Growth Differentiation Factor 15 Is Associated with Platelet Reactivity in Patients with Acute Coronary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Sampling
2.3. Multiple Electrode Aggregometry (MEA)
2.4. Growth Differentiation Factor 15 (GDF)-15
2.5. Statistical Analysis
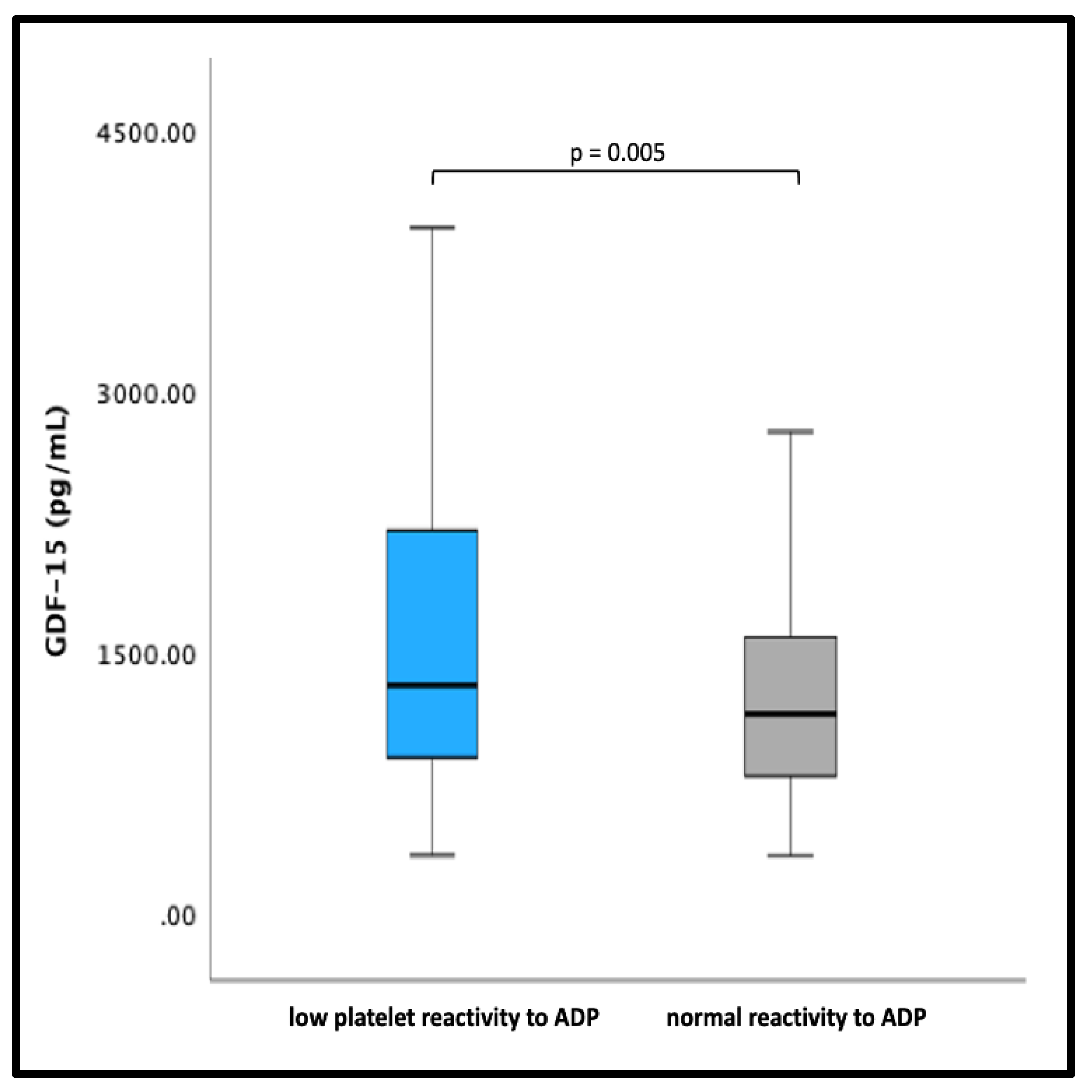
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R.P. Acute coronary syndromes. Lancet 2022, 399, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Tersalvi, G.; Biasco, L.; Cioffi, G.M.; Pedrazzini, G. Acute Coronary Syndrome, Antiplatelet Therapy, and Bleeding: A Clinical Perspective. J. Clin. Med. 2020, 9, 2064. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Michelson, A.D.; Frelinger, A.L., III; Bhatt, D.L. Novel aspects of antiplatelet therapy in cardiovascular disease. Res. Pract. Thromb. Haemost. 2018, 2, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Eslam, R.B.; Koppensteiner, R.; Lang, I.M.; Panzer, S. Prasugrel reduces agonists’ inducible platelet activation and leukocyte-platelet interaction more efficiently than clopidogrel. Cardiovasc. Ther. 2013, 31, e40–e45. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Juni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016, 134, e123–e155. [Google Scholar] [CrossRef]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef]
- Farhan, S.; Freynhofer, M.K.; Brozovic, I.; Bruno, V.; Vogel, B.; Tentzeris, I.; Baumgartner-Parzer, S.; Huber, K.; Kautzky-Willer, A. Determinants of growth differentiation factor 15 in patients with stable and acute coronary artery disease. A prospective observational study. Cardiovasc. Diabetol. 2016, 15, 60. [Google Scholar] [CrossRef]
- Kempf, T.; Zarbock, A.; Widera, C.; Butz, S.; Stadtmann, A.; Rossaint, J.; Bolomini-Vittori, M.; Korf-Klingebiel, M.; Napp, L.C.; Hansen, B.; et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat. Med. 2011, 17, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Suriben, R.; Chen, M.; Higbee, J.; Oeffinger, J.; Ventura, R.; Li, B.; Mondal, K.; Gao, Z.; Ayupova, D.; Taskar, P.; et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat. Med. 2020, 26, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Hijazi, Z.; Andersson, U.; Alexander, J.H.; De Caterina, R.; Hanna, M.; Horowitz, J.D.; Hylek, E.M.; Lopes, R.D.; Asberg, S.; et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: Insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation 2014, 130, 1847–1858. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Hagstrom, E.; James, S.K.; Bertilsson, M.; Becker, R.C.; Himmelmann, A.; Husted, S.; Katus, H.A.; Steg, P.G.; Storey, R.F.; Siegbahn, A.; et al. Growth differentiation factor-15 level predicts major bleeding and cardiovascular events in patients with acute coronary syndromes: Results from the PLATO study. Eur. Heart J. 2016, 37, 1325–1333. [Google Scholar] [CrossRef]
- Rossaint, J.; Vestweber, D.; Zarbock, A. GDF-15 prevents platelet integrin activation and thrombus formation. J. Thromb. Haemost. 2013, 11, 335–344. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Danese, E.; Brocco, G.; Gelati, M.; Montagnana, M.; Sanchis-Gomar, F.; Favaloro, E.J. Serum Concentration of Growth Differentiation Factor-15 Is Independently Associated with Global Platelet Function and Higher Fibrinogen Values in Adult Healthy Subjects. Semin. Thromb. Hemost. 2017, 43, 621–628. [Google Scholar] [CrossRef]
- Arauna, D.; Garcia, F.; Rodriguez-Manas, L.; Marrugat, J.; Saez, C.; Alarcon, M.; Wehinger, S.; Espinosa-Parrilla, Y.; Palomo, I.; Fuentes, E. Older adults with frailty syndrome present an altered platelet function and an increased level of circulating oxidative stress and mitochondrial dysfunction biomarker GDF-15. Free. Radic. Biol. Med. 2020, 149, 64–71. [Google Scholar] [CrossRef]
- Tscharre, M.; Wittmann, F.; Kitzmantl, D.; Lee, S.; Eichelberger, B.; Wadowski, P.P.; Laufer, G.; Wiedemann, D.; Panzer, S.; Perkmann, T.; et al. Growth Differentiation Factor-15 Correlates Inversely with Protease-Activated Receptor-1-Mediated Platelet Reactivity in Patients with Left Ventricular Assist Devices. Pharmaceuticals 2022, 15, 484. [Google Scholar] [CrossRef]
- Wadowski, P.P.; Pultar, J.; Weikert, C.; Eichelberger, B.; Tscharre, M.; Koppensteiner, R.; Panzer, S.; Gremmel, T. Platelet-to-Lymphocyte Ratio as Marker of Platelet Activation in Patients on Potent P2Y12 Inhibitors. J. Cardiovasc. Pharmacol. Ther. 2022, 27, 1096524. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Wendon, J.; Cordoba, J.; Dhawan, A.; Larsen, F.S.; Manns, M.; Nevens, F.; Samuel, D.; Simpson, K.J.; Yaron, I.; Bernardi, M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J. Hepatol. 2017, 66, 1047–1081. [Google Scholar] [CrossRef] [PubMed]
- Tscharre, M.; Wittmann, F.; Kitzmantl, D.; Lee, S.; Eichelberger, B.; Wadowski, P.P.; Laufer, G.; Wiedemann, D.; Forstner-Bergauer, B.; Ay, C.; et al. Platelet activation and aggregation in different centrifugal-flow left ventricular assist devices. Platelets 2022, 33, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Kopp, C.W.; Seidinger, D.; Koppensteiner, R.; Panzer, S.; Sunder-Plassmann, R.; Mannhalter, C.; Steiner, S. Differential impact of cytochrome 2C9 allelic variants on clopidogrel-mediated platelet inhibition determined by five different platelet function tests. Int. J. Cardiol. 2013, 166, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Kopp, C.W.; Seidinger, D.; Giurgea, G.A.; Koppensteiner, R.; Steiner, S.; Panzer, S. The formation of monocyte-platelet aggregates is independent of on-treatment residual agonists’-inducible platelet reactivity. Atherosclerosis 2009, 207, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Aradi, D.; Alexopoulos, D.; Ten Berg, J.; Bhatt, D.L.; Bonello, L.; Collet, J.P.; Cuisset, T.; Franchi, F.; Gross, L.; et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y(12) Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Toth, O.; Calatzis, A.; Penz, S.; Losonczy, H.; Siess, W. Multiple electrode aggregometry: A new device to measure platelet aggregation in whole blood. Thromb. Haemost. 2006, 96, 781–788. [Google Scholar]
- Mayer, K.; Bernlochner, I.; Braun, S.; Schulz, S.; Orban, M.; Morath, T.; Cala, L.; Hoppmann, P.; Schunkert, H.; Laugwitz, K.L.; et al. Aspirin treatment and outcomes after percutaneous coronary intervention: Results of the ISAR-ASPI registry. J. Am. Coll. Cardiol. 2014, 64, 863–871. [Google Scholar] [CrossRef]
- Sibbing, D.; Braun, S.; Morath, T.; Mehilli, J.; Vogt, W.; Schomig, A.; Kastrati, A.; von Beckerath, N. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J. Am. Coll. Cardiol. 2009, 53, 849–856. [Google Scholar] [CrossRef]
- Sibbing, D.; Schulz, S.; Braun, S.; Morath, T.; Stegherr, J.; Mehilli, J.; Schomig, A.; von Beckerath, N.; Kastrati, A. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J. Thromb. Haemost. 2010, 8, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Gelbenegger, G.; Postula, M.; Pecen, L.; Halvorsen, S.; Lesiak, M.; Schoergenhofer, C.; Jilma, B.; Hengstenberg, C.; Siller-Matula, J.M. Aspirin for primary prevention of cardiovascular disease: A meta-analysis with a particular focus on subgroups. BMC Med. 2019, 17, 198. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Benenati, S.; Franchi, F.; Rollini, F.; Capodanno, D.; Biondi-Zoccai, G.; Vescovo, G.M.; Cavallari, L.H.; Bikdeli, B.; Ten Berg, J.; et al. Comparative effects of guided vs. potent P2Y12 inhibitor therapy in acute coronary syndrome: A network meta-analysis of 61 898 patients from 15 randomized trials. Eur. Heart J. 2022, 43, 959–967. [Google Scholar] [CrossRef]
- Kato, E.T.; Morrow, D.A.; Guo, J.; Berg, D.D.; Blazing, M.A.; Bohula, E.A.; Bonaca, M.P.; Cannon, C.P.; de Lemos, J.A.; Giugliano, R.P.; et al. Growth differentiation factor 15 and cardiovascular risk: Individual patient meta-analysis. Eur. Heart J. 2022, 44, 293–300. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Grosso, A.; Abu-Assi, E.; Kinnaird, T.; Ariza-Sole, A.; Manzano-Fernandez, S.; Templin, C.; Velicki, L.; Xanthopoulou, I.; Cerrato, E.; et al. Incidence and predictors of bleeding in ACS patients treated with PCI and prasugrel or ticagrelor: An analysis from the RENAMI registry. Int. J. Cardiol. 2018, 273, 29–33. [Google Scholar] [CrossRef]
- Gremmel, T.; Steiner, S.; Seidinger, D.; Koppensteiner, R.; Panzer, S.; Kopp, C.W. In vivo and protease-activated receptor-1-mediated platelet activation but not response to antiplatelet therapy predict two-year outcomes after peripheral angioplasty with stent implantation. Thromb. Haemost. 2014, 111, 474–482. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Prasugrel (n = 116) | Ticagrelor (n = 90) | p |
|---|---|---|---|
| GDF-15, pg/mL | 1136 [865–1564] | 1492 [868–2027] | 0.014 |
| Age, years | 56 [48–64] | 59 [51–70] | 0.011 |
| Male patients, No. (%) | 94 (81.0) | 71 (78.9) | 0.702 |
| Body mass index, kg/m2 | 27.7 [25.2–31.0] | 26.8 [24.3–29.7] | 0.293 |
| Prior myocardial infarction, No. (%) | 18 (15.5) | 15 (16.7) | 0.762 |
| Prior stroke or TIA, No. (%) | 4 (3.4) | 2 (2.2) | 0.647 |
| Arterial hypertension, No. (%) | 74 (63.8) | 61 (67.8) | 0.510 |
| Hyperlipidemia, No. (%) | 87 (75.0) | 64 (71.1) | 0.757 |
| Peripheral artery disease, No. (%): | 8 (6.9) | 5 (5.6) | 0.349 |
| ACS, No. % | |||
| STEMI | 108 (93.1) | 27 (30.0) | <0.01 |
| NSTEMI | 7 (6.0) | 62 (68.9) | |
| Diabetes mellitus type II, No. (%): | 24 (20.7) | 27 (30.0) | 0.097 |
| Smoker, No. (%): | 69 (59.5) | 47 (52.2) | 0.517 |
| Serum creatinine, mg/dl | 0.89 [0.76–1.01] | 1.00 [0.82–1.17] | 0.001 |
| Platelet count, G/l | 221 [194–251] | 227 [191–269] | 0.567 |
| High-sensitivity CRP, mg/dL | 1.35 [0.69–3.85] | 1.29 [0.82–3.84] | 0.299 |
| Hemoglobin, mmol/L | 14.0 [13.2–14.9] | 13.6 [12.7–14.6] | 0.197 |
| proBNP, pg/mL | 748 [289–1494] | 603 [220–1113] | 0.096 |
| Statin, No. (%) | 114 (98.2) | 88 (97.8) | 0.855 |
| Beta blocker, No. (%) | 111 (95.7) | 87 (96.7) | 0.606 |
| ACE inhibitor, No. (%) | 97 (83.6) | 70 (77.8) | 0.569 |
| ARB, No. (%) | 16 (13.8) | 17 (18.9) | 0.318 |
| Calcium channel blocker, No. (%) | 10 (8.6) | 8 (8.9) | 0.942 |
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β | p | β | p | |
| GDF-15 | −0.171 | 0.013 | −0.124 | 0.324 |
| Age | −0.090 | 0.191 | ||
| Sex | 0.088 | 0.202 | ||
| BMI | 0.013 | 0.853 | ||
| Type of ACS | −0.013 | 0.853 | ||
| HLP | −0.061 | 0.376 | ||
| History of smoking | −0.091 | 0.192 | ||
| Arterial hypertension | 0.047 | 0.499 | ||
| Previous MCI | 0.095 | 0.174 | ||
| P2Y12 antagonist | −0.034 | 0.625 | ||
| CKD | −0.128 | 0.074 | −0.024 | 0.843 |
| Diabetes mellitus | 0.121 | 0.085 | 0.065 | 0.407 |
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β | p | β | p | |
| GDF-15 | 0.070 | 0.312 | ||
| Age | −0.083 | 0.228 | ||
| Sex | 0.130 | 0.134 | ||
| BMI | −0.016 | 0.821 | ||
| Type of ACS | −0.128 | 0.065 | −0.101 | 0.152 |
| HLP | −0.045 | 0.520 | ||
| History of smoking | −0.149 | 0.032 | −0.115 | 0.106 |
| Arterial hypertension | 0.123 | 0.077 | 0.088 | 0.217 |
| Previous MCI | 0.100 | 0.153 | ||
| P2Y12 antagonist | 0.009 | 0.904 | ||
| CKD | 0.094 | 0.192 | ||
| Diabetes mellitus | 0.001 | 0.990 | ||
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β | p | β | p | |
| GDF-15 | −0.200 | 0.004 | −0.150 | 0.044 |
| Age | −0.180 | 0.009 | −0.029 | 0.712 |
| Sex | −0.042 | 0.544 | ||
| BMI | 0.038 | 0.589 | ||
| Type of ACS | −0.051 | 0.463 | ||
| HLP | −0.144 | 0.037 | −0.149 | 0.031 |
| History of smoking | −0.225 | 0.001 | −2.116 | 0.036 |
| Arterial hypertension | 0.125 | 0.072 | 0.092 | 0.194 |
| Previous MCI | 0.084 | 0.231 | ||
| P2Y12 antagonist | 0.042 | 0.552 | ||
| CKD | −0.096 | 0.181 | ||
| Diabetes mellitus | 0.050 | 0.477 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutschlechner, D.; Tscharre, M.; Wadowski, P.P.; Pultar, J.; Weikert, C.; Lee, S.; Eichelberger, B.; Panzer, S.; Perkmann, T.; Gremmel, T. Growth Differentiation Factor 15 Is Associated with Platelet Reactivity in Patients with Acute Coronary Syndrome. J. Clin. Med. 2023, 12, 1627. https://doi.org/10.3390/jcm12041627
Mutschlechner D, Tscharre M, Wadowski PP, Pultar J, Weikert C, Lee S, Eichelberger B, Panzer S, Perkmann T, Gremmel T. Growth Differentiation Factor 15 Is Associated with Platelet Reactivity in Patients with Acute Coronary Syndrome. Journal of Clinical Medicine. 2023; 12(4):1627. https://doi.org/10.3390/jcm12041627
Chicago/Turabian StyleMutschlechner, David, Maximilian Tscharre, Patricia P. Wadowski, Joseph Pultar, Constantin Weikert, Silvia Lee, Beate Eichelberger, Simon Panzer, Thomas Perkmann, and Thomas Gremmel. 2023. "Growth Differentiation Factor 15 Is Associated with Platelet Reactivity in Patients with Acute Coronary Syndrome" Journal of Clinical Medicine 12, no. 4: 1627. https://doi.org/10.3390/jcm12041627
APA StyleMutschlechner, D., Tscharre, M., Wadowski, P. P., Pultar, J., Weikert, C., Lee, S., Eichelberger, B., Panzer, S., Perkmann, T., & Gremmel, T. (2023). Growth Differentiation Factor 15 Is Associated with Platelet Reactivity in Patients with Acute Coronary Syndrome. Journal of Clinical Medicine, 12(4), 1627. https://doi.org/10.3390/jcm12041627





