Non-Diabetic Kidney Disease in Type 2 Diabetes Mellitus: A Changing Spectrum with Therapeutic Ascendancy
Abstract
:1. Introduction
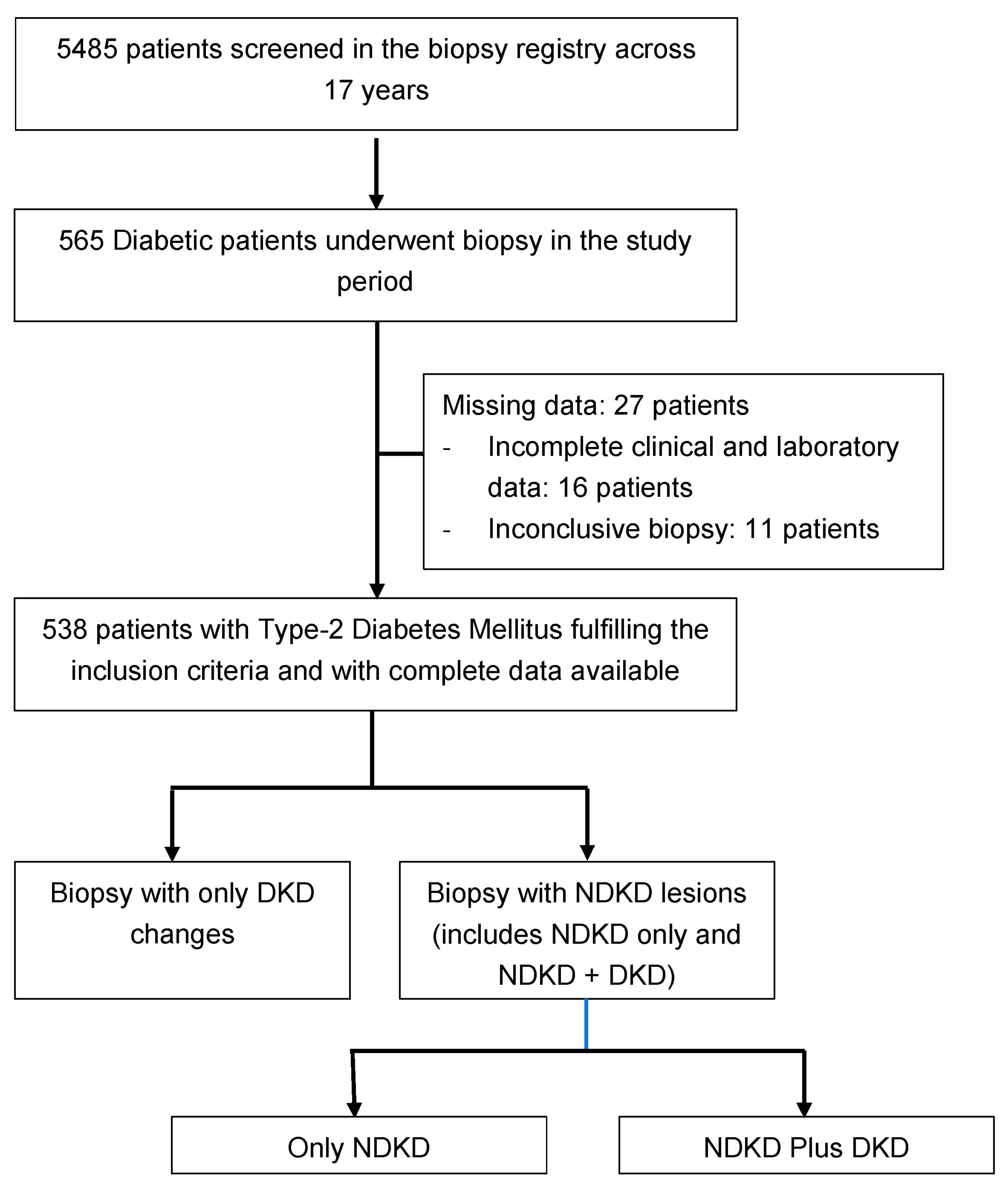
2. Materials and Methods
Statistical Analysis
3. Results
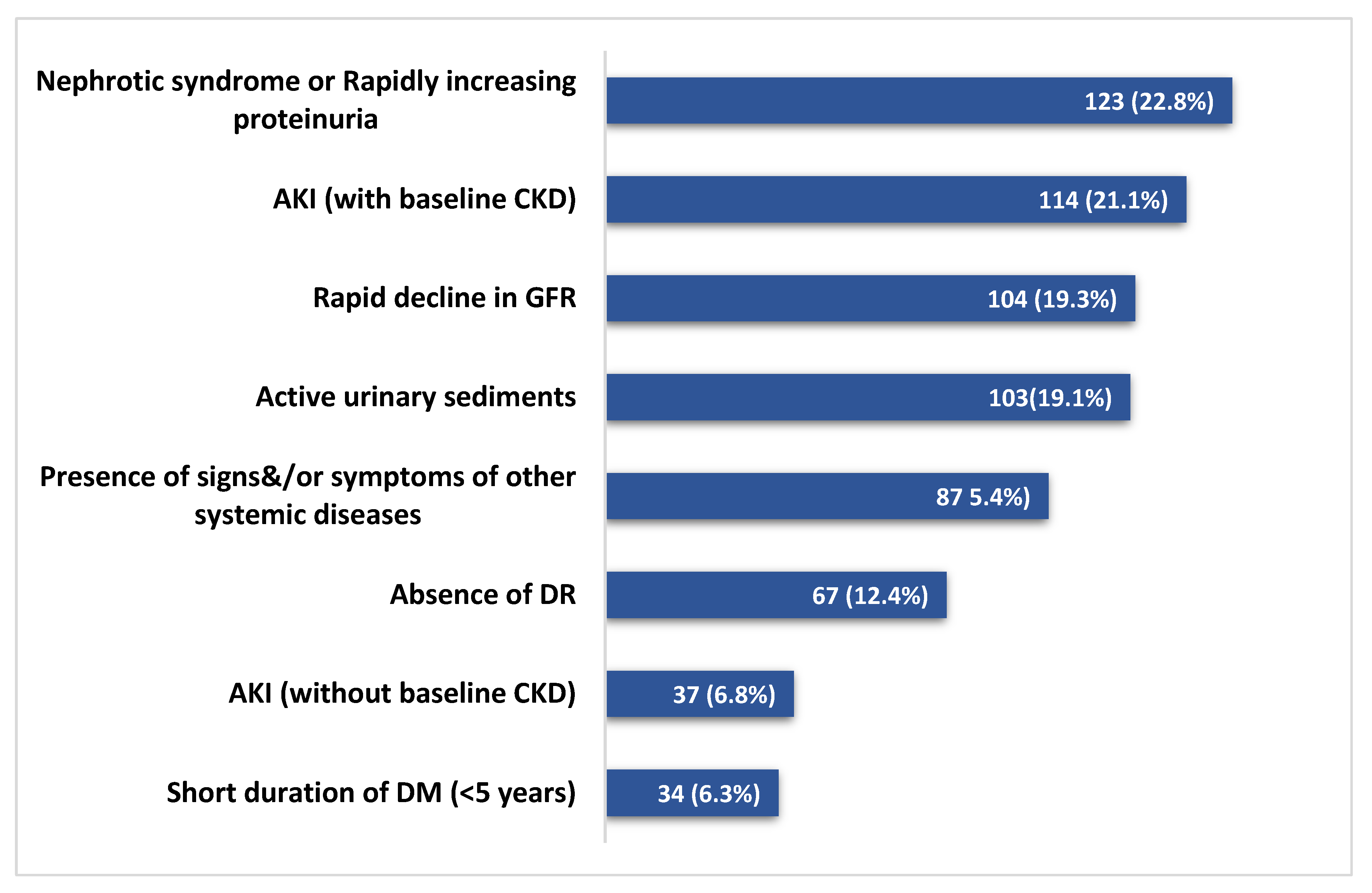
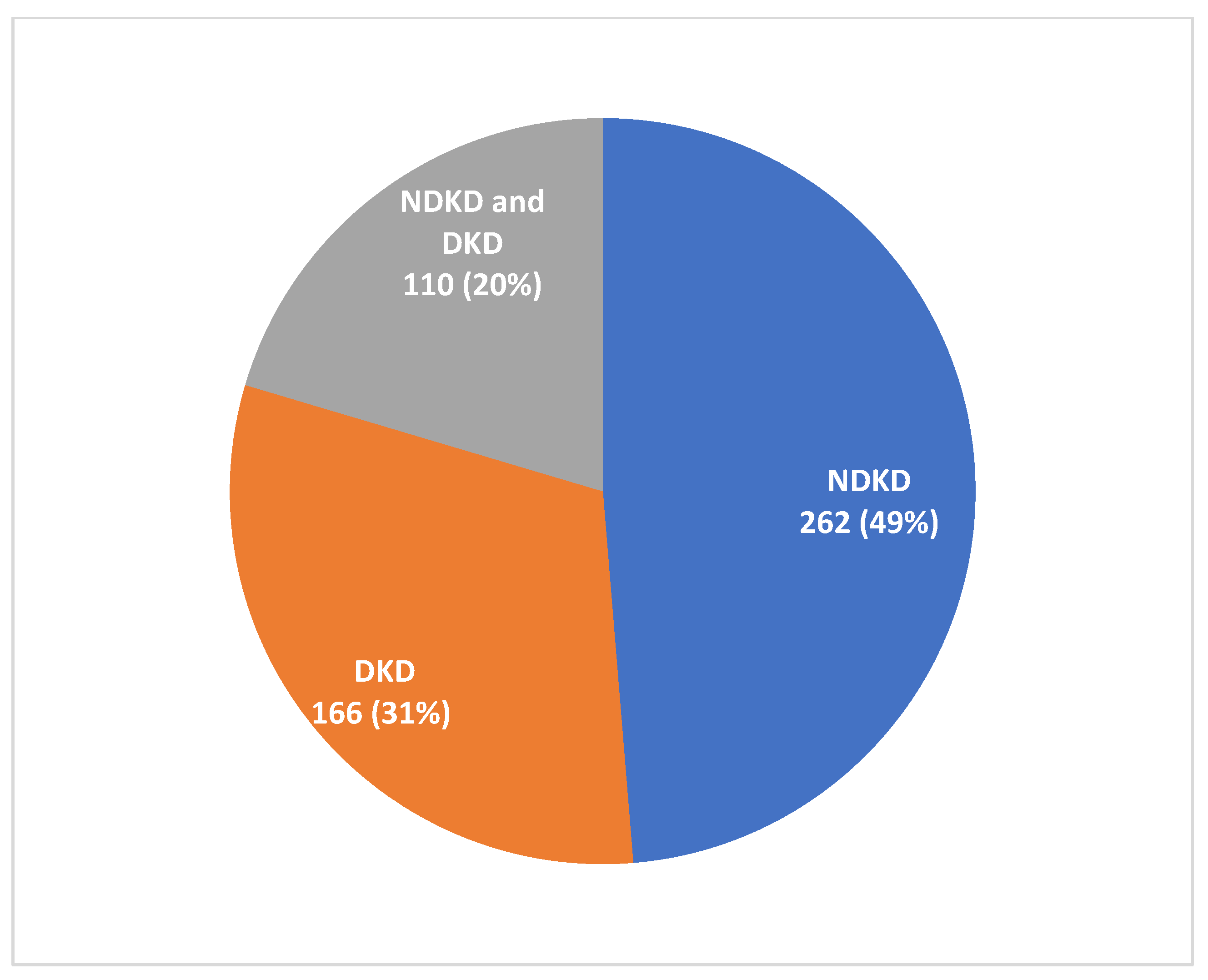
3.1. Indication and Histological Findings of Biopsies
3.2. Clinicopathological Comparison between DKD and NDKD
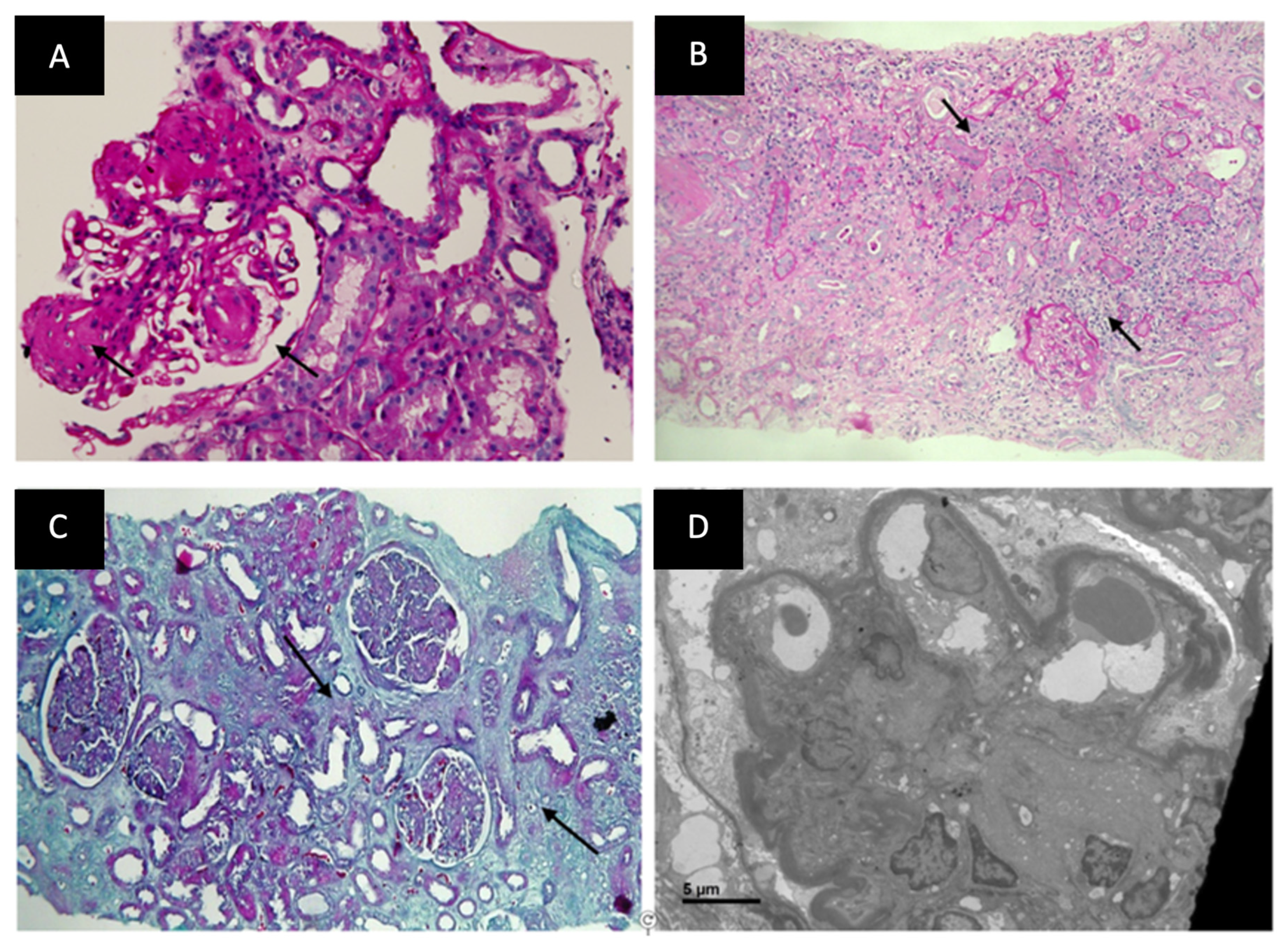
3.3. Histological Comparison between DKD and NDKD
3.4. Histological Comparison between DKD and NDKD plus DKD
3.5. Association of RAASB and SGLT2I Treatment with Histological Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Bhave, N.; Bragg-Gresham, J.; Balkrishnan, R.; Dietrich, X.; Eckard, A.; Eggers, P.W.; et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2018, 71 (Suppl. S1), A7, Correction on Am. J. Kidney Dis. 2018, 71, 501. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, S.F.; Roark, T.; Zelnick, L.R.; Najafian, B.; Andeen, N.K.; Alpers, C.E.; Pichler, R.; Ayers, E.; de Boer, I.H. Histopathologic and Clinical Features in Patients with Diabetes and Kidney Disease. Kidney360 2020, 1, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.G.; Bomback, A.S.; Radhakrishnan, J.; Herlitz, L.C.; Stokes, M.B.; Markowitz, G.S.; D’Agati, V.D. The modern spectrum of renal biopsy findings in patients with diabetes. Clin. J. Am. Soc. Nephrol. 2013, 8, 1718–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentino, M.; Bolignano, D.; Tesar, V.; Pisano, A.; Biesen, W.V.; Tripepi, G.; D’Arrigo, G.; Gesualdo, L. ERA-EDTA Immunonephrology Working Group. Renal biopsy in patients with diabetes: A pooled meta-analysis of 48 studies. Nephrol. Dial. Transplant. 2017, 32, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Prasad, N.; Patel, M.R. Infection-Induced Kidney Diseases. Front. Med. 2018, 5, 327. [Google Scholar] [CrossRef] [Green Version]
- Kaveeshwar, S.A.; Cornwall, J. The current state of diabetes mellitus in India. Australas Med. J. 2014, 7, 45–48. [Google Scholar] [CrossRef]
- Liew, A.; Bavanandan, S.; Prasad, N.; Wong, M.G.; Chang, J.M.; Eiam-Ong, S.; Hao, C.M.; Lim, C.Y.; Lim, S.K.; Oh, K.H.; et al. Asian Pacific Society of Nephrology Clinical Practice Guideline on Diabetic Kidney Disease—An Executive Summary. Nephrology 2020, 25, 809–817. [Google Scholar] [CrossRef]
