A Systematic Review
Abstract
1. Introduction
1.1. sVNS Models
1.1.1. Fiber Selective Stimulation
1.1.2. Spatially Selective Stimulation
1.1.3. Kiliohertz Electrical Stimulation (KES)
1.1.4. Neural Titration
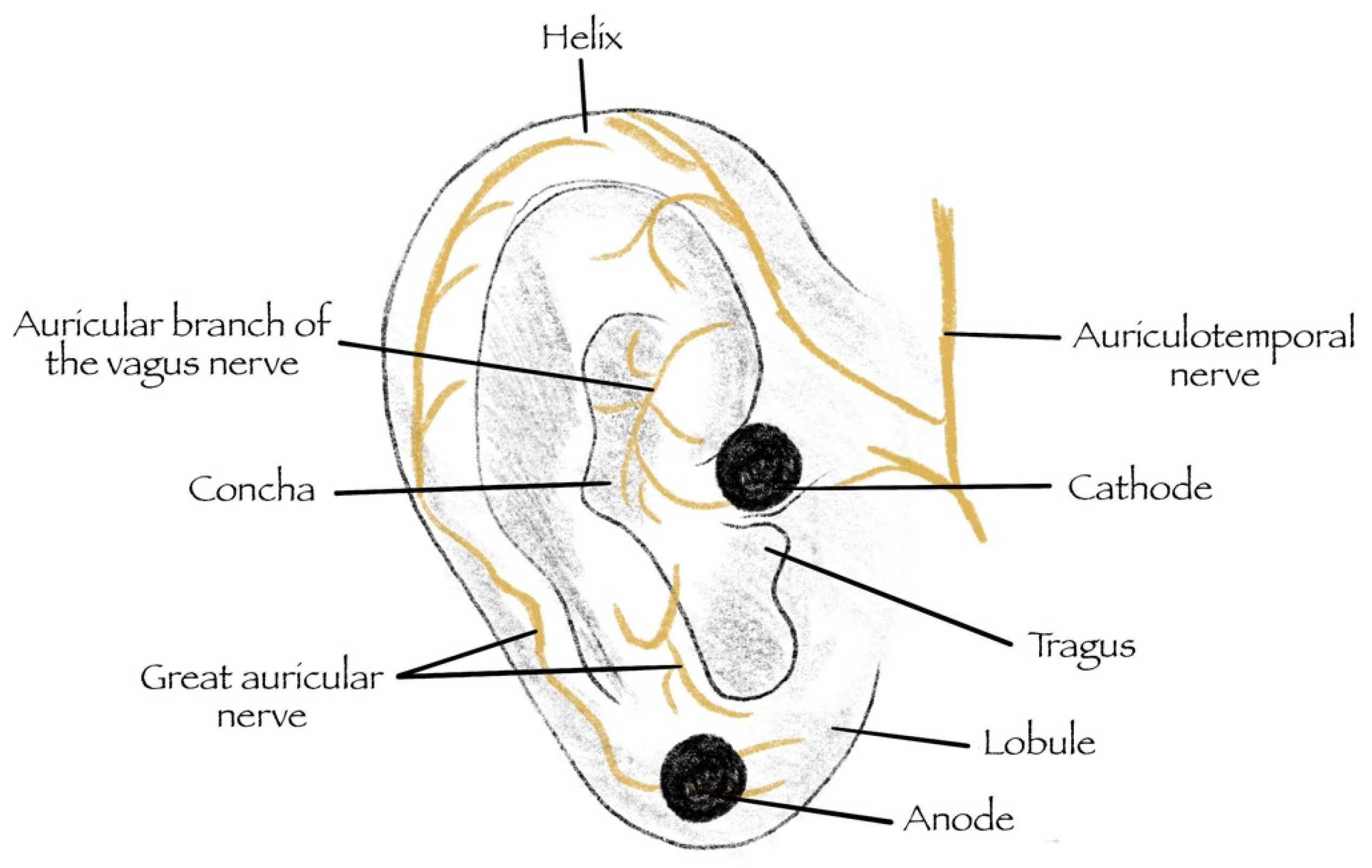
1.1.5. Transcutaneous—Vagus Nerve Stimulation (tVNS)
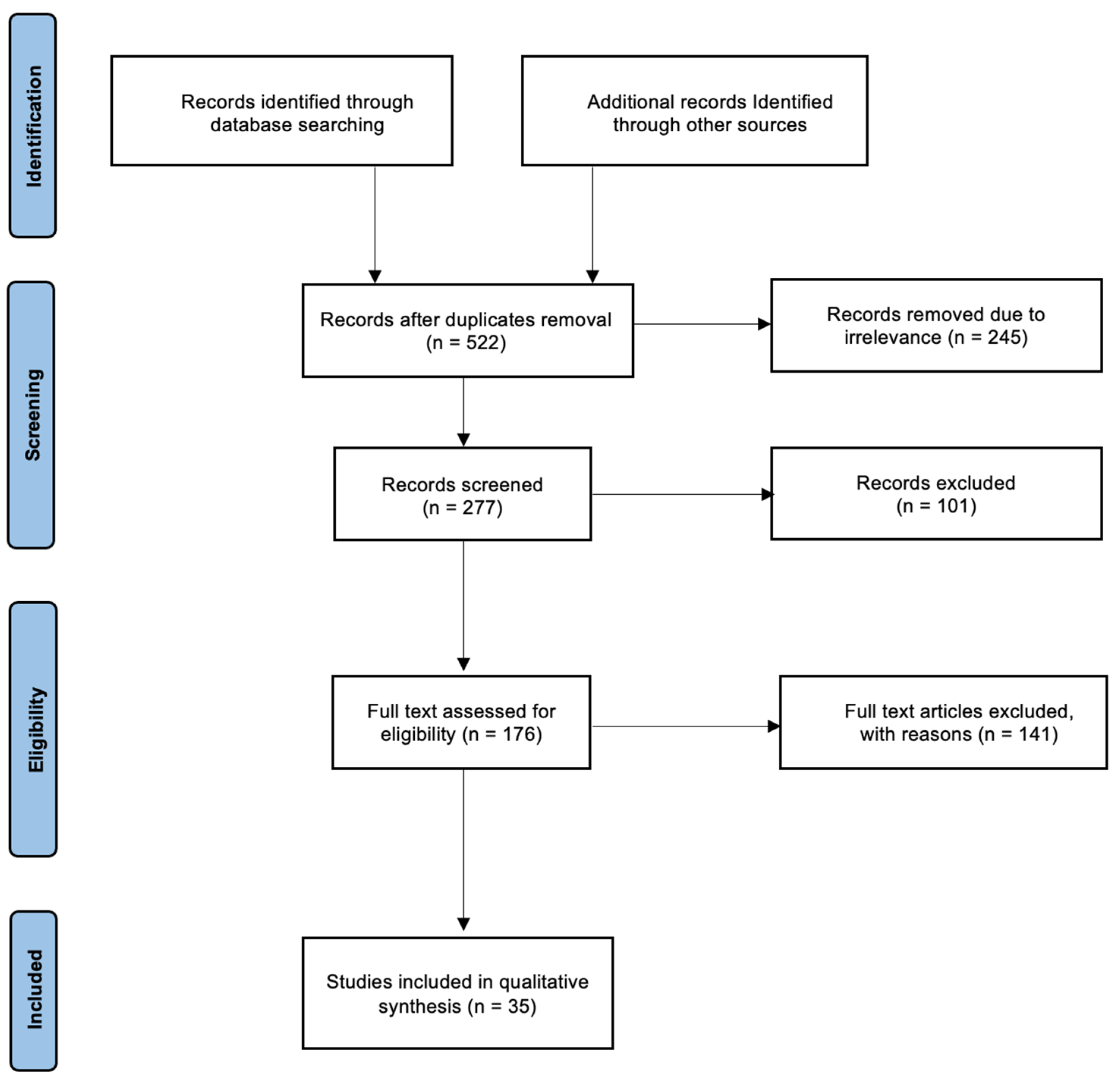
2. Materials and Methods
3. Results
3.1. VNS Trial in Cardiac Diseases
3.1.1. Myocardial Ischemia
3.1.2. Atrial Arrhythmia
3.1.3. Ventricular Arrhythmias
3.1.4. Cardiac Arrest
3.1.5. Heart Failure
3.2. sVNS Methods
3.2.1. Fiber-Selective Stimulation
3.2.2. Spatially-Selective Stimulation
3.2.3. Kilohertz Electrical Stimulation Block (KES)
3.2.4. Neural Titration
3.2.5. Transcutaneous—Vagus Nerve Stimulation (tVNS)
4. Discussion
4.1. Cardiac Diseases
4.1.1. Myocardial Ischemia
4.1.2. Atrial Arrhythmias
4.1.3. Ventricular Arrhythmias
4.1.4. Cardiac Arrest
4.1.5. Heart Failure
4.2. Cellular Mechanism of VNS
4.3. Limitations and Bias
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Câmara, R.; Griessenauer, C.J. Anatomy of the Vagus Nerve. In Nerves and Nerve Injuries; Elsevier: Amsterdam, The Netherlands, 2015; pp. 385–397. [Google Scholar]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Capilupi, M.J.; Kerath, S.M.; Becker, L.B. Vagus Nerve Stimulation and the Cardiovascular System. Cold Spring Harb. Perspect. Med. 2020, 10, a034173. [Google Scholar] [CrossRef]
- Stakenborg, N.; Gomez-Pinilla, P.J.; Verlinden, T.J.M.; Wolthuis, A.M.; D’Hoore, A.; Farré, R.; Herijgers, P.; Matteoli, G.; Boeckxstaens, G.E. Comparison between the Cervical and Abdominal Vagus Nerves in Mice, Pigs, and Humans. Neurogastroenterol. Motil. 2020, 32, e13889. [Google Scholar] [CrossRef]
- Aristovich, K.; Donega, M.; Fjordbakk, C.; Tarotin, I.; Chapman, C.A.R.; Viscasillas, J.; Stathopoulou, T.R.; Crawford, A.; Chew, D.; Perkins, J.; et al. Model-Based Geometrical Optimisation and in Vivo Validation of a Spatially Selective Multielectrode Cuff Array for Vagus Nerve Neuromodulation. J. Neurosci. Methods 2021, 352, 109079. [Google Scholar] [CrossRef]
- Gold, M.R.; van Veldhuisen, D.J.; Hauptman, P.J.; Borggrefe, M.; Kubo, S.H.; Lieberman, R.A.; Milasinovic, G.; Berman, B.J.; Djordjevic, S.; Neelagaru, S.; et al. Vagus Nerve Stimulation for the Treatment of Heart Failure. J. Am. Coll. Cardiol. 2016, 68, 149–158. [Google Scholar] [CrossRef]
- Vaseghi, M.; Salavatian, S.; Rajendran, P.S.; Yagishita, D.; Woodward, W.R.; Hamon, D.; Yamakawa, K.; Irie, T.; Habecker, B.A.; Shivkumar, K. Parasympathetic Dysfunction and Antiarrhythmic Effect of Vagal Nerve Stimulation Following Myocardial Infarction. JCI Insight 2017, 2, e86715. [Google Scholar] [CrossRef]
- Konstam, M.A.; Mann, D.L.; Udelson, J.J.E.; Ardell, J.L.; de Ferrari, G.M.; Cowie, M.R.; Klein, H.U.; Gregory, D.D.; Massaro, J.M.; Libbus, I.; et al. Advances in Our Clinical Understanding of Autonomic Regulation Therapy Using Vagal Nerve Stimulation in Patients Living With Heart Failure. Front. Physiol. 2022, 13, 857538. [Google Scholar] [CrossRef]
- Johnson, R.L.; Wilson, C.G. A Review of Vagus Nerve Stimulation as a Therapeutic Intervention. J. Inflamm. Res. 2018, 11, 203–213. [Google Scholar] [CrossRef]
- Fitchett, A.; Mastitskaya, S.; Aristovich, K. Selective Neuromodulation of the Vagus Nerve. Front. Neurosci. 2021, 15, 685872. [Google Scholar] [CrossRef]
- Howland, R.H. Vagus Nerve Stimulation. Curr. Behav. Neurosci. Rep. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Yap, J.Y.Y.; Keatch, C.; Lambert, E.; Woods, W.; Stoddart, P.R.; Kameneva, T. Critical Review of Transcutaneous Vagus Nerve Stimulation: Challenges for Translation to Clinical Practice. Front. Neurosci. 2020, 14, 284. [Google Scholar] [CrossRef]
- Ottaviani, M.M.; Vallone, F.; Micera, S.; Recchia, F.A. Closed-Loop Vagus Nerve Stimulation for the Treatment of Cardiovascular Diseases: State of the Art and Future Directions. Front. Cardiovasc. Med. 2022, 9, 866957. [Google Scholar] [CrossRef]
- Mertens, A.; Raedt, R.; Gadeyne, S.; Carrette, E.; Boon, P.; Vonck, K. Recent Advances in Devices for Vagus Nerve Stimulation. Expert Rev. Med. Devices 2018, 15, 527–539. [Google Scholar] [CrossRef]
- Dietrich, S.; Smith, J.; Scherzinger, C.; Hofmann-Preiß, K.; Freitag, T.; Eisenkolb, A.; Ringler, R. A Novel Transcutaneous Vagus Nerve Stimulation Leads to Brainstem and Cerebral Activations Measured by Functional MRI/Funktionelle Magnetresonanztomographie Zeigt Aktivierungen Des Hirnstamms Und Weiterer Zerebraler Strukturen Unter Transkutaner Vagusnervstimulation. Biomed. Tech./Biomed. Eng. 2008, 53, 104–111. [Google Scholar] [CrossRef]
