Techniques and Outcomes of Endoscopic Ultrasound Guided—Pancreatic Duct Drainage (EUS- PDD)
Abstract
1. Introduction
2. Indications for EUS- PDD
3. Contraindications for EUS- PDD
- a.
- difficult puncture with standard needles due to the small caliber of the dilated PD embedded within a fibrotic pancreas;
- b.
- unstable scope position and consequent poor force transmission to the puncturing needle;
- c.
- difficult wire manipulation through the needle (due to PD stricture, unfavorable needle to duct angle, preferential passage into a dilated side branch [7]), and risk of wire shearing during manipulation;
- d.
- difficulty passing devices such as balloons or cystotomes into the PD for tract dilatation;
- e.
- fragility of the pancreas and associated adverse events after aggressive manipulation.
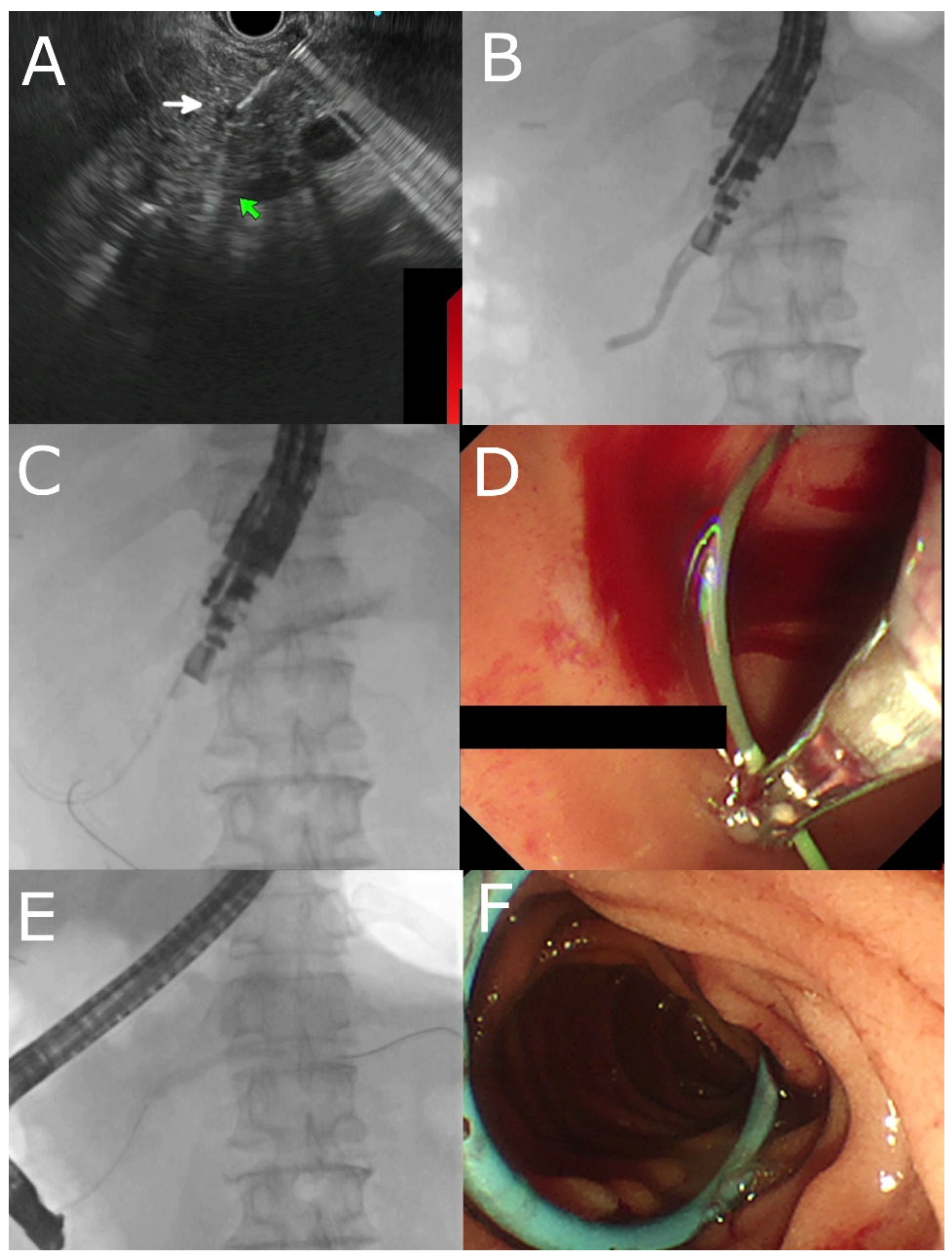
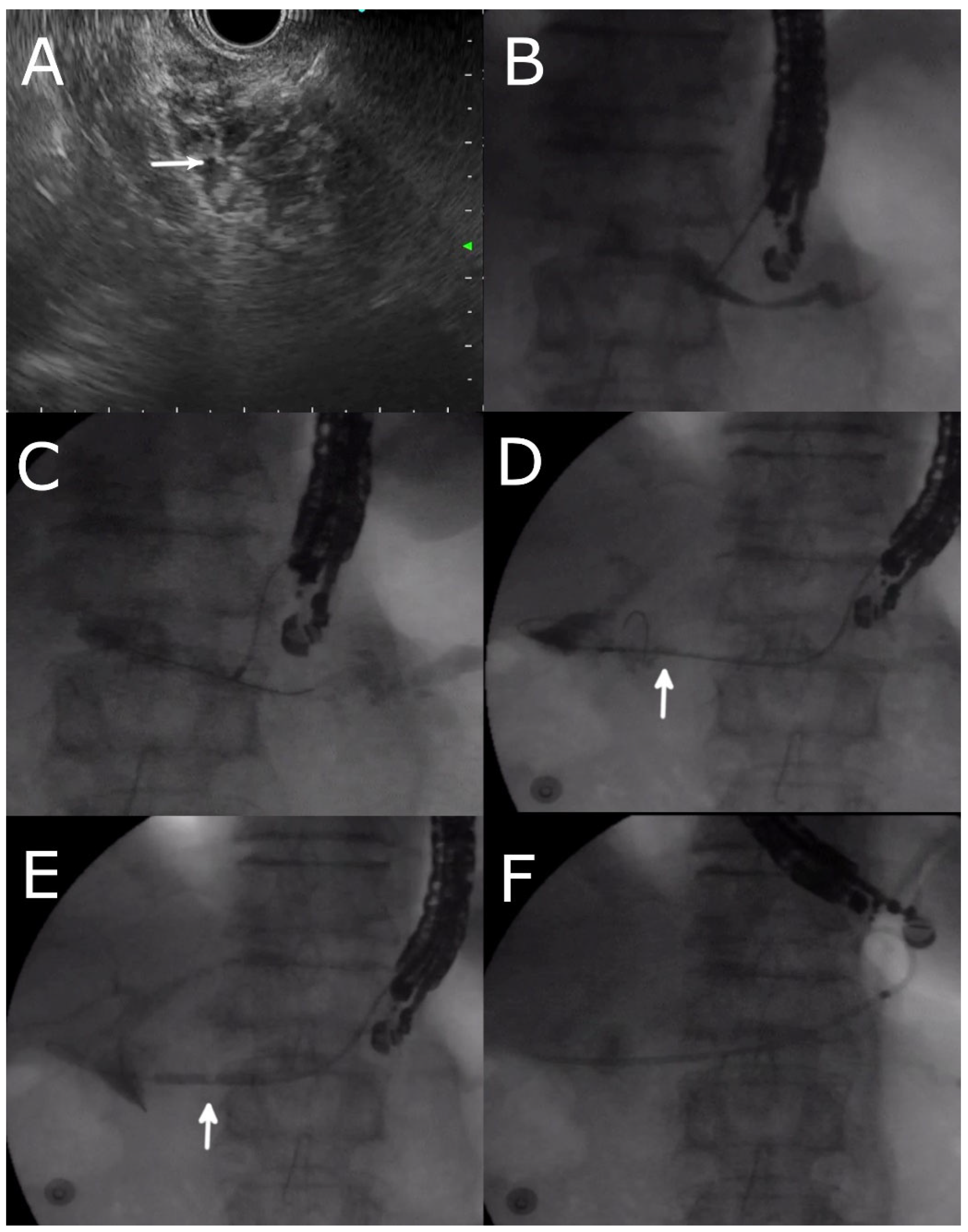
4. Technique of EUS- PDD
4.1. Patient Preparation
4.2. Approaches and Equipment
4.3. Pancreatic Duct Access
4.4. EUS- RV ERP
4.5. Transmural Approaches with Transpapillary or Trans-Anastomotic Stenting
4.6. Transmural Stenting with Antegrade/Retrograde Stenting
4.7. Per Oral Pancreaticoscopy
5. Outcomes
5.1. Long-Term Outcomes
5.2. Post-Operative Pancreatic Fistula
5.3. Comparison between e-ERP vs. EUS- PDD
5.4. Comparison between EUS- RV and EUS- TMD
5.5. Overall Outcomes from Meta-Analyses and Systemic Reviews
6. EUS- PDD Training
7. Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bataille, L.; Deprez, P. A new application for therapeutic EUS: Main pancreatic duct drainage with a “pancreatic rendezvous technique”. Gastrointest. Endosc. 2002, 55, 740–743. [Google Scholar] [CrossRef]
- François, E.; Kahaleh, M.; Giovannini, M.; Matos, C.; Devière, J. EUS-guided pancreaticogastrostomy. Gastrointest. Endosc. 2002, 56, 128–133. [Google Scholar] [CrossRef]
- Devière, J. EUS-guided pancreatic duct drainage: A rare indication in need of prospective evidence. Gastrointest. Endosc. 2017, 85, 178–180. [Google Scholar] [CrossRef]
- Kahaleh, M.; Hernandez, A.J.; Tokar, J.; Adams, R.B.; Shami, V.M.; Yeaton, P. EUS-guided pancreaticogastrostomy: Analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest. Endosc. 2007, 65, 224–230. [Google Scholar] [CrossRef]