- Yu, A.; Chertow, G.; Luyckx, V.; Marsden, P.; Skorecki, K.; Taal, M. Brenner & Rector’s The Kidney, 11th ed.; Elsevier Publications: Philadelphia, PA, USA, 2020. [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 2011, 34 (Suppl. S1), S11–S61. [Google Scholar] [CrossRef] [Green Version]
- Dabelea, D.; Pihoker, C.; Talton, J.W.; D’Agostino, R.B., Jr.; Fujimoto, W.; Klingensmith, G.J.; Lawrence, J.M.; Linder, B.; Marcovina, S.M.; Mayer-Davis, E.J.; et al. Etiological approach to characterization of diabetes type: The SEARCH for Diabetes in Youth Study. Diabetes Care 2011, 34, 1628–1633. [Google Scholar] [CrossRef] [Green Version]
- Steck, A.K.; Johnson, K.; Barriga, K.J.; Miao, D.; Yu, L.; Hutton, J.C.; Eisenbarth, G.S.; Rewers, M.J. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: Diabetes autoimmunity study in the young. Diabetes Care 2011, 34, 1397–1399. [Google Scholar] [CrossRef] [Green Version]
- Klingensmith, G.J.; Pyle, L.; Arslanian, S.; Copeland, K.C.; Cuttler, L.; Kaufman, F.; Laffel, L.; Marcovina, S.; Tollefsen, S.E.; Weinstock, R.S.; et al. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: Results from the TODAY study. Diabetes Care 2010, 33, 1970–1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int. Suppl. 2013, 3, 63–72. [CrossRef] [PubMed] [Green Version]
- Bennet, W.G.; Chapman, J.R. Oxford Textbook of Clinical Nephrology, 4th ed.; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Wilkinson, C.P.; Ferris, F.L., 3rd; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, S1–S87. [Google Scholar] [CrossRef]
- Smith, J.N.; Negrelli, J.M.; Manek, M.B.; Hawes, E.M.; Viera, A.J. Diagnosis and management of acute coronary syndrome: An evidence-based update. J. Am. Board Fam. Med. 2015, 28, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089, Correction on Stroke 2019, 50, e239. [Google Scholar] [CrossRef] [Green Version]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006, 113, e463–e654. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.G.; D’Agati, V.D.; Olson, J.L.; Jennette, J.C. Heptinstall’s Pathology of the Kidney; Wolters Kluwer Health: Philadelphia, PA, USA, 2014. [Google Scholar]