- Altavilla, R.; Paolucci, M.; Altamura, C.; Vernieri, F. P038. Effects of Non-Invasive Vagus Nerve Stimulation on Cerebral Vasomotor Reactivity in Patients with Chronic Migraine during Intercritical Phase: A Pilot Study. J. Headache Pain 2015, 16, A62. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Calhoun, A.H.; Lipton, R.B.; Grosberg, B.M.; Cady, R.K.; Dorlas, S.; Simmons, K.A.; Mullin, C.; Liebler, E.J.; Goadsby, P.J.; et al. Chronic Migraine Headache Prevention with Noninvasive Vagus Nerve Stimulation. Neurology 2016, 87, 529–538. [Google Scholar] [CrossRef]
- Grazzi, L.; Egeo, G.; Calhoun, A.H.; McClure, C.K.; Liebler, E.; Barbanti, P. Non-Invasive Vagus Nerve Stimulation (NVNS) as Mini-Prophylaxis for Menstrual/Menstrually Related Migraine: An Open-Label Study. J. Headache Pain 2016, 17, 91. [Google Scholar] [CrossRef]
- Colzato, L.S.; Ritter, S.M.; Steenbergen, L. Transcutaneous Vagus Nerve Stimulation (TVNS) Enhances Divergent Thinking. Neuropsychologia 2018, 111, 72–76. [Google Scholar] [CrossRef]
- Barbanti, P.; Grazzi, L.; Egeo, G.; Padovan, A.M.; Liebler, E.; Bussone, G. Non-Invasive Vagus Nerve Stimulation for Acute Treatment of High-Frequency and Chronic Migraine: An Open-Label Study. J. Headache Pain 2015, 16, 61. [Google Scholar] [CrossRef]
- Jacobs, H.I.L.; Riphagen, J.M.; Razat, C.M.; Wiese, S.; Sack, A.T. Transcutaneous Vagus Nerve Stimulation Boosts Associative Memory in Older Individuals. Neurobiol. Aging 2015, 36, 1860–1867. [Google Scholar] [CrossRef]
- Ben-Menachem, E. Vagus-Nerve Stimulation for the Treatment of Epilepsy. Lancet Neurol. 2002, 1, 477–482. [Google Scholar] [CrossRef]
- Plachta, D.T.T.; Gierthmuehlen, M.; Cota, O.; Espinosa, N.; Boeser, F.; Herrera, T.C.; Stieglitz, T.; Zentner, J. Blood Pressure Control with Selective Vagal Nerve Stimulation and Minimal Side Effects. J. Neural Eng. 2014, 11, 036011. [Google Scholar] [CrossRef]
- Dali, M.; Rossel, O.; Andreu, D.; Laporte, L.; Hernández, A.; Laforet, J.; Marijon, E.; Hagège, A.; Clerc, M.; Henry, C.; et al. Model Based Optimal Multipolar Stimulation without a Priori Knowledge of Nerve Structure: Application to Vagus Nerve Stimulation. J. Neural Eng. 2018, 15, 046018. [Google Scholar] [CrossRef]
- Vuckovic, A.; Tosato, M.; Struijk, J.J. A Comparative Study of Three Techniques for Diameter Selective Fiber Activation in the Vagal Nerve: Anodal Block, Depolarizing Prepulses and Slowly Rising Pulses. J. Neural Eng. 2008, 5, 275–286. [Google Scholar] [CrossRef]
- Ardell, J.L.; Rajendran, P.S.; Nier, H.A.; KenKnight, B.H.; Armour, J.A. Central-Peripheral Neural Network Interactions Evoked by Vagus Nerve Stimulation: Functional Consequences on Control of Cardiac Function. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H1740–H1752. [Google Scholar] [CrossRef]
- Peuker, E.T.; Filler, T.J. The Nerve Supply of the Human Auricle. Clin. Anat. 2002, 15, 35–37. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, N71. [Google Scholar] [CrossRef]
- Yi, C.; Zhang, C.; Hu, X.; Li, Y.; Jiang, H.; Xu, W.; Lu, J.; Liao, Y.; Ma, R.; Li, X.; et al. Vagus Nerve Stimulation Attenuates Myocardial Ischemia/Reperfusion Injury by Inhibiting the Expression of Interleukin-17A. Exp. Ther. Med. 2016, 11, 171–176. [Google Scholar] [CrossRef]
- Beaumont, E.; Southerland, E.M.; Hardwick, J.C.; Wright, G.L.; Ryan, S.; Li, Y.; KenKnight, B.H.; Andrew Armour, J.; Ardell, J.L. Vagus Nerve Stimulation Mitigates Intrinsic Cardiac Neuronal and Adverse Myocyte Remodeling Postmyocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1198. [Google Scholar] [CrossRef]
- Zhang, R.; Wugeti, N.; Sun, J.; Yan, H.; Guo, Y.; Zhang, L.; Ma, M.; Guo, X.; Jiao, C.; Xu, W.; et al. Effects of Vagus Nerve Stimulation via Cholinergic Anti-Inflammatory Pathway Activation on Myocardial Ischemia/Reperfusion Injury in Canine. Int. J. Clin. Exp. Med. 2014, 7, 2615. [Google Scholar]
- Shinlapawittayatorn, K.; Chinda, K.; Palee, S.; Surinkaew, S.; Kumfu, S.; Kumphune, S.; Chattipakorn, S.; Kenknight, B.H.; Chattipakorn, N. Vagus Nerve Stimulation Initiated Late during Ischemia, but Not Reperfusion, Exerts Cardioprotection via Amelioration of Cardiac Mitochondrial Dysfunction. Heart Rhythm. 2014, 11, 2278–2287. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, L.; Wang, S.; Huang, B.; Liao, K.; Saren, G.; Tan, T.; Jiang, H. Chronic Intermittent Low-Level Transcutaneous Electrical Stimulation of Auricular Branch of Vagus Nerve Improves Left Ventricular Remodeling in Conscious Dogs With Healed Myocardial Infarction. Circ. Heart Fail. 2014, 7, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.S.; Liu, J.J.; Hwang, T.C.; Yu, X.J.; Lu, Y.; Zang, W.J. Tumour Necrosis Factor-α and Its Receptors in the Beneficial Effects of Vagal Stimulation after Myocardial Infarction in Rats. Clin. Exp. Pharmacol. Physiol. 2011, 38, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Yokota, H.; Edama, M.; Hirabayashi, R.; Sekine, C.; Otsuru, N.; Saito, K.; Kojima, S.; Miyaguchi, S.; Onishi, H. Effects of Stimulus Frequency, Intensity, and Sex on the Autonomic Response to Transcutaneous Vagus Nerve Stimulation. Brain Sci. 2022, 12, 1038. [Google Scholar] [CrossRef]
- Stavrakis, S.; Stoner, J.A.; Humphrey, M.B.; Morris, L.; Filiberti, A.; Reynolds, J.C.; Elkholey, K.; Javed, I.; Twidale, N.; Riha, P.; et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. JACC Clin. Electrophysiol. 2020, 6, 282–291. [Google Scholar] [CrossRef]
- Naggar, I.; Nakase, K.; Lazar, J.; Salciccioli, L.; Selesnick, I.; Stewart, M. Vagal Control of Cardiac Electrical Activity and Wall Motion during Ventricular Fibrillation in Large Animals. Auton. Neurosci. 2014, 183, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Anderson, A.; Guzman, P.A.; Nakano, A.; Tolkacheva, E.G.; Wickman, K. Atrial GIRK Channels Mediate the Effects of Vagus Nerve Stimulation on Heart Rate Dynamics and Arrhythmogenesis. Front. Physiol. 2018, 9, 943. [Google Scholar] [CrossRef]
- Lee, S.W.; Kulkarni, K.; Annoni, E.M.; Libbus, I.; KenKnight, B.H.; Tolkacheva, E.G. Stochastic Vagus Nerve Stimulation Affects Acute Heart Rate Dynamics in Rats. PLoS ONE 2018, 13, e0194910. [Google Scholar] [CrossRef]
- Stavrakis, S.; Humphrey, M.B.; Scherlag, B.; Iftikhar, O.; Parwani, P.; Abbas, M.; Filiberti, A.; Fleming, C.; Hu, Y.; Garabelli, P.; et al. Low-Level Vagus Nerve Stimulation Suppresses Post-Operative Atrial Fibrillation and Inflammation: A Randomized Study. JACC Clin. Electrophysiol. 2017, 3, 929–938. [Google Scholar] [CrossRef]
- Stavrakis, S.; Humphrey, M.B.; Scherlag, B.J.; Hu, Y.; Jackman, W.M.; Nakagawa, H.; Lockwood, D.; Lazzara, R.; Po, S.S. Low-Level Transcutaneous Electrical Vagus Nerve Stimulation Suppresses Atrial Fibrillation. J. Am. Coll. Cardiol. 2015, 65, 867–875. [Google Scholar] [CrossRef]
- Rossi, P.; Ricci, A.; de Paulis, R.; Papi, E.; Pavaci, H.; Porcelli, D.; Monari, G.; Maselli, D.; Bellisario, A.; Turani, F.; et al. Epicardial Ganglionated Plexus Stimulation Decreases Postoperative Inflammatory Response in Humans. Heart Rhythm. 2012, 9, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.C.; Ahmed, U.; Shoaib, M.; Alper, E.; Rehman, A.; Kim, J.; Shinozaki, K.; Volpe, B.T.; Chavan, S.; Zanos, S.; et al. Threshold Adjusted Vagus Nerve Stimulation after Asphyxial Cardiac Arrest Results in Neuroprotection and Improved Survival. Bioelectron. Med. 2022, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, I.; Lee, J.H.; Kim, S.; Jang, D.H.; Jo, Y.H. Vagus Nerve Stimulation Improves Mitochondrial Dysfunction in Post-Cardiac Arrest Syndrome in the Asphyxial Cardiac Arrest Model in Rats. Front. Neurosci. 2022, 16, 762007. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.; Zhao, S.; Yang, Z.; Tang, Z.; Ravindra, N.; Bradley, J.; Ornato, J.P.; Peberdy, M.A.; Tang, W. Improved Outcomes of Cardiopulmonary Resuscitation in Rats Treated With Vagus Nerve Stimulation and Its Potential Mechanism. Shock 2018, 49, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Elkholey, K.; Morris, L.; Niewiadomska, M.; Asad, Z.U.A.; Humphrey, M.B. Neuromodulation of Inflammation to Treat Heart Failure With Preserved Ejection Fraction: A Pilot Randomized Clinical Trial. J. Am. Heart Assoc. 2022, 11, 23582. [Google Scholar] [CrossRef]
- Zhou, L.; Filiberti, A.; Humphrey, M.B.; Fleming, C.D.; Scherlag, B.J.; Po, S.S.; Stavrakis, S. Low-Level Transcutaneous Vagus Nerve Stimulation Attenuates Cardiac Remodelling in a Rat Model of Heart Failure with Preserved Ejection Fraction. Exp. Physiol. 2019, 104, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Asad, Z.; Elkholey, K.; Scherlag, B.J.; Po, S.S.; Stavrakis, S. Autonomic Neuromodulation Acutely Ameliorates Left Ventricular Strain in Humans. J. Cardiovasc. Transl. Res. 2018, 12, 221–230. [Google Scholar] [CrossRef]
- Nearing, B.D.; Libbus, I.; Amurthur, B.; Kenknight, B.H.; Verrier, R.L. Acute Autonomic Engagement Assessed by Heart Rate Dynamics During Vagus Nerve Stimulation in Patients With Heart Failure in the ANTHEM-HF Trial. J. Cardiovasc. Electrophysiol. 2016, 27, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; de Ferrari, G.M.; Tuinenburg, A.E.; Wright, D.; Brugada, J.; Butter, C.; Klein, H.; Stolen, C.; Meyer, S.; Stein, K.M.; et al. Chronic Vagal Stimulation for the Treatment of Low Ejection Fraction Heart Failure: Results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) Randomized Controlled Trial. Eur. Heart J. 2015, 36, 425–433. [Google Scholar] [CrossRef]
- Zhang, Y.; Popović, Z.B.; Bibevski, S.; Fakhry, I.; Sica, D.A.; van Wagoner, D.R.; Mazgalev, T.N. Chronic Vagus Nerve Stimulation Improves Autonomic Control and Attenuates Systemic Inflammation and Heart Failure Progression in a Canine High-Rate Pacing Model. Circ. Heart Fail. 2009, 2, 692–699. [Google Scholar] [CrossRef]