- Tyberg, A.; Sharaiha, R.Z.; Kedia, P.; Kumta, N.; Gaidhane, M.; Artifon, E.; Giovannini, M.; Kahaleh, M. EUS-guided pancreatic drainage for pancreatic strictures after failed ERCP: A multicenter international collaborative study. Gastrointest. Endosc. 2017, 85, 164–169. [Google Scholar] [CrossRef]
- Toshima, T.; Fujimori, N.; Yoshizumi, T.; Itoh, S.; Nagao, Y.; Harada, N.; Oono, T.; Mori, M. A Novel Strategy of Endoscopic Ultrasonography-Guided Pancreatic Duct Drainage for Pancreatic Fistula After Pancreaticoduodenectomy. Pancreas 2021, 50, e21–e22. [Google Scholar] [CrossRef]
- Chapman, C.G.; Waxman, I.; Siddiqui, U.D. Endoscopic Ultrasound (EUS)-Guided Pancreatic Duct Drainage: The Basics of When and How to Perform EUS-Guided Pancreatic Duct Interventions. Clin. Endosc. 2016, 49, 161–167. [Google Scholar] [CrossRef]
- García-Alonso, F.J.; Peñas-Herrero, I.; Sanchez-Ocana, R.; Villarroel, M.; Cimavilla, M.; Bazaga, S.; De Benito Sanz, M.; Gil-Simon, P.; de la Serna-Higuera, C.; Perez-Miranda, M. The role of endoscopic ultrasound guidance for biliary and pancreatic duct access and drainage to overcome the limitations of ERCP: A retrospective evaluation. Endoscopy 2021, 53, 691–699. [Google Scholar] [CrossRef]
- Ghandour, B.; Akshintala, V.S.; Bejjani, M.; Szvarca, D.; Khashab, M.A. A modified approach for endoscopic ultrasound-guided management of disconnected pancreatic duct syndrome via drainage of a communicating collection. Endoscopy 2022, 54, 917–919. [Google Scholar] [CrossRef]
- Miyata, T.; Kamata, K.; Takenaka, M. Endoscopic ultrasonography-guided transenteric pancreatic duct drainage without cautery for obstructive pancreatitis as a result of ampullary carcinoma. Dig. Endosc. 2018, 30, 403–404. [Google Scholar] [CrossRef]
- Krafft, M.R.; Croglio, M.P.; James, T.W.; Baron, T.H.; Nasr, J.Y. Endoscopic endgame for obstructive pancreatopathy: Outcomes of anterograde EUS-guided pancreatic duct drainage. A dual-center study. Gastrointest. Endosc. 2020, 92, 1055–1066. [Google Scholar] [CrossRef]
- Fujii, L.L.; Topazian, M.D.; Abu Dayyeh, B.K.; Baron, T.H.; Chari, S.T.; Farnell, M.B.; Gleeson, F.C.; Gostout, C.J.; Kendrick, M.L.; Pearson, R.K.; et al. EUS-guided pancreatic duct intervention: Outcomes of a single tertiary-care referral center experience. Gastrointest. Endosc. 2013, 78, 854–864.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.I.; Saxena, P.; Ngamruengphong, S.; Haito-Chavez, Y.; Bukhari, M.; Artifon, E.; Khashab, M.A. Endoscopic ultrasound-guided pancreatic duct drainage: Technical approaches to a challenging procedure. Endoscopy 2016, 48 (Suppl. 1), E192–E193. [Google Scholar] [CrossRef] [PubMed]