- Tervaert, T.W.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; Cook, H.T.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; de Heer, E.; et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 556–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haneda, M.; Utsunomiya, K.; Koya, D.; Babazono, T.; Moriya, T.; Makino, H.; Kimura, K.; Suzuki, Y.; Wada, T.; Ogawa, S.; et al. A new classification of Diabetic Nephropathy 2014: A report from Joint Committee on Diabetic Nephropathy. Clin. Exp. Nephrol. 2015, 6, 242–246. [Google Scholar] [CrossRef] [Green Version]
- Nasr, S.H.; Markowitz, G.S.; Stokes, M.B.; Said, S.M.; Valeri, A.M.; D’Agati, V.D. Acute postinfectious glomerulonephritis in the modern era: Experience with 86 adults and review of the literature. Medicine 2008, 87, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.H.; Fidler, M.E.; Valeri, A.M.; Cornell, L.D.; Sethi, S.; Zoller, A.; Stokes, M.B.; Markowitz, G.S.; D’Agati, V.D. Postinfectious glomerulonephritis in the elderly. J. Am. Soc. Nephrol. 2011, 22, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasr, S.H.; Markowitz, G.S.; Whelan, J.D.; Albanese, J.J.; Rosen, R.M.; Fein, D.A.; Kim, S.S.; D’Agati, V.D. IgA-dominant acute poststaphylococcal glomerulonephritis complicating diabetic nephropathy. Hum. Pathol. 2003, 34, 1235–1241. [Google Scholar] [CrossRef]
- Nasr, S.H.; D’Agati, V.D. IgA-dominant postinfectious glomerulonephritis: A new twist on an old disease. Nephron. Clin. Pract. 2011, 119, c18–c26. [Google Scholar] [CrossRef]
- Sethi, S.; Nester, C.M.; Smith, R.J. Membranoproliferative glomerulonephritis and C3 glomerulopathy: Resolving the confusion. Kidney Int. 2012, 81, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Prakash, J.; Gupta, T.; Prakash, S.; Bhushan, P.; Usha Sivasankar, M.; Singh, S.P. Non-diabetic renal disease in type 2 diabetes mellitus: Study of renal—Retinal relationship. Indian J. Nephrol. 2015, 25, 222–228. [Google Scholar] [CrossRef]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Pedro, R.A.; Ramon, S.A.; Marc, B.B.; Juan, F.B.; Isabel, M.M. Prevalence and relationship between diabetic retinopathy and nephropathy, and its risk factors in the North-East of Spain, a population-based study. Ophthalmic Epidemiol. 2010, 17, 251–265. [Google Scholar] [CrossRef]
- Arora, P.; Roychaudhury, A.; Pandey, R. Non-diabetic Renal Diseases in Patients with Diabetes Mellitus Clinicopathological Correlation. Indian J. Nephrol. 2020, 30, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Erdogmus, S.; Kiremitci, S.; Celebi, Z.K.; Akturk, S.; Duman, N.; Ates, K.; Erturk, S.; Nergizoglu, G.; Kutlay, S.; Sengul, S.; et al. Non-Diabetic Kidney Disease in Type 2 Diabetic Patients: Prevalence, Clinical Predictors and Outcomes. Kidney Blood Press Res. 2017, 42, 886–893. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Xia, X.; Wu, X.F.; Yu, X.Q.; Huang, F.X. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: A meta-analysis. Diabetologia 2013, 56, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, X.G.; Cai, G.Y.; Zhu, H.Y.; Zhou, J.H.; Wu, J.; Chen, P.; Lin, S.P.; Qiu, Q.; Chen, X.M. Identifying parameters to distinguish non-diabetic renal diseases from diabetic nephropathy in patients with type 2 diabetes mellitus: A meta-analysis. PLoS ONE 2013, 8, e64184. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, X.G.; Cai, G.Y.; Zhu, H.Y.; Zhou, J.H.; Wu, J.; Chen, P.; Lin, S.P.; Qiu, Q.; Chen, X.M. Correlations among Diabetic Microvascular Complications: A Systematic Review and Meta-analysis. Sci. Rep. 2019, 9, 3137. [Google Scholar]
- McClellan, W.M.; Langston, R.D.; Presley, R. Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J. Am. Soc. Nephrol. 2004, 15, 1912–1919. [Google Scholar] [CrossRef]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; de Boer, I.H. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Okada, T.; Nagao, T.; Matsumoto, H.; Nagaoka, Y.; Wada, T.; Nakao, T. Clinical significance of microscopic haematuria in diabetic nephropathy in type 2 diabetes patients with overt proteinuria. Nephrology 2013, 18, 563–568. [Google Scholar] [CrossRef]
- Bash, L.D.; Selvin, E.; Steffes, M.; Coresh, J.; Astor, B.C. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Arch. Intern. Med. 2008, 168, 2440–2447. [Google Scholar] [CrossRef]
- Mottl, A.K.; Kwon, K.S.; Mauer, M.; Mayer-Davis, E.J.; Hogan, S.L.; Kshirsagar, A.V. Normoalbuminuric diabetic kidney disease in the U.S. population. J. Diabetes Complications 2013, 27, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.; Becker, B.; Strzelczyk, P.; Ritz, E. Renal disease and hypertension in non-insulin-dependent diabetes mellitus. Kidney Int. 1999, 55, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, C.K.; Bergis, K.H.; Fliser, D.; Ritz, E. Renal findings in patients with short-term type 2 diabetes. J. Am. Soc. Nephrol. 