COVID-19-Related Neuropathic Pain: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methodology
2.1. Protocol and Registration
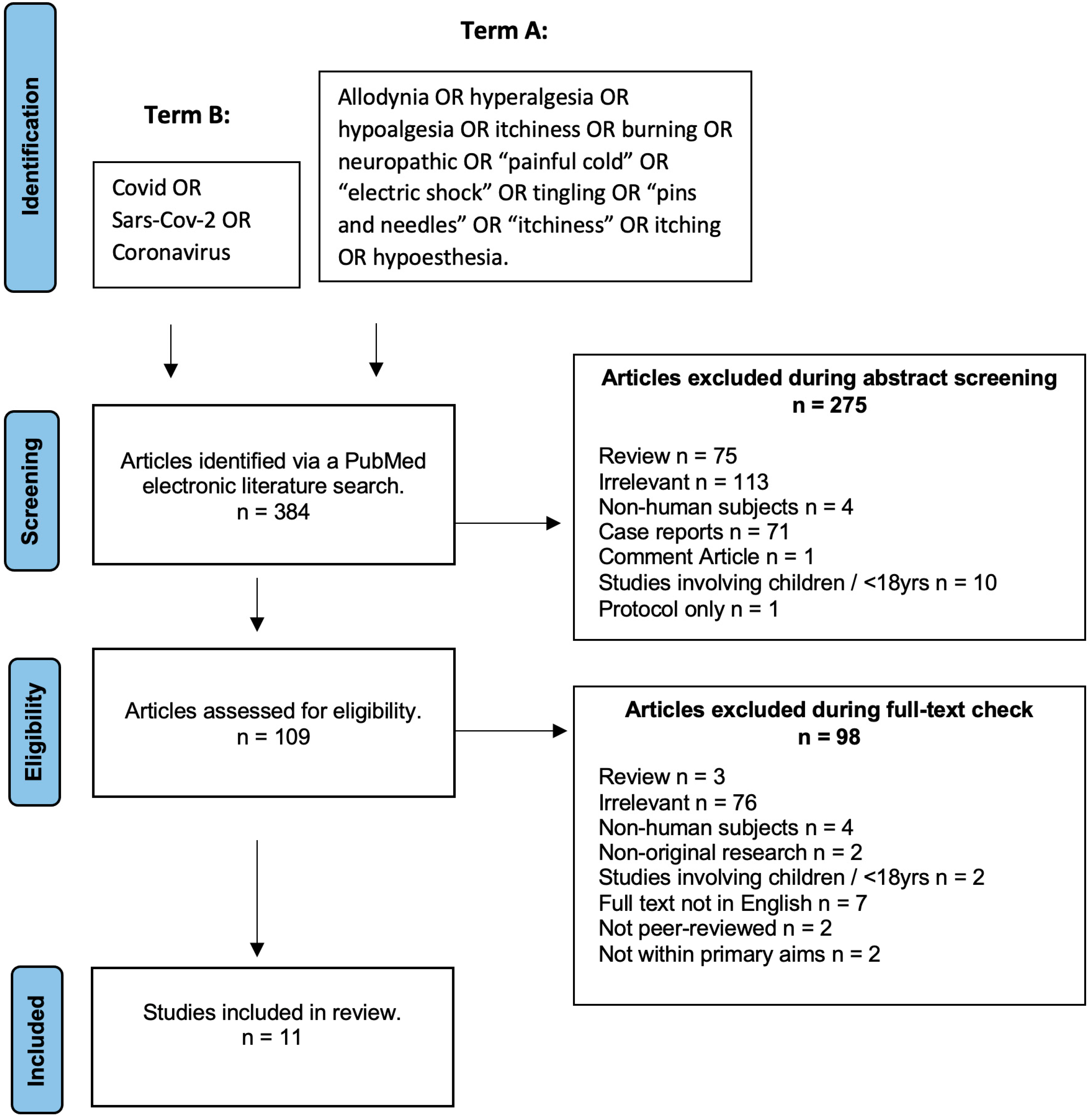
2.2. Literature Search Strategy
2.3. Inclusion/Exclusion Criteria
2.4. Data Extraction
2.5. Synthesis of Results
2.6. Statistical Analysis
2.7. Assessment of Bias
3. Results
3.1. COVID-19-Related Neuropathic Pain
3.2. Epidemiology
3.3. Risk Factors for Neuropathic Pain
3.3.1. Demographics
3.3.2. COVID-19 Severity
3.3.3. Treatment with Azithromycin
3.3.4. Depression, Anxiety and Psychological Distress
3.4. Locations
3.5. Natural History
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baud, D.; Qi, X.; Nielsen-Saines, K.; Musso, D.; Pomar, L.; Favre, G. Real estimates of mortality following COVID-19 infection. Lancet Infect. Dis. 2020, 20, 773. [Google Scholar] [CrossRef]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef]
- Joshi, D.; Gyanpuri, V.; Pathak, A.; Chaurasia, R.N.; Mishra, V.N.; Kumar, A.; Singh, V.K.; Dhiman, N.R. Neuropathic Pain Associated with COVID-19: A Systematic Review of Case Reports. Curr. Pain Headache Rep. 2022, 26, 595–603. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Bannister, K.; Sachau, J.; Baron, R.; Dickenson, A.H. Neuropathic pain: Mechanism-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 257–274. [Google Scholar] [CrossRef]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 1–10. [Google Scholar] [CrossRef]
- Jaggi, A.S.; Singh, N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology 2012, 291, 1–9. [Google Scholar] [CrossRef]
- Mearns, E.S.; Taylor, A.; Thomas Craig, K.J.; Puglielli, S.; Cichewicz, A.B.; Leffler, D.A.; Sanders, D.S.; Lebwohl, B.; Hadjivassiliou, M. Neurological manifestations of neuropathy and ataxia in celiac disease: A systematic review. Nutrients 2019, 11, 380. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Y.; Xie, K.; Chen, Y.; Wang, H.; Bian, Y.; Wang, Y.; Dong, A.; Yu, Y. Effect of autophagy on allodynia, hyperalgesia and astrocyte activation in a rat model of neuropathic pain. Int. J. Mol. Med. 2018, 42, 2009–2019. [Google Scholar] [CrossRef]
- Wu, S.; Bono, J.; Tao, Y.X. Long noncoding RNA (lncRNA): A target in neuropathic pain. Expert Opin. Ther. Targets 2019, 23, 15–20. [Google Scholar] [CrossRef]
- Bakkers, M.; Faber, C.G.; Hoeijmakers, J.G.; Lauria, G.; Merkies, I.S. Small fibers, large impact: Quality of life in small-fiber neuropathy. Muscle Nerve 2014, 49, 329–336. [Google Scholar] [CrossRef]