- Chandan, S.; Mohan, B.P.; Khan, S.R.; Kassab, L.L.; Ponnada, S.; Ofosu, A.; Bhat, I.; Singh, S.; Adler, D.G. Efficacy and safety of endoscopic ultrasound-guided pancreatic duct drainage (EUS-PDD): A systematic review and meta-analysis of 714 patients. Endosc. Int. Open 2020, 8, E1664–E1672. [Google Scholar] [CrossRef]
- Vila, J.J.; Pérez-Miranda, M.; Vazquez-Sequeiros, E.; Abadia, M.A.; Pérez-Millán, A.; González-Huix, F.; Gornals, J.; Iglesias-Garcia, J.; De la Serna, C.; Aparicio, J.R.; et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest. Endosc. 2012, 76, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Dalal, A.; Patil, G.; Maydeo, A. Six-year retrospective analysis of endoscopic ultrasonography-guided pancreatic ductal interventions at a tertiary referral center. Dig. Endosc. 2020, 32, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Veitch, A.M.; Radaelli, F.; Alikhan, R.; Dumonceau, J.M.; Eaton, D.; Jerrome, J.; Lester, W.; Nylander, D.; Thoufeeq, M.; Vanbiervliet, G.; et al. Endoscopy in patients on antiplatelet or anticoagulant therapy: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guideline update. Gut 2021, 70, 1611–1628. [Google Scholar] [CrossRef]
- Nakai, Y.; Kogure, H.; Isayama, H.; Koike, K. Endoscopic ultrasound-guided pancreatic duct drainage. Saudi J. Gastroenterol. 2019, 25, 210–217. [Google Scholar] [CrossRef]
- Imoto, A.; Ogura, T.; Higuchi, K. Endoscopic Ultrasound-Guided Pancreatic Duct Drainage: Techniques and Literature Review of Transmural Stenting. Clin. Endosc. 2020, 53, 525–534. [Google Scholar] [CrossRef]
- Kurihara, T.; Itoi, T.; Sofuni, A.; Itokawa, F.; Moriyasu, F. Endoscopic ultrasonography-guided pancreatic duct drainage after failed endoscopic retrograde cholangiopancreatography in patients with malignant and benign pancreatic duct obstructions. Dig. Endosc. 2013, 25 (Suppl. 2), 109–116. [Google Scholar] [CrossRef]