1996, 7, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Sasaki, K.; Lin, M.Y.; Smith, K.D.; Nicosia, R.F.; Alpers, C.E.; Najafian, B. Interstitial eosinophilic aggregates in diabetic nephropathy: Allergy or not? Nephrol. Dial. Transplant. 2015, 30, 1370–1376. [Google Scholar] [CrossRef] [Green Version]
- Chong, Y.B.; Keng, T.C.; Tan, L.P.; Ng, K.P.; Kong, W.Y.; Wong, C.M.; Cheah, P.L.; Looi, L.M.; Tan, S.Y. Clinical predictors of non-diabetic renal disease and role of renal biopsy in diabetic patients with renal involvement: A single centre review. Ren. Fail. 2012, 34, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.K.; Gwi, E.; Chan, K.W.; Wong, P.N.; Lo, K.Y.; Lee, K.F.; Wong, A.K. Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Nephrol. Dial. Transplant. 1997, 12, 2588–2591. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.M.; Lewis, E.J.; Leonard-Martin, T.; Lewis, J.B.; Batlle, D. Renal pathology patterns in type II diabetes mellitus: Relationship with retinopathy. The Collaborative Study Group. Nephrol. Dial. Transplant. 1998, 13, 2547–2552. [Google Scholar] [CrossRef] [Green Version]
| Variable | Frequency |
|---|---|
| SD) | 11.5 |
Gender
| 436 (81%) 102 (19%) |
| SD) | 6.1 |
Glycemic control (HbA1C%)
| 230 (42.7%) 308 (57.3%) |
Other comorbidities
| 419 (78%) 72 (13.3%) 23 (4.2%) |
| Uncontrolled hypertension (n, %) | 158 (29.4%) |
| SD) | 4.9 |
Hypertension onset in relation to Diabetes
| 244 (45.4%) 197 (36.6%) |
Macrovascular complications
| 40 (7.4%) 17 (3.2%) 16 (3%) |
Microvascular complications
| 160 (29.7%) 26 (4.8%) |
Presenting complaints
| 496 (92.2%) 207 (38.5%) 16 (3%) 86 (16%) |
Syndromic presentation
| 214 (39.8%) 183 (34%) 42 (7.8%) |
| eGFR at presentation (median, IQR); mL/min/1.73 m2 | 17.2 (9.5–41.7) |
| Dialysis requiring renal failure at presentation (%) | 157 (29.2%) |
| SD) | 3.9 |
| Microscopic hematuria (>3 RBC/HPF) | 249 (46.3%) |
Laboratory parameters
| 2.3 3809 1.1 0.7 |
| Renal Biopsy Diagnosis | Total (N = 372) | NDKD Only (n = 262) | NDKD and DKD (n = 110) |
|---|---|---|---|
ATIN
| 126 (33.8%) 79 (21.2%) 47 (12.6%) | 70 (26.7%) 42 (16%) 28 (10.7) | 56 (50.9%) 37 (33.6%) 19 (17.2%) |
| IRGN | 52 (13.9%) | 32 (12.2%) | 20 (18.2%) |
| Membranous nephropathy | 36 (9.6%) | 34 (12.9%) | 2 (1.8%) |