- Sabbah, H.N.; Imai, M.; Zaretsky, A.; Rastogi, S.; Wang, M.; Jiang, A.; Zaca, V. 509 Therapy with Vagus Nerve Electrical Stimulation Combined with Beta-Blockade Improves Left Ventricular Systolic Function in Dogs with Heart Failure beyond That Seen with Beta-Blockade Alone. Eur. J. Heart Fail. Suppl. 2007, 6, 114. [Google Scholar] [CrossRef]
- Blanz, S.L.; Musselman, E.D.; Settell, M.L.; Knudsen, B.E.; Nicolai, E.N.; Trevathan, J.K.; Verner, R.S.; Begnaud, J.; Suminski, A.J.; Williams, J.C.; et al. Spatially Selective Stimulation of the Pig Vagus Nerve to Modulate Target Effect versus Side Effect. bioRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Huang, B.; Po, S.S.; Tan, T.; Wang, M.; Zhou, L.; Meng, G.; Yuan, S.; Zhou, X.; Li, X.; et al. Low-Level Tragus Stimulation for the Treatment of Ischemia and Reperfusion Injury in Patients With ST-Segment Elevation Myocardial Infarction: A Proof-of-Concept Study. JACC Cardiovasc. Interv. 2017, 10, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Stauss, H.M. Differential Hemodynamic and Respiratory Responses to Right and Left Cervical Vagal Nerve Stimulation in Rats. Physiol. Rep. 2017, 5, e13244. [Google Scholar] [CrossRef]
- Patel, Y.A.; Butera, R.J. Differential Fiber-Specific Block of Nerve Conduction in Mammalian Peripheral Nerves Using Kilohertz Electrical Stimulation. J. Neurophysiol. 2015, 113, 3923–3929. [Google Scholar] [CrossRef] [PubMed]
- Ordelman, S.C.M.A.; Kornet, L.; Cornelussen, R.; Buschman, H.P.J.; Veltink, P.H. Selectivity for Specific Cardiovascular Effects of Vagal Nerve Stimulation With a Multi-Contact Electrode Cuff. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Yoshida, K.; Toft, E.; Struijk, J.J. Quasi-Trapezoidal Pulses to Selectively Block the Activation of Intrinsic Laryngeal Muscles during Vagal Nerve Stimulation. J. Neural Eng. 2007, 4, 205–212. [Google Scholar] [CrossRef]
- Wallick, D.W.; Zhang, Y.; Tabata, T.; Zhuang, S.; Mowrey, K.A.; Watanabe, J.; Greenberg, N.L.; Grimm, R.A.; Mazgalev, T.N. Selective AV Nodal Vagal Stimulation Improves Hemodynamics during Acute Atrial Fibrillation in Dogs. Am. J. Physiol.-Heart Circ. Physiol. 2001, 281, H1490–H1497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, Y.; Xue, F.S.; Yuan, Y.J.; Xiong, J.; Li, R.P.; Liao, X.; Liu, J.H. Postconditioning with Vagal Stimulation Attenuates Local and Systemic Inflammatory Responses to Myocardial Ischemia Reperfusion Injury in Rats. Inflamm. Res. 2012, 61, 1273–1282. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amelia, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, N.; Ulloa, L.; et al. Nicotinic Acetylcholine Receptor Alpha7 Subunit Is an Essential Regulator of Inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Lu, X.; Costantini, T.; Lopez, N.E.; Wolf, P.L.; Hageny, A.M.; Putnam, J.; Eliceiri, B.; Coimbra, R. Vagal Nerve Stimulation Protects Cardiac Injury by Attenuating Mitochondrial Dysfunction in a Murine Burn Injury Model. J. Cell. Mol. Med. 2013, 17, 664–671. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics--2015 Update: A Report from the American Heart Association. Circulation 2015, 131, e29–e39. [Google Scholar] [CrossRef]
- Takahashi, N.; Zipes, D.P. Vagal Modulation of Adrenergic Effects on Canine Sinus and Atrioventricular Nodes. Am. J. Physiol. 1983, 244, H775–H781. [Google Scholar] [CrossRef]
- Jiang, Y.; Po, S.S.; Amil, F.; Dasari, T.W. Non-Invasive Low-Level Tragus Stimulation in Cardiovascular Diseases. Arrhythm. Electrophysiol. Rev. 2020, 9, 40. [Google Scholar] [CrossRef]
- Zhang, S.J.; Huang, C.X.; Zhao, Q.Y.; Zhang, S.; Dai, Z.X.; Zhao, H.Y.; Qian, Y.S.; Zhang, Y.J.; Wang, Y.C.; He, B.; et al. The Role of A7nAChR-Mediated Cholinergic Anti-Inflammatory Pathway in Vagal Nerve Regulated Atrial Fibrillation. Int. Heart J. 2021, 62, 607–615. [Google Scholar] [CrossRef]
- Stavrakis, S.; Po, S. Ganglionated Plexi Ablation: Physiology and Clinical Applications. Arrhythm. Electrophysiol. Rev. 2017, 6, 186. [Google Scholar] [CrossRef]
- Ng, G.A. Vagal Modulation of Cardiac Ventricular Arrhythmia. Exp. Physiol. 2014, 99, 295–299. [Google Scholar] [CrossRef]
- Winter, J.; Tipton, M.J.; Shattock, M.J. Autonomic Conflict Exacerbates Long QT Associated Ventricular Arrhythmias. J. Mol. Cell. Cardiol. 2018, 116, 145–154. [Google Scholar] [CrossRef]
- Kishi, T. Heart Failure as an Autonomic Nervous System Dysfunction. J. Cardiol. 2012, 59, 117–122. [Google Scholar] [CrossRef]
- Scridon, A.; Halaţiu, V.B.; Balan, A.I.; Cozac, D.A.; Moldovan, V.; Bănescu, C.; Perian, M.; Şerban, R.C. Long-Term Effects of Ivabradine on Cardiac Vagal Parasympathetic Function in Normal Rats. Front. Pharmacol. 2021, 12, 596956. [Google Scholar] [CrossRef]