- Smart, K.M.; Blake, C.; Staines, A.; Doody, C. Self-reported pain severity, quality of life, disability, anxiety and depression in patients classified with ‘nociceptive’, ‘peripheral neuropathic’ and ‘central sensitisation’ pain. The discriminant validity of mechanisms-based classifications of low back (±leg) pain. Man. Ther. 2012, 17, 119–125. [Google Scholar]
- Clark, A.K.; Old, E.A.; Malcangio, M. Neuropathic pain and cytokines: Current perspectives. J. Pain Res. 2013, 6, 803. [Google Scholar]
- Attal, N.; Martinez, V.; Bouhassira, D. Potential for increased prevalence of neuropathic pain after the COVID-19 pandemic. Pain Rep. 2021, 6, e884. [Google Scholar] [CrossRef]
- McFarland, A.J.; Yousuf, M.S.; Shiers, S.; Price, T.J. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: Implications for COVID-19 and pain. Pain Rep. 2021, 6, e885. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef]
- Davies, K.S. Formulating the evidence-based practice question: A review of the frameworks. Evid. Based Libr. Inf. Pract. 2011, 6, 75–80. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised IASP definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef]
- Liampas, A.; Velidakis, N.; Georgiou, T.; Vadalouca, A.; Varrassi, G.; Hadjigeorgiou, G.M.; Tsivgoulis, G.; Zis, P. Prevalence and management challenges in central post-stroke neuropathic pain: A systematic review and meta-analysis. Adv. Ther. 2020, 37, 3278–3291. [Google Scholar] [CrossRef]
- Ali, S.T.; Kang, A.K.; Patel, T.R.; Clark, J.R.; Perez-Giraldo, G.S.; Orban, Z.S.; Lim, P.H.; Jimenez, M.; Graham, E.L.; Batra, A.; et al. Evolution of neurologic symptoms in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2022, 9, 950–961. [Google Scholar] [CrossRef]
- Jena, D.; Sahoo, J.; Barman, A.; Gupta, A.; Patel, V. Musculoskeletal and Neurological Pain Symptoms Among Hospitalized COVID-19 Patients. Am. J. Phys. Med. Rehabil. 2022, 101, 411–416. [Google Scholar] [CrossRef]
- Miller, C.; O’Sullivan, J.; Jeffrey, J.; Power, D. Brachial Plexus Neuropathies during the COVID-19 Pandemic: A Retrospective Case Series of 15 Patients in Critical Care. Phys. Ther. 2021, 101, pzaa191. [Google Scholar] [CrossRef]
- Novak, P.; Mukerji, S.S.; Alabsi, H.S.; Systrom, D.; Marciano, S.P.; Felsenstein, D.; Mullally, W.J.; Pilgrim, D.M. Multisystem Involvement in Post-Acute Sequelae of Coronavirus Disease 19. Ann. Neurol. 2022, 91, 367–379. [Google Scholar] [CrossRef]
- Ocak, O.; Sahin, E.M. Evaluation of Neuropathic Pain Features in COVID-19 Patients. Neurol. India 2022, 70, 591. [Google Scholar]
- Ojeda, A.; Calvo, A.; Cuñat, T.; Mellado-Artigas, R.; Comino-Trinidad, O.; Aliaga, J.; Arias, M.; Ferrando, C.; Martinez-Pallí, G.; Dürsteler, C. Characteristics and influence on quality of life of new-onset pain in critical COVID-19 survivors. Eur. J. Pain 2022, 26, 680–694. [Google Scholar] [CrossRef]
- Scherlinger, M.; Felten, R.; Gallais, F.; Nazon, C.; Chatelus, E.; Pijnenburg, L.; Mengin, A.; Gras, A.; Vidailhet, P.; Arnould-Michel, R.; et al. Refining “Long-COVID” by a prospective multimodal evaluation of patients with long-term symptoms attributed to SARS-CoV-2 infection. Infect. Dis. Ther. 2021, 10, 1747–1763. [Google Scholar] [CrossRef]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021, 31, 385–394. [Google Scholar] [CrossRef]
- Magdy, R.; Eid, R.A.; Fathy, W.; Abdel-Aziz, M.M.; Ibrahim, R.E.; Yehia, A.; Sheemy, M.S.; Hussein, M. Characteristics and risk factors of persistent neuropathic pain in recovered COVID-19 patients. Pain Med. 2022, 23, 774–781. [Google Scholar] [CrossRef]
- Oguz-Akarsu, E.; Gullu, G.; Kilic, E.; Dinc, Y.; Ursavas, A.; Yilmaz, E.; Zarifoglu, M.; Karli, N.; Pandemic Study Team; Akalın, H.; et al. Insight into pain syndromes in acute phase of mild-to-moderate COVID-19: Frequency, clinical characteristics, and associated factors. Eur. J. Pain 2022, 26, 492–504. [Google Scholar] [CrossRef]
- Starace, M.; Iorizzo, M.; Sechi, A.; Alessandrini, A.M.; Carpanese, M.; Bruni, F.; Vara, G.; Apalla, Z.; Asz-Sigall, D.; Barruscotti, S.; et al. Trichodynia and telogen effluvium in COVID-19 patients: Results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021, 5, 11–18. [Google Scholar] [CrossRef]
- O’Dowd, A. COVID-19: Third of people infected have long term symptoms. BMJ Br. Med. J. 2021, 373, n1626. [Google Scholar] [CrossRef]



| Population | Adults Only (≥18 Years of Age) |
|---|---|
| Intervention | Neuropathic pain following COVID-19 infection. |
| Comparison |
|
| Outcome | Prevalence (COVID-19-related neuropathic pain). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, L.D.; Zis, P. COVID-19-Related Neuropathic Pain: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1672. https://doi.org/10.3390/jcm12041672
Williams LD, Zis P. COVID-19-Related Neuropathic Pain: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(4):1672. https://doi.org/10.3390/jcm12041672
Chicago/Turabian StyleWilliams, Laura Dawn, and Panagiotis Zis. 2023. "COVID-19-Related Neuropathic Pain: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 4: 1672. https://doi.org/10.3390/jcm12041672
APA StyleWilliams, L. D., & Zis, P. (2023). COVID-19-Related Neuropathic Pain: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(4), 1672. https://doi.org/10.3390/jcm12041672