| IgA nephropathy | 34 (9.1%) | 29 (11.1%) | 5 (4.5%) |
FSGS
| 29 (7.8%) 15 (4%) 14 (3.7%) | 27 (10.3%) 15 (5.7%) 12 (4.5%) | 2 (1.8%) 0 2 (1.8%) |
| MPGN | 17 (4.5%) | 13 (4.9%) | 4 (3.6%) |
| Cast Nephropathy | 15 (4%) | 13 (4.9%) | 2 (1.8%) |
| Crescentic GN—Immune complex associated | 15 (4%) | 5 (1.9%) | 10 (9.1%) |
| Pauci-immune Crescentic GN | 14 (3.7%) | 14 (5.3%) | 0 |
| MCD | 8 (2.1%) | 8 (3.1%) | 0 |
| Granulomatous interstitial nephritis | 6 (1.6%) | 4 (1.4%) | 2 (1.8%) |
| Chronic interstitial nephritis | 6 (1.6%) | 2 (0.7%) | 4 (3.6%) |
| Lupus nephritis | 3 (0.8%) | 3 (1.1%) | 0 |
| Amyloidosis | 4 (1%) | 4 (1.4%) | 0 |
| Thrombotic microangiopathy | 3 (0.8%) | 2 (0.7%) | 1 (0.9%) |
| Monoclonal immune deposition disease | 2 (0.5%) | 1 (0.4%) | 1 (0.9%) |
| Anti-GBM disease | 2 (0.5%) | 1 (0.4%) | 1 (0.9%) |
| Variable | DKD Only (n = 166) | NDKD (n = 372) | p-Value | ||
|---|---|---|---|---|---|
| †NDKD Only (n = 262) | ††NDKD + DKD (n = 110) | Total (n = 372) († + ††) | |||
| SD) | 55.4 11.2 | 57.2 12.0 | 10.3 | 56.9 11.5 | 0.06 |
Gender
| 131 (79%) 35 (21%) | 212 (81%) 50 (19%) | 88 (80%) 22 (20%) | 305 (82%) 67 (18%) | 0.79 |
| Duration of Diabetes mellitus * | 6.6 | 5.4 | 6.5 | 5.5 5.4 | <0.001 |
| Diabetes onset within 5 years | 73 (44%) | 193 (73.6%) | 53 (48.2%) | 247 (66.3%) | <0.001 |
| Glycemic control SD) Poor glycemic control (>8%) | 2.3 98 (59%) | 2.6 134 (51.1%) | 2.7 76 (69.1%) | 6.7 2.6 210 (56.4%) | 0.96 0.22 |
| Hypertension (%) Uncontrolled hypertension (%) | 143 (86.1%) 49 (29.5%) | 178 (74.1%) 57 (29.3%) | 98 (89%) 47 (42.7%) | 276 (74.1%) 109 (29.3%) | <0.001 0.81 |
| SD) | 3.5 | 2.8 | 2.6 | 3.6 4.8 | 0.35 |
HTN onset in relation to diabetes
| 92 (55.4%) 51 (30.7%) | 87 (33.2%) 93 (35.4%) | 50 (45.4%) 46 (41.8%) | 137 (36.8%) 139 (37.3%) | <0.001 0.13 |
Diabetes related macrovascular complications
| 19 (11.4%) 4 (2.4%) 5 (3%) | 15 (5.7%) 5 (1.9%) 3 (1.1%) | 6 (5.4%) 8 (7.2%) 8 (7.2%) | 21 (5.6%) 13 (3.5%) 11 (2.9%) | 0.01 0.53 0.93 |
Diabetes related microvascular complications
| 76 (45.8%) 12 (7.2%) | 56 (21.3%) 9 (3.4%) | 28 (25.4%) 5 (4.5%) | 84 (22.5%) 14 (3.7%) | <0.001 0.07 |
Presenting complaints
| 155 (93.3%) 34 (20.4%) 4 (2.4%) 13 (7.8%) 16 (9.6%) | 236 (90%) 111 (42.3%) 4 (1.5%) 54 (20.6%) 23 (8.7%) | 105 (96.3%) 56 (50.9%) 8 (7.2%) 19 (17.2%) 18 (16.3%) | 341 (91.6%) 173 (46.5%) 12 (3.2%) 73 (19.6%) 71 (19%) | 0.09 <0.001 0.64 0.001 0.008 |