- Bawa, P.N.S.; Jones, K.E.; Stein, R.B. Assessment of Size Ordered Recruitment. Front Hum. Neurosci. 2014, 8, 532. [Google Scholar] [CrossRef]
- Ahmed, U.; Chang, Y.-C.; Cracchiolo, M.; Lopez, M.F.; Tomaio, J.N.; Datta-Chaudhuri, T.; Zanos, T.P.; Rieth, L.; Al-Abed, Y.; Zanos, S. Anodal Block Permits Directional Vagus Nerve Stimulation. Sci. Rep. 2020, 10, 9221. [Google Scholar] [CrossRef]
- Gorman, P.H.; Mortimer, J.T. The Effect of Stimulus Parameters on the Recruitment Characteristics of Direct Nerve Stimulation. IEEE Trans. Biomed. Eng. 1983, BME-30, 407–414. [Google Scholar] [CrossRef]
- Pečlin, P.; Rozman, J. Alternative Paradigm of Selective Vagus Nerve Stimulation Tested on an Isolated Porcine Vagus Nerve. Sci. World J. 2014, 2014, 310283. [Google Scholar] [CrossRef]
- Neudorfer, C.; Chow, C.T.; Boutet, A.; Loh, A.; Germann, J.; Elias, G.J.B.; Hutchison, W.D.; Lozano, A.M. Kilohertz-Frequency Stimulation of the Nervous System: A Review of Underlying Mechanisms. Brain Stimul. 2021, 14, 513–530. [Google Scholar] [CrossRef]
- Fallen, E.L.; Kamath, M.V.; Tougas, G.; Upton, A. Afferent Vagal Modulation: Clinical Studies of Visceral Sensory Input. Auton. Neurosci. 2001, 90, 35–40. [Google Scholar] [CrossRef]
- Kreuzer, P.M.; Landgrebe, M.; Husser, O.; Resch, M.; Schecklmann, M.; Geisreiter, F.; Poeppl, T.B.; Prasser, S.J.; Hajak, G.; Langguth, B. Transcutaneous Vagus Nerve Stimulation: Retrospective Assessment of Cardiac Safety in a Pilot Study. Front. Psychiatry 2012, 3, 70. [Google Scholar] [CrossRef]
- Sclocco, R.; Garcia, R.G.; Kettner, N.W.; Isenburg, K.; Fisher, H.P.; Hubbard, C.S.; Ay, I.; Polimeni, J.R.; Goldstein, J.; Makris, N.; et al. The Influence of Respiration on Brainstem and Cardiovagal Response to Auricular Vagus Nerve Stimulation: A Multimodal Ultrahigh-Field (7T) FMRI Study. Brain Stimul. 2019, 12, 911–921. [Google Scholar] [CrossRef]
- Jayaraj, J.C.; Davatyan, K.; Subramanian, S.S.; Priya, J. Epidemiology of Myocardial Infarction. Minerva Med. 2018, 54, 2083–2087. [Google Scholar] [CrossRef]
- Deng, D.; Liu, L.; Xu, G.; Gan, J.; Shen, Y.; Shi, Y.; Zhu, R.; Lin, Y. Epidemiology and Serum Metabolic Characteristics of Acute Myocardial Infarction Patients in Chest Pain Centers. Iran J. Public Health 2018, 47, 1017. [Google Scholar]
- Berg, D.D.; Wiviott, S.D.; Braunwald, E.; Guo, J.; Im, K.; Kashani, A.; Gibson, C.M.; Cannon, C.P.; Morrow, D.A.; Bhatt, D.L.; et al. Modes and Timing of Death in 66 252 Patients with Non-ST-Segment Elevation Acute Coronary Syndromes Enrolled in 14 TIMI Trials. Eur. Heart J. 2018, 39, 3810–3820. [Google Scholar] [CrossRef]
- Mechanic, O.J.; Gavin, M.; Grossman, S.A. Acute Myocardial Infarction; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T. Myocardial Ischemia Reperfusion Injury: From Basic Science to Clinical Bedside. Semin. Cardiothorac. Vasc. Anesth. 2012, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Vinten-Johansen, J. Involvement of Neutrophils in the Pathogenesis of Lethal Myocardial Reperfusion Injury. Cardiovasc. Res. 2004, 61, 481–497. [Google Scholar] [CrossRef]
- Nesheiwat, Z.; Goyal, A.; Jagtap, M. Atrial Fibrillation; StatPearls: Treasure Island, FL, USA, 2022; pp. 1–8. [Google Scholar]
- Markides, V.; Schilling, R.J. Atrial Fibrillation: Classification, Pathophysiology, Mechanisms and Drug Treatment. Heart 2003, 89, 939. [Google Scholar] [CrossRef]
- Chugh, S.S.; Reinier, K.; Teodorescu, C.; Evanado, A.; Kehr, E.; al Samara, M.; Mariani, R.; Gunson, K.; Jui, J. Epidemiology of Sudden Cardiac Death: Clinical and Research Implications. Prog. Cardiovasc. Dis. 2008, 51, 213. [Google Scholar] [CrossRef] [PubMed]
- Foth, C.; Gangwani, M.K.; Alvey, H. Ventricular Tachycardia; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Koplan, B.A.; Stevenson, W.G. Ventricular Tachycardia and Sudden Cardiac Death. Mayo Clin. Proc. 2009, 84, 289. [Google Scholar] [CrossRef]
- Patel, K.; Hipskind, J.E. Cardiac Arrest; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Fox, C.S.; Evans, J.C.; Larson, M.G.; Kannel, W.B.; Levy, D. The Framingham Heart Study. Circulation 2004, 110, 522–527. [Google Scholar] [CrossRef]
- Weisfeldt, M.L.; Becker, L.B. Resuscitation after Cardiac Arrest: A 3-Phase Time-Sensitive Model. JAMA 2002, 288, 3035–3038. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Inamdar, A.C. Heart Failure: Diagnosis, Management and Utilization. J. Clin. Med. 2016, 5, 62. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of Heart Failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal Trends and Patterns in Heart Failure Incidence: A Population-Based Study of 4 Million Individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Dassanayaka, S.; Jones, S.P. Recent Developments in Heart Failure. Circ. Res. 2015, 117, e58. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef]