- Abdelqader, A.; Kahaleh, M. When ERCP Fails: EUS-Guided Access to Biliary and Pancreatic Ducts. Dig. Dis. Sci. 2022, 67, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Elmunzer, B.J.; Piraka, C.R. EUS-Guided Methylene Blue Injection to Facilitate Pancreatic Duct Access After Unsuccessful ERCP. Gastroenterology 2016, 151, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Aneese, A.M.; Ghaith, G.; Cannon, M.E.; Manuballa, V.; Cappell, M.S. EUS-guided methylene blue injection to facilitate endoscopic cannulation of an obscured pancreatic duct orifice after ampullectomy. Am. J. Gastroenterol. 2018, 113, 782–783. [Google Scholar] [CrossRef] [PubMed]
- Will, U.; Meyer, F.; Manger, T.; Wanzar, I. Endoscopic ultrasound-assisted rendezvous maneuver to achieve pancreatic duct drainage in obstructive chronic pancreatitis. Endoscopy 2005, 37, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Kato, H.; Saragai, Y.; Takada, S.; Mizukawa, S.; Muro, S.; Akimoto, Y.; Tomoda, T.; Matsumoto, K.; Horiguchi, S.; et al. Indications for Endoscopic Ultrasound-Guided Pancreatic Drainage: For Benign or Malignant Cases? Can. J. Gastroenterol. Hepatol. 2018, 2018, 8216109. [Google Scholar] [CrossRef]
- Itoi, T.; Yasuda, I.; Kurihara, T.; Itokawa, F.; Kasuya, K. Technique of endoscopic ultrasonography-guided pancreatic duct intervention (with videos). J. Hepatobiliary Pancreat. Sci. 2014, 21, E4–E9. [Google Scholar] [CrossRef]
- Park, D.H.; Jang, J.W.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest. Endosc. 2011, 74, 1276–1284. [Google Scholar] [CrossRef]
- Honjo, M.; Itoi, T.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Mukai, S.; Sofuni, A.; Nagakawa, Y.; Iwasaki, H.; Kanai, T. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video). Endosc. Ultrasound. 2018, 7, 376–382. [Google Scholar] [CrossRef]
- Nakai, Y.; Kogure, H.; Koike, K. Double-guidewire technique for endoscopic ultrasound-guided pancreatic duct drainage. Dig. Endosc. 2019, 31 (Suppl. 1), 65–66. [Google Scholar] [CrossRef]
- Krafft, M.R.; Nasr, J.Y. Anterograde Endoscopic Ultrasound-Guided Pancreatic Duct Drainage: A Technical Review. Dig. Dis. Sci. 2019, 64, 1770–1781. [Google Scholar] [CrossRef]