Syndromic presentation
| 69 (41.5%) 4 (8.4%) 4 (2.4%) | 84 (32%) 83 (31.6%) 23 (8.7%) | 25 (22.7%) 62 (56.3%) 11 (10%) | 167 (44.8%) 188 (50.5%) 34 (9.1%) | 0.83 <0.001 0.005 |
| eGFR at presentation (median, IQR); mL/min/1.73 m2 | 23.1 (11.5–45.5) | 19.4 (10–51.5) | 18.5 (9.1–22.3) | 15 23.1 | 0.08 |
| Dialysis requiring renal failure at presentation | 30 (18%) | 75 (28.6%) | 48 (43.6%) | 127 (34.1%) | <0.001 |
| SD) | 4.4 | 3.5 | 3.4 | 4.1 3.5 | <0.001 |
Degree of proteinuria
| 8 (4.8%) 60 (36.1%) 98 (59%) | 27 (10.3%) 131 (51.5%) 123 (46.9%) | 13 (11.8%) 61 (55.4%) 43 (39%) | 170 (45.6%) | 0.02 0.001 0.001 |
| Microscopic hematuria (>3 RBC/HPF) | 62 (37.3%) | 136 (51.9%) | 48 (43.6%) | 187 (50.2%) | 0.01 |
Laboratory parameters
Lipid profile
| 9.6 2.2 9017 3240 1.32 1.06 3.22 0.7 176 89.6 185 73.2 8 (4.8%) 2 (2.4%) | 9.8 2.4 9852 4014 1.25 1.13 3.19 0.7 195 118 189 90.3 41 (15.6%) 16 (6.1%) | 9.5 1.8 9761 4021 1.32 0.96 3.2 0.7 188.4 98.5 176.4 104.6 31 (28.2%) 1 (0.9%) | 9.8 2.4 9852 4014 1.25 1.13 3.19 0.7 195 118 189 90.3 74 (19.8%) 17 (4.5%) | 0.24 0.07 0.52 0.64 0.07 0.62 <0.001 0.04 |
| Variable | p-Value | Odds Ratio (95% Confidence Interval) |
|---|---|---|
| 5 years | 0.003 | 1.9 (1.26–3.14) |
| Hypertension | 0.22 | 0.73 (0.3–2.11) |
| Hypertension onset after diabetes | 0.94 | 0.11 (0.03–1.86) |
| Absence of CAD | 0.05 | 2.1 (1.11–4.96) |
| Absence of DR | <0.001 | 4.9 (2.9–8.4) |
| Oliguria | 0.02 | 1.8 (1.1–3.1) |
| Evidence of infection (ongoing/recent past) | 0.63 | 1.2 (0.52–1.44) |
| Presence of extra-renal manifestations | 0.64 | 1.8 (0.82–2.26) |
| Acute kidney Injury/Acute on Chronic kidney disease | <0.001 | 6.2 (3.4–11.0) |
| Rapidly progressive renal failure | 0.58 | 1.23 (0.6–2.68) |
| Dialysis requiring renal failure at presentation | 0.06 | 1.79 (0.96–3.3) |
| Microscopic hematuria | 0.07 | 1.51 (0.95–2.41) |
| Nephrotic range proteinuria | 0.66 | 0.48 (0.2–1.8) |
| Sub-nephrotic proteinuria | 0.52 | 1.18 (0.4–3.1) |
| Low serum C3 level | <0.001 | 4.9 (2.08–11.6) |
| Low serum C4 level | 0.77 | 1.6 (0.8–4.6) |
| Diabetic Kidney Disease (n = 166) | RAASB | RAASB + SGLT2I | ||||
|---|---|---|---|---|---|---|
| Yes (n = 44) | No (n = 122) | p-Value | Yes (n = 38) | No (n = 128) | p-Value | |