- Carandina, A.; Rodrigues, G.D.; di Francesco, P.; Filtz, A.; Bellocchi, C.; Furlan, L.; Carugo, S.; Montano, N.; Tobaldini, E. Effects of Transcutaneous Auricular Vagus Nerve Stimulation on Cardiovascular Autonomic Control in Health and Disease. Auton. Neurosci. 2021, 236, 2920–2925. [Google Scholar] [CrossRef]
- Poppa, T.; Benschop, L.; Horczak, P.; Vanderhasselt, M.A.; Carrette, E.; Bechara, A.; Baeken, C.; Vonck, K. Auricular Transcutaneous Vagus Nerve Stimulation Modulates the Heart-Evoked Potential. Brain Stimul. 2022, 15, 260–269. [Google Scholar] [CrossRef]

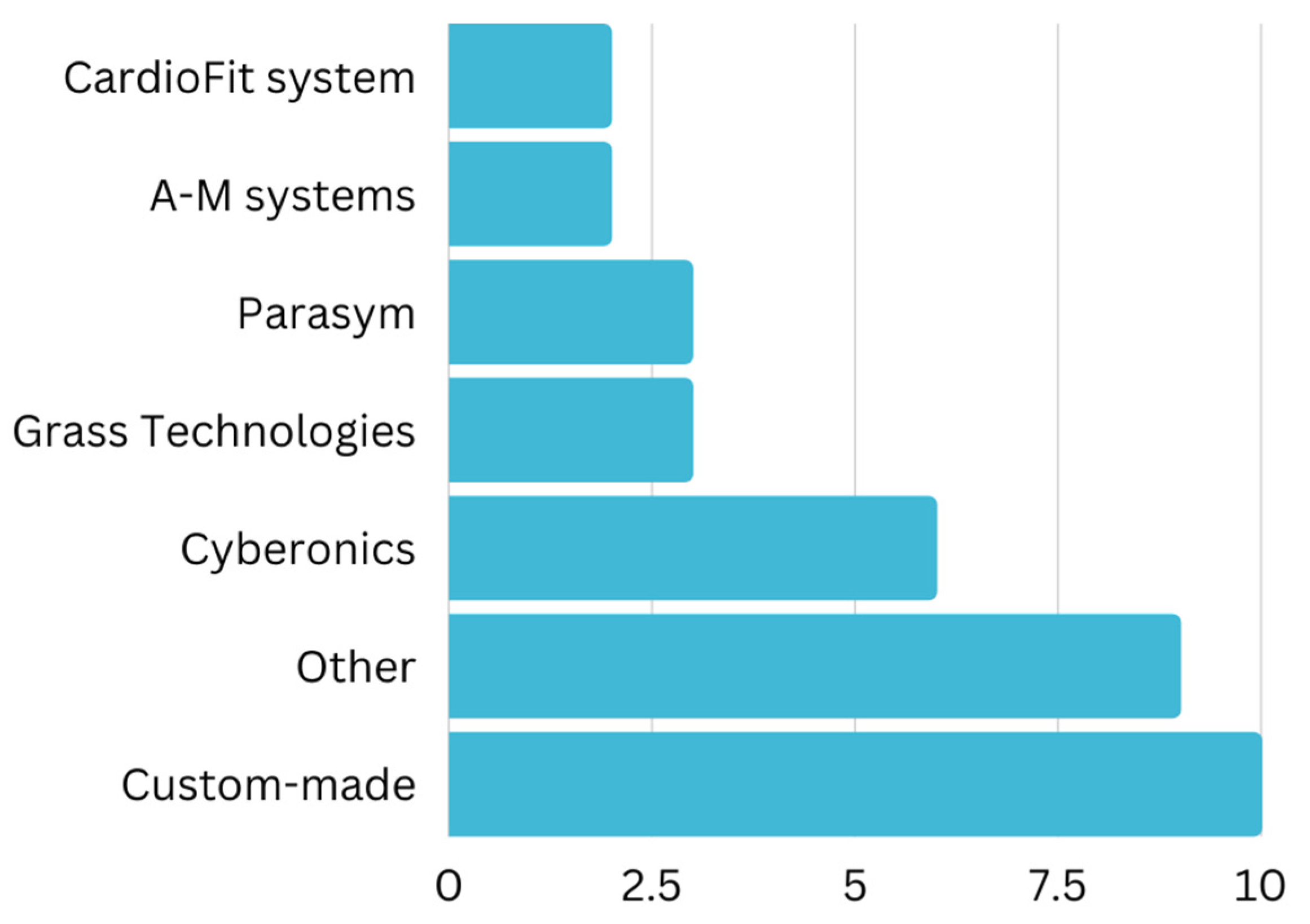
| Study | Year | Model | CVS Disease | Characteristics | Device | VNS Tool | Outcome/Result |
|---|---|---|---|---|---|---|---|
| Yi et al. [29] | 2016 | Rat | I/R injury | I/R rats subjected to occlusion of the left anterior descending artery for 30 min, followed by reperfusion for 4 h | Grass technologies | Rectangular electrical pulses (10 Hz for 2 ms) | Significant reduction in infarct size following I/R injury. |
| Beaumont et al. [30] | 2015 | Pig | MI | Surgical induction of MI by ligation of ventral descending coronary artery and associated vein | Cyberonics | Bipolar VNS electrode (cyclical stimulation 14 s on and 48 s off for 80 days, frequency 20 Hz, 500 μs pulse duration) | Focal mitigation of remodelling of the intrinsic cardiac nervous system |
| Zhang et al. [31] | 2014 | Dog | I/R injury | Surgical occlusion of the left anterior descending artery for 1 h, followed by reperfusion for 6 h | SEN-7103, Nihon-Kohden | Special silver-argentic chloride stimulation electrode (frequency 10 Hz, pulse width 0.5 ms and stimulation strength 1.5–3 V) | Inhibition of pro-inflammatory products like TNF-α |
| Shinlapawittayatorn et al. [32] | 2014 | Pig | I/R injury | Occlusion of the left anterior descending artery for 60 min, followed by reperfusion for 120 min, with VNS received either after 30 | N/A | Bipolar pacing lead and anchor lead for cyclical stimulation 20 s on and 30 s off (frequency 20 Hz, current 3.5 mA) | Cardioprotective effects during ischemia but not reperfusion. |
| Wang et al. [33] | 2014 | Dog | MI | Harris 2-stage occlusion of the left anterior descending artery (partial occlusion for 20 min then completely for 3 h) | N/A | Custom-made stimulator for cyclical low-level transcutaneous stimulation on bilateral tragus, 5 s on and 5 s off (frequency 20 Hz, and pulse width 1 ms) | Significant improvement in alleviated cardiac fibrosis, cardiac function, and attenuated LV remodeling |
| Kong et al. [34] | 2011 | Rat | MI | Occlusion of the left anterior descending artery for 240 min continuously | N/A | Bipolar electrodes attached to the cardiac end of right vagus nerve (frequency 5 Hz, voltage 2–6 V) | Significant attenuation of TNF-α signaling pathway |
| Study | Year | Model | CVS Disease/Parameter | Characteristics | Device | VNS Tool | Outcome/Results |
|---|---|---|---|---|---|---|---|
| Yokota et al. [35] | 2022 | Human | Heart rate modulation | Healthy adults monitored with two different ECG devices | NEMOS | hemispheric titanium-stimulating electrodes on cymba concha for transcutaneous stimulation (experimented with different sets of stimulation frequencies at 100 Hz, 25 Hz, 10 Hz, 1 Hz, and 0 Hz) | A more significant decrease in heart rate at 100 Hz signifies a frequency-dependent manner for the vagal transmission |
| Stavrakis et al. [36] | 2020 | Human | Atrial fibrillation | Paroxysmal AF patients, documented by an ECG with in a 3-month span (2 separate occasions) | Parasym device | Ear clip onto the tragus for low level transcutaneous stimulation (frequency 20 Hz and pulse width 200 ms) for 1 h continuously every day for 6 months | 85% decrease in AF and a 23% reduction of TNF-α in those using LLTS compared to controls |
| Naggar et al. [37] | 2018 | Pig and sheep | Ventricular fibrillation | VF was induced by large, low impedance electrodes on the ventricles with a direct current of 3–5 volts for several seconds | A-M Systems model 2100 | Platinum strip electrodes. Bilateral innervation of cervical vagus nerve (frequency 50 Hz, current 1–10 mA, voltage 3–5 V) | A direct electrical effect on the ventricles |
| Lee et al. [38] | 2018 | Rat | Heart rate modulation | Healthy rats. Samples and recordings taken before, during, and after VNS. | Demipulse Model 103 VNS pulse generator | Helical lead bipolar cuff electrodes on the right cervical vagus nerve (frequency 10 Hz, current 0.25 mA, 500 μs pulse duration) for 1 min | GIRK proved feasible as a tool for VNS action |
| Lee et al. [39] | 2018 | Rat | Heart rate modulation | Healthy rats. Samples and recordings taken before, during, and after VNS. | Cyberonics | Gaussian distribution of stimulation frequencies for stochastic VNS, on the right cervical vagus nerve (frequency 10,20 or 30 Hz, current 0.1 mA) for 2 min | Further attenuation heart rate with stochastic VNS compared to Standard VNS method |
