Fetal Growth Restriction and Long-Term Cardiovascular Morbidity of Offspring in Dichorionic–Diamniotic Twin Pregnancies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
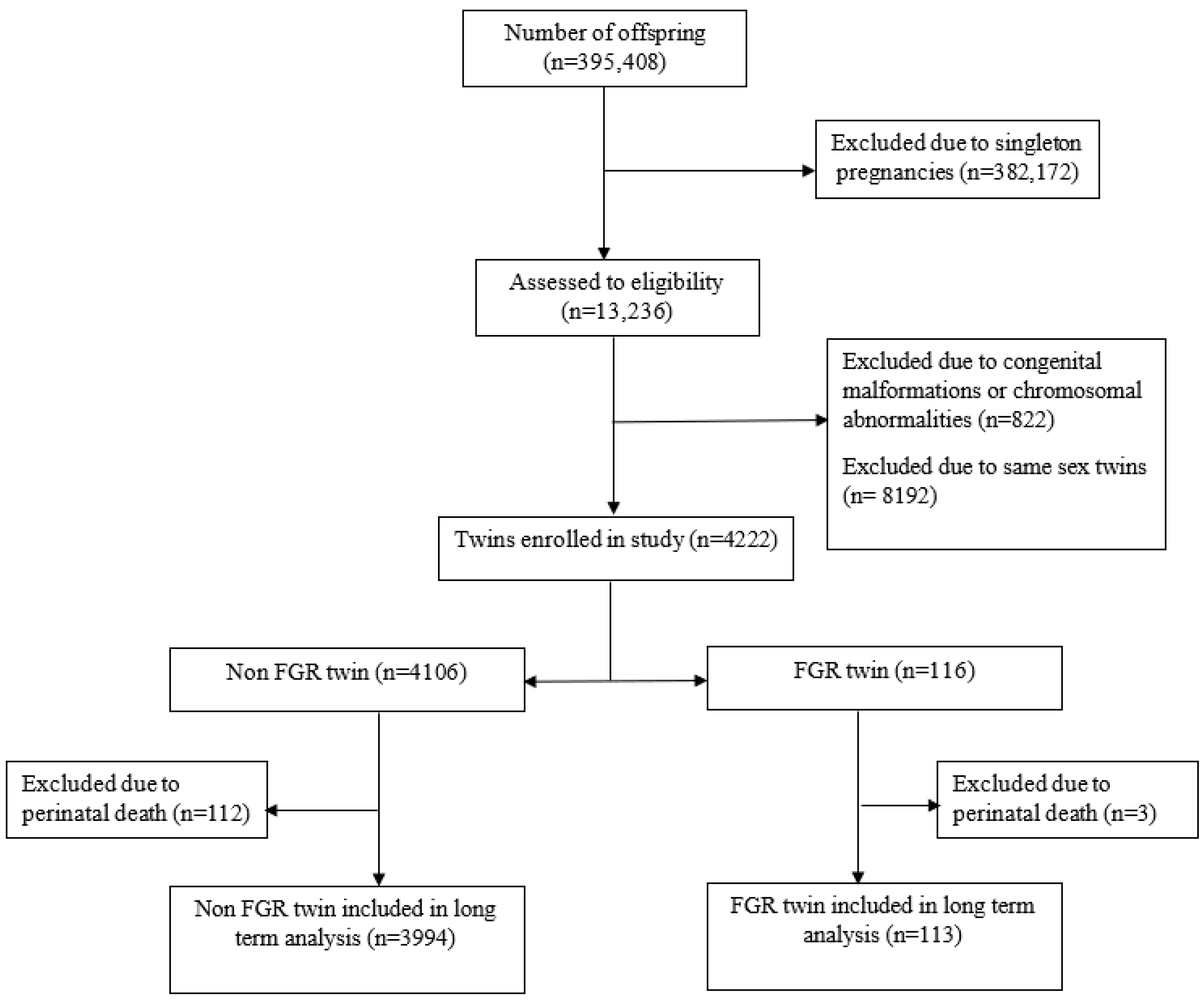
2.3. Study Population
2.4. Data Collection Method
2.5. Study Size
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Curtin, S.C.; Mathews, T.J. Births: Final data for 2013. Natl. Vital Stat. Rep. 2021, 70, 1–51. [Google Scholar] [PubMed]
- Adashi, E.Y.; Gutman, R. Delayed Childbearing as a Growing, Previously Unrecognized Contributor to the National Plural Birth Excess. Obstet. Gynecol. 2018, 132, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Okby, R.; Harlev, A.; Sacks, K.N.; Sergienko, R.; Sheiner, E. Preeclampsia acts differently in in vitro fertilization versus spontaneous twins. Arch. Gynecol. Obstet. 2018, 297, 653–658. [Google Scholar] [CrossRef]
- Adashi, E.Y. Seeing double: A nation of twins from sea to shining sea. Am. J. Obstet. Gynecol. 2016, 214, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.M.; Hauth, J.; Caritis, S.; Lindheimer, M.D.; MacPherson, C.; Klebanoff, M.; Van Dorsten, J.; Landon, M.; Miodovnik, M.; Paul, R.; et al. Hypertensive disorders in twin versus singleton gestations. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am. J. Obstet. Gynecol. 2000, 182, 938–942. [Google Scholar] [CrossRef]
- Schwartz, D.B.; Daoud, Y.; Zazula, P.; Goyert, G.; Bronsteen, R.; Wright, D.; Copes, J. Gestational diabetes mellitus: Metabolic and blood glucose parameters in singleton versus twin pregnancies. Am. J. Obstet. Gynecol. 1999, 181, 912–914. [Google Scholar] [CrossRef]
- Chauhan, S.P.; Scardo, J.A.; Hayes, E.; Abuhamad, A.Z.; Berghella, V. Twins: Prevalence, problems, and preterm births. Am. J. Obstet. Gynecol. 2010, 203, 305–315. [Google Scholar] [CrossRef]
- Sharma, D.; Shastri, S.; Farahbakhsh, N.; Sharma, P. Intrauterine growth restriction—Part 1. J. Matern.-Fetal Neonatal Med. 2016, 29, 3977–3987. [Google Scholar] [CrossRef]
- Campbell, D.; Templeton, A. Maternal complications of twin pregnancy. Int. J. Gynecol. Obstet. 2003, 84, 71–73. [Google Scholar] [CrossRef]
- Doctor, B.A.; O’Riordan, M.A.; Kirchner, H.; Shah, D.; Hack, M. Perinatal correlates and neonatal outcomes of small for gestational age infants born at term gestation. Am. J. Obstet. Gynecol. 2001, 185, 652–659. [Google Scholar] [CrossRef]
- Sacchi, C.; Marino, C.; Nosarti, C.; Vieno, A.; Visentin, S.; Simonelli, A. Association of Intrauterine Growth Restriction and Small for Gestational Age Status with Childhood Cognitive Outcomes: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020, 174, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Peles, G.; Paz-Levy, D.; Wainstock, T.; Goldbart, A.; Kluwgant, D.; Sheiner, E. Pediatric respiratory hospitalizations in small for gestational age neonates born at term. Pediatr. Pulmonol. 2021, 57, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Neimark, E.; Wainstock, T.; Sheiner, E.; Fischer, L.; Pariente, G. Long-term cardiovascular hospitalizations of small for gestational age (SGA) offspring born to women with and without gestational diabetes mellitus (GDM). Gynecol. Endocrinol. 2019, 35, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Pariente, G.; Shoham-Vardi, I.; Kessous, R.; Sherf, M.; Sheiner, E. Placental Abruption as a Significant Risk Factor for Long-term Cardiovascular Mortality in a Follow-up Period of More Than a Decade. Paediatr. Périnat. Epidemiol. 2014, 28, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Sacks, K.N.; Friger, M.; Shoham-Vardi, I.; Spiegel, E.; Sergienko, R.; Landau, D.; Sheiner, E. Prenatal exposure to preeclampsia as an independent risk factor for long-term cardiovascular morbidity of the offspring. Pregnancy Hypertens. 2018, 13, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Groom, K.M.; Oyston, C.; Chamley, L.W.; Clark, A.R.; James, J.L. The placenta in fetal growth restriction: What is going wrong? Placenta 2020, 96, 10–18. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C.; Simmonds, S.J.; Wield, G.A. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ 1993, 306, 422–426. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef]
- Hadar, O.; Sheiner, E.; Wainstock, T. The Association between Delivery of Small-for-Gestational-Age Neonate and Their Risk for Long-Term Neurological Morbidity. J. Clin. Med. 2020, 9, 3199. [Google Scholar] [CrossRef]
- Spiegel, E.; Shoham-Vardi, I.; Sergienko, R.; Landau, D.; Sheiner, E. The association between birth weight at term and long-term endocrine morbidity of the offspring. J. Matern. Neonatal Med. 2018, 32, 2657–2661. [Google Scholar] [CrossRef]
- Barker, D.J.P. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Hiersch, L.; Barrett, J.; Fox, N.S.; Rebarber, A.; Kingdom, J.; Melamed, N. Should twin-specific growth charts be used to assess fetal growth in twin pregnancies? Am. J. Obstet. Gynecol. 2022, 227, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Powell, T. Role of the placenta in fetal programming: Underlying mechanisms and potential interventional approaches. Clin. Sci. 2007, 113, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gutvirtz, G.; Wainstock, T.; Sheiner, E.; Landau, D.; Walfisch, A. Pediatric Cardiovascular Morbidity of the Early Term Newborn. J. Pediatr. 2018, 194, 81–86.e2. [Google Scholar] [CrossRef]

| Characteristics | Mother to FGR Twin (n = 116) N (%) | Mother to Non-FGR Twin (n = 4106) N (%) | p Value | |
|---|---|---|---|---|
| Maternal age, years (mean + SD), | 30.74 + 6.4 | 29.95 + 5.5 | 0.125 | |
| gravidity | 1 | 38 (32.8%) | 1034 (25.2%) | 0.179 |
| 2–4 | 48 (41.4%) | 1866 (45.4%) | ||
| 5+ | 30 (25.9%) | 1206 (29.4%) | ||
| Parity | 1 | 52 (44.8%) | 1312 (32.0%) | 0.011 |
| 2–4 | 46 (39.7%) | 1888 (46.0%) | ||
| 5+ | 18 (15.5%) | 906 (22.1%) | ||
| Pregnancy following fertility treatment | 48 (41.4%) | 1226 (29.9%) | 0.008 | |
| Hypertensive disorders * | 30 (25.9%) | 412 (10.0%) | <0.001 | |
| Diabetes mellitus ** | 8 (6.9%) | 394 (9.6%) | 0.329 | |
| Preterm delivery | 90 (78.9%) | 2208 (53.8%) | <0.001 | |
| Cesarean delivery | 84 (72.4%) | 2261 (55.1%) | <0.001 | |
| Gender | Male | 58(50.0%) | 2053 (50.0%) | 1.00 |
| Female | 58 (50.0%) | 2053 (50.0%) | ||
| Mean birthweight, gr (mean + SD) | 1904.69 + 525.0 | 2339.79 + 555.9 | <0.001 | |
| 5th minute Apgar score <7 | 1 (0.9%) | 88 (2.2%) | 0.519 | |
| Perinatal mortality | 3 (2.6%) | 112 (2.7%) | 0.926 |
| Dichorionic–Diamniotic Twin with FGR (n = 113) N (%) | Dichorionic–Diamniotic Twin without FGR (n = 3994) N (%) | OR | 95% CI | p Value | |
|---|---|---|---|---|---|
| Structural valvular disease | 1 (0.9%) | 2 (0.1%) | 17.8 | 1.60–197.98 | 0.001 |
| Childhood hypertension | 2 (1.8%) | 2 (0.1%) | 36 | 5.02–257.63 | <0.001 |
| Arrhythmia | 0 (<0.1%) | 13 (0.3%) | 1 | 0.99–1.00 | 0.544 |
| Rheumatic fever | 0 (<0.1%) | 1 (<0.1%) | 1 | 0.99–1.00 | 0.866 |
| Pulmonary heart disease | 1 (0.9%) | 3 (0.1%) | 11.9 | 1.23–115.08 | 0.006 |
| Peri-, myo-, endocarditis | 0 (<0.1%) | 4 (0.1%) | 1 | 0.99–1.00 | 0.736 |
| Ischemic heart diseases | 1 (0.9%) | 2 (<0.1%) | 17.8 | 1.60–197.98 | 0.001 |
| Diastolic heart failure | 0 (<0.1%) | 1 (<0.1%) | 1 | 0.99–1.00 | 0.866 |
| Cardiac heart disease, not-otherwise specified | 0 (<0.1%) | 3 (0.1%) | 1 | 0.99–1.00 | 0.771 |
| Total cardiovascular hospitalizations | 5 (4.4%) | 53 (1.3%) | 3.4 | 1.35–8.78 | 0.006 |
| Variables | Adjusted HR | 95% CI | p Value |
|---|---|---|---|
| FGR dichorionic–diamniotic twin (vs. non-FGR dichorionic–diamniotic twin) | 3.3 | 1.31–8.19 | 0.011 |
| Order of birth | 1.3 | 0.79–2.23 | 0.289 |
| Gender | 1.1 | 0.64–1.80 | 0.784 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzafrir, T.; Wainstock, T.; Sheiner, E.; Miodownik, S.; Pariente, G. Fetal Growth Restriction and Long-Term Cardiovascular Morbidity of Offspring in Dichorionic–Diamniotic Twin Pregnancies. J. Clin. Med. 2023, 12, 1628. https://doi.org/10.3390/jcm12041628
Tzafrir T, Wainstock T, Sheiner E, Miodownik S, Pariente G. Fetal Growth Restriction and Long-Term Cardiovascular Morbidity of Offspring in Dichorionic–Diamniotic Twin Pregnancies. Journal of Clinical Medicine. 2023; 12(4):1628. https://doi.org/10.3390/jcm12041628
Chicago/Turabian StyleTzafrir, Tuval, Tamar Wainstock, Eyal Sheiner, Shayna Miodownik, and Gali Pariente. 2023. "Fetal Growth Restriction and Long-Term Cardiovascular Morbidity of Offspring in Dichorionic–Diamniotic Twin Pregnancies" Journal of Clinical Medicine 12, no. 4: 1628. https://doi.org/10.3390/jcm12041628
APA StyleTzafrir, T., Wainstock, T., Sheiner, E., Miodownik, S., & Pariente, G. (2023). Fetal Growth Restriction and Long-Term Cardiovascular Morbidity of Offspring in Dichorionic–Diamniotic Twin Pregnancies. Journal of Clinical Medicine, 12(4), 1628. https://doi.org/10.3390/jcm12041628