- Itoi, T.; Kasuya, K.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Yasuda, I.; Nakai, Y.; Isayama, H.; Moriyasu, F. Endoscopic ultrasonography-guided pancreatic duct access: Techniques and literature review of pancreatography, transmural drainage and rendezvous techniques. Dig. Endosc. 2013, 25, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Park, D.H.; Cho, M.K.; Nam, K.; Song, T.J.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. Feasibility and safety of a fully covered self-expandable metal stent with antimigration properties for EUS-guided pancreatic duct drainage: Early and midterm outcomes (with video). Gastrointest. Endosc. 2016, 83, 366–373.e2. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Park, D.H.; Song, T.J.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. Long-term outcome of endoscopic ultrasound-guided pancreatic duct drainage using a fully covered self-expandable metal stent for pancreaticojejunal anastomosis stricture. J. Gastroenterol. Hepatol. 2020, 35, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Hayat, U.; Freeman, M.L.; Trikudanathan, G.; Azeem, N.; Amateau, S.K.; Mallery, J. Endoscopic ultrasound-guided pancreatic duct intervention and pancreaticogastrostomy using a novel cross-platform technique with small-caliber devices. Endosc. Int. Open 2020, 8, E196–E202. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Ishii, S.; Fujisawa, T.; Saito, H.; Takasaki, Y.; Takahashi, S.; Yamagata, W.; Ochiai, K.; Tomishima, K.; Isayama, H. Efficacy and Safety of Peroral Pancreatoscopy Through the Fistula Created by Endoscopic Ultrasound-Guided Pancreaticogastrostomy. Pancreas 2022, 51, 228–233. [Google Scholar] [CrossRef]
- Will, U.; Fueldner, F.; Thieme, A.K.; Goldmann, B.; Gerlach, R.; Wanzar, I.; Meyer, F. Transgastric pancreatography and EUS-guided drainage of the pancreatic duct. J. Hepatobiliary Pancreat. Surg. 2007, 14, 377–382. [Google Scholar] [CrossRef]
- Tessier, G.; Bories, E.; Arvanitakis, M.; Hittelet, A.; Pesenti, C.; Le Moine, O.; Giovannini, M.; Deviere, J. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest. Endosc. 2007, 65, 233–241. [Google Scholar] [CrossRef]
- Barkay, O.; Sherman, S.; McHenry, L.; Yoo, B.M.; Fogel, E.L.; Watkins, J.L.; DeWitt, J.; Al-Haddad, M.A.; Lehman, G.A. Therapeutic EUS-assisted endoscopic retrograde pancreatography after failed pancreatic duct cannulation at ERCP. Gastrointest. Endosc. 2010, 71, 1166–1173. [Google Scholar] [CrossRef]