| Stavrakis et al. [40] | 2017 | Human | Atrial fibrillation | Patients undergoing cardiac surgery, including coronary artery bypass graft and valve surgery | N/A | Bipolar wire for low level vagus nerve stimulation, adjacent to the superior vena cava (frequency 20 Hz, pulse duration 0.1 ms) upto 72 h | Decrease of 24% in the incidence of postoperative atrial fibrillation in the active group compared to the sham controls (12% vs. 36%) |
| Stavrakis et al. [41] | 2015 | Human | Atrial fibrillation | Paroxysmal AF patients that were referred to an electrophysiology lab for AF ablation | Grass S88 stimulator | A flat metal clip onto the tragus for low level transcutaneous stimulation (frequency 20 Hz, square wave 1 ms) | Suppression of atrial fibrillation and decreased inflammatory cytokines |
| Rossi et al. [42] | 2012 | Human | Atrial fibrillation | Patients undergoing coronary artery bypass graft | LabView system (Streamline Convenience model 6495 Medtronic) | Bipolar temporary wire for low level vagus nerve stimulation (frequency 50 Hz, pulse duration 1 ms) | Reduced incidence of postoperative atrial fibrillation from 25% to 7% in controls, with significant decrease in inflammatory cytokines |
| Study | Year | Model | CVS Disease | Characteristics | Device | VNS Tool | Outcome/Results |
|---|---|---|---|---|---|---|---|
| Choudhary et al. [43] | 2022 | Rat | Cardiac arrest | 12 min of untreated asphyxia-CA via vecuronium bromide injection | N/A | Threshold adjusted VNS, biphasic rectangular electrical pulses left cervical vagus nerve | Significant improvement in survivability rate to 72 h |
| Kim et al. [44] | 2022 | Rat | Cardiac arrest | 450 s of untreated asphyxia-CA via vecuronium bromide injection | Model 2100 isolated pulse stimulator, A-M Systems | Platinum electrode for stable electrical stimulation, left cervical vagus nerve (frequency 1 Hz, current 1 mA, pulse duration 10 ms) for 3 h | Relieve of mitochondrial dysfunction after post cardiac arrest syndrome |
| Sun et al. [45] | 2018 | Rat | Cardiac arrest | Ventricular fibrillation was induced and untreated for 8 min, leading to CA | Model 8002A pulse generator; Hewlett-Packard | Electrical rectangular pulses, right cervical vagus nerve (frequency 10 Hz, pulse duration 0.5 ms, voltage 2–6 V) | Improved CPR outcomes |
| Study | Year | Model | CVS Disease | Characteristics | Device | VNS Tool | Outcome/Results |
|---|---|---|---|---|---|---|---|
| Stavrakis et al. [46] | 2022 | Human | Heart failure | Heart failure patients with preserved ejection fraction and at least 2 co-morbidities | Parasym device | low-level transcutaneous vagus nerve stimulation, Active (tragus) or sham (earlobe), (frequency 20 Hz, current 22.9 ± 13.4 mA, pulse dura-tion 0.2 ms) | Significant improvement in inflammatory cytokines, global longitudinal strain, and quality of life |
| Zhou et al. [47] | 2019 | Rat | Heart failure | Dahl salt sensitive rats were feed a high salt diet to induce HF | InTENSity™ Twin Stim® | Oppositely charged magnetic electrodes for low-level transcutaneous stimulation (frequency 20 Hz, current 2.0 mA, pulse duration 0.2 ms) 30 min daily for 4 weeks. | Significant improvement in cardiac diastolic dysfunction and attenuation of cardiac inflammation and fibrosis |
| Tran et al. [48] | 2018 | Human | Heart failure | Patients diagnosed with diastolic dysfunction via echocardiogram, within 24 months from study enrollment | Parasym device, Parasym Health | Ear clip electrode for low level transcutaneous stimulation (frequency 20 Hz, current 1 mA below the discomfort threshold, pulse width 200 μs) | Improved left ventricular global longitudinal stain (a marker for early recognition of left ventricular systolic dysfunction) |
| Dali et al. [24] | 2018 | Sheep | Heart failure | Sheep infarcted to create significant HF | Custom-made | 12-pole cylindrical cuff electrode for spatially selective control (frequency 25.6 Hz, current 1.33 mA) | 62% reduction in side effects compared to non-selective VNS |
| Gold et al. [6] | 2016 | Human | Heart failure | Patients with NYHA class III symptoms of HF | BioControl CardioFit system | Nerve stimulation cuff on the right vagus nerve (current 3.5–5.5 mA) | No reduction in rate of death in HF, but improved quality of life. |
| Nearing et al. [49] | 2016 | Human | Heart failure | Patients with NYHA class II or III symptoms of HF | Demipulse Model 103 pulse generator, Cyberonics | Lead placement randomized 1:1 to either the right or left cervical vagus nerve, with cyclical stimulation 14 s on and 66 s off. Initially (frequency 10 Hz, pulse width 130 μs, current 1.5–3.0 mA) then intensity was increased in 0.25 mA steps until it was intolerable | Ability to modulate the heart in both left- and right- sided stimulation |
| Zannad et al. [50] | 2015 | Human | Heart failure | Patients with NYHA class II or III symptoms of HF | NECTAR-HF system | Self-sizing bipolar helical lead by cyclical stimulation of 10 s on and 50 s off (frequency 20 Hz, current 4 mA, pulse duration of 300 μs) | Having no effect on cardiac remodeling or function |
| Zhang et al. [51] | 2009 | Dog | Heart failure | Dogs underwent 8 weeks of VF (4 weeks to develop HF and 4 weeks to maintain HF) | Cyberonics | Right cervical vagus nerve electrode with cyclical stimulation of 14 s on and 12 s off (frequency 20 Hz, current 0.75 to 2.5 mA, pulse width 0.5 ms) | Improved cardiac autonomic control whilst attenuating HF development |
| Sabbah et al. [52] | 2007 | Dog | Heart failure | Dogs with chronic HF undergoing 3 months therapy by the experiment | CardioFit VNES system | Vagus nerve stimulation combined with beta-blockade therapy | Improved left ventricular systolic function with an increased EF of 9.8 ± 0.6% compared to controls |
| Study | Year | Model | Application | Characteristics | Device | VNS Tool | Outcome/Results |
|---|---|---|---|---|---|---|---|
| Blanz S et al. [53] | 2022 | Pig | Heart rate modulation | Healthy pigs | ImThera | Spatially-selective control, right cervical vagus nerve | Reduced side effects |
| Yu et al. [54] | 2017 | Human | Myocardial infarction | Patients with STEMI, who presented with 12 h of symptoms from onset and underwent PCI | S20, Jinjiang | Low-level tragus stimulation (frequency 20 Hz, pulsed duration 1 ms) | Significant improvement in myocardial injury biomarkers, inflammatory responses, and reperfusion-related ventricular arrhythmias. |
| Stauss et al. [55] | 2017 | Rat | Hypertension | Stroke-prone, spontaneously hypertensive rats | Grass instruments | Fiber selective control, bilateral cervical vagus nerve (experimented with various frequencies and pulsed durations) | Stronger cardiorespiratory response in left-sided cervical VNS compared to the right. |
| Patel et al. [56] | 2015 | Rat | Neuromodulation | Healthy rats | N/A | KES block technique (pulse width 0.2 ms, voltage 5 V) | Possible application of KES block for selective stimulation of nerves |
| Adrell et al. [26] | 2015 | Dog | Heart rate modulation | Healthy dogs | PerenniaFlex model, Cyberonics | Bipolar stimulating helical cuff electrodes for neural titration method (frequency 10 Hz, pulse width 500 μs) a range of different frequencies were experimented. | Simultaneous stimulation of efferent and afferent fibers resulted in bradycardia |
| Ordelman et al. [57] | 2013 | Pig | Heart rate modulation | Healthy pigs | custom-made | Biphasic pulses for spatially-selective control (frequency 10–50 Hz, pulse amplitude 1–10 mA, pulse width 300 μs) for 60 s | Successful reduction in heart rate and respiratory rate |
| Vuckovic et al. [25] | 2008 | Pig | Neuromodulation | Healthy pigs | N/A | Fiber selective control with anodal block, depolarizing pre-pulses, and slowly rising pulses techniques | Anodal block requiring high currents. Depolarizing pre-pulses has safe limits in its charge per phase but most sensitive to current amplitude. |
| Tosato et al. [58] | 2007 | Pig | Heart rate modulation | Healthy pigs | N/A | Fiber selective control with anodal block technique | Successful reduction in heart rate with reduced laryngeal side effects by 77% |
| Wallick et al. [59] | 2001 | Dog | Atrial fibrillation | Healthy dogs subjected to AF initiated by brief burst of right atrial stimulation then maintained by sinus node fat-pad stimulation for at least 15 min | Master-8, AMPI | Fiber selective control (frequency 20 Hz, current >3 mA, pulse duration 50 μs) | Hemodynamic improvement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elamin, A.B.A.; Forsat, K.; Senok, S.S.; Goswami, N. Vagus Nerve Stimulation and Its Cardioprotective Abilities: A Systematic Review. J. Clin. Med. 2023, 12, 1717. https://doi.org/10.3390/jcm12051717
Elamin ABA, Forsat K, Senok SS, Goswami N. Vagus Nerve Stimulation and Its Cardioprotective Abilities: A Systematic Review. Journal of Clinical Medicine. 2023; 12(5):1717. https://doi.org/10.3390/jcm12051717
Chicago/Turabian StyleElamin, Ahmed Banibella Abdelmagied, Kowthar Forsat, Solomon Silas Senok, and Nandu Goswami. 2023. "Vagus Nerve Stimulation and Its Cardioprotective Abilities: A Systematic Review" Journal of Clinical Medicine 12, no. 5: 1717. https://doi.org/10.3390/jcm12051717
APA StyleElamin, A. B. A., Forsat, K., Senok, S. S., & Goswami, N. (2023). Vagus Nerve Stimulation and Its Cardioprotective Abilities: A Systematic Review. Journal of Clinical Medicine, 12(5), 1717. https://doi.org/10.3390/jcm12051717