Degree of IFTA
| 1 (2.3%) 32 (72.7%) 11 (25%) | 1 (0.8%) 40 (32.8%) 81 (66.4%) | 0.43 <0.001 <0.001 | 1 (2.6%) 28 (73.6%) 9 (23.6%) | 1 (0.7%) 69 (53.9%) 58 (45.3%) | 0.33 0.03 0.01 |
Vascular changes
| 5 (11.3%) 34 (77.2%) 5 (11.3%) | 7 (5.7%) 103 (84.4%) 12 (9.7%) | 0.21 0.28 0.76 | 5 (13.1%) 18 (47.3%) 15 (39.5%) | 7 (5.4%) 86 (67.1%) 35 (27.3%) | 0.10 0.02 0.15 |
| Non-diabetic kidney disease (n = 372) | RAASB | RAASB + SGLT2I | ||||
| Yes (n = 83) | No (n = 289) | p-value | Yes (n = 46) | No (n = 326) | p-value | |
Degree of IFTA
| 28 (33.7%) 37 (44.5%) 18 (21.7%) | 31 (10.7%) 131 (45.3%) 127 (40%) | <0.001 0.89 0.002 | 21 (45.6%) 23 (50%) 2 (4.3%) | 38 (11.6%) 145 (44.4%) 143 (43.8%) | <0.001 0.47 <0.001 |
Vascular changes
| 38 (45.7%) 26 (31.3%) 19 (22.8%) | 104 (35.9%) 63 (21.7%) 122 (43.9%) | 0.10 0.07 <0.001 | 29 (63%) 4 (8.6%) 13 (28.2%) | 116 (35.5%) 85 (26%) 125 (38.3%) | <0.001 0.009 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, N.; Veeranki, V.; Bhadauria, D.; Kushwaha, R.; Meyyappan, J.; Kaul, A.; Patel, M.; Behera, M.; Yachha, M.; Agrawal, V.; et al. Non-Diabetic Kidney Disease in Type 2 Diabetes Mellitus: A Changing Spectrum with Therapeutic Ascendancy. J. Clin. Med. 2023, 12, 1705. https://doi.org/10.3390/jcm12041705
Prasad N, Veeranki V, Bhadauria D, Kushwaha R, Meyyappan J, Kaul A, Patel M, Behera M, Yachha M, Agrawal V, et al. Non-Diabetic Kidney Disease in Type 2 Diabetes Mellitus: A Changing Spectrum with Therapeutic Ascendancy. Journal of Clinical Medicine. 2023; 12(4):1705. https://doi.org/10.3390/jcm12041705
Chicago/Turabian StylePrasad, Narayan, Vamsidhar Veeranki, Dharmendra Bhadauria, Ravi Kushwaha, Jeyakumar Meyyappan, Anupama Kaul, Manas Patel, Manas Behera, Monika Yachha, Vinita Agrawal, and et al. 2023. "Non-Diabetic Kidney Disease in Type 2 Diabetes Mellitus: A Changing Spectrum with Therapeutic Ascendancy" Journal of Clinical Medicine 12, no. 4: 1705. https://doi.org/10.3390/jcm12041705
APA StylePrasad, N., Veeranki, V., Bhadauria, D., Kushwaha, R., Meyyappan, J., Kaul, A., Patel, M., Behera, M., Yachha, M., Agrawal, V., & Jain, M. (2023). Non-Diabetic Kidney Disease in Type 2 Diabetes Mellitus: A Changing Spectrum with Therapeutic Ascendancy. Journal of Clinical Medicine, 12(4), 1705. https://doi.org/10.3390/jcm12041705