- Ergun, M.; Aouattah, T.; Gillain, C.; Gigot, J.F.; Hubert, C.; Deprez, P.H. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: Long-term outcome. Endoscopy 2011, 43, 518–525. [Google Scholar] [CrossRef]
- Shah, J.N.; Marson, F.; Weilert, F.; Bhat, Y.M.; Nguyen-Tang, T.; Shaw, R.E.; Binmoeller, K.F. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest. Endosc. 2012, 75, 56–64. [Google Scholar] [CrossRef]
- Will, U.; Reichel, A.; Fueldner, F.; Meyer, F. Endoscopic ultrasonography-guided drainage for patients with symptomatic obstruction and enlargement of the pancreatic duct. World J. Gastroenterol. 2015, 21, 13140–13151. [Google Scholar] [CrossRef]
- Chen, Y.I.; Levy, M.J.; Moreels, T.G.; Hajijeva, G.; Will, U.; Artifon, E.L.; Hara, K.; Kitano, M.; Topazian, M.; Abu Dayyeh, B.; et al. An international multicenter study comparing EUS-guided pancreatic duct drainage with enteroscopy-assisted endoscopic retrograde pancreatography after Whipple surgery. Gastrointest. Endosc. 2017, 85, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Aviña, F.I.; Casasola-Sánchez, L.E.; Ramírez-Luna, M.; Saúl, Á.; Murcio-Pérez, E.; Chan, C.; Uscanga, L.; Duarte-Medrano, G.; Valdovinos-Andraca, F. Permanent Indwelling Transmural Stents for Endoscopic Treatment of Patients with Disconnected Pancreatic Duct Syndrome: Long-term Results. J. Clin. Gastroenterol. 2018, 52, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, Y.; Itoi, T.; Sofuni, A.; Tsuchiya, T.; Kamada, K.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Fujita, M.; et al. Evaluation of a new stent for EUS-guided pancreatic duct drainage: Long-term follow-up outcome. Endosc. Int. Open 2018, 6, E505–E512. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Koshita, S.; Kanno, Y.; Ogawa, T.; Kusunose, H.; Yonamine, K.; Miyamoto, K.; Kozakai, F.; Okano, H.; Ohira, T.; et al. Early and long-term clinical outcomes of endoscopic interventions for benign pancreatic duct stricture/obstruction-the possibility of additional clinical effects of endoscopic ultrasonography-guided pancreatic drainage. Pancreatology 2022, 22, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Onodera, M.; Kawakami, H.; Kuwatani, M.; Kudo, T.; Haba, S.; Abe, Y.; Kawahata, S.; Eto, K.; Nasu, Y.; Tanaka, E.; et al. Endoscopic ultrasound-guided transmural drainage for pancreatic fistula or pancreatic duct dilation after pancreatic surgery. Surg. Endosc. 2012, 26, 1710–1717. [Google Scholar] [CrossRef]
- Kogure, H.; Sato, T.; Nakai, Y.; Ishigaki, K.; Hakuta, R.; Saito, K.; Saito, T.; Takahara, N.; Hamada, T.; Mizuno, S.; et al. Endoscopic management of pancreatic diseases in patients with surgically altered anatomy: Clinical outcomes of combination of double-balloon endoscopy- and endoscopic ultrasound-guided interventions. Dig. Endosc. 2021, 33, 441–450. [Google Scholar] [CrossRef]
- Bhurwal, A.; Tawadros, A.; Mutneja, H.; Gjeorgjievski, M.; Shah, I.; Bansal, V.; Patel, A.; Sarkar, A.; Bartel, M.; Brahmbhatt, B. EUS guided pancreatic duct decompression in surgically altered anatomy or failed ERCP—A systematic review, meta-analysis and meta-regression. Pancreatology 2021, 21, 990–1000. [Google Scholar] [CrossRef]
- Basiliya, K.; Veldhuijzen, G.; Gerges, C.; Maubach, J.; Will, U.; Elmunzer, B.J.; Stommel, M.W.J.; Akkermans, R.; Siersema, P.D.; van Geenen, E.M. Endoscopic retrograde pancreatography-guided versus endoscopic ultrasound-guided technique for pancreatic duct cannulation in patients with pancreaticojejunostomy stenosis: A systematic literature review. Endoscopy 2021, 53, 266–276. [Google Scholar] [CrossRef]
- Tyberg, A.; Bodiwala, V.; Kedia, P.; Tarnasky, P.R.; Khan, M.A.; Novikov, A.; Gaidhane, M.; Ardengh, J.C.; Kahaleh, M. EUS-guided pancreatic drainage: A steep learning curve. Endosc. Ultrasound. 2020, 9, 175–179. [Google Scholar] [CrossRef]
- van der Merwe, S.W.; van Wanrooij, R.L.J.; Bronswijk, M.; Everett, S.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Kunda, R.; Badaoui, A.; Law, R.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.Y.B.; Dhir, V.; Kida, M.; Yasuda, I.; Jin, Z.D.; Seo, D.W.; Almadi, M.; Ang, T.L.; Hara, K.; Hilmi, I.; et al. Consensus guidelines on the optimal management in interventional EUS procedures: Results from the Asian EUS group RAND/UCLA expert panel. Gut 2018, 67, 1209–1228. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.W.; Moon, J.H.; Lee, Y.N.; Song, Y.H.; Yang, J.K.; Lee, T.H.; Cha, S.W.; Cho, Y.D.; Park, S.H. Peroral cholecystoscopy using a multibending ultraslim endoscope through a lumen-apposing metal stent for endoscopic ultrasound-guided gallbladder drainage: A feasibility study. Endoscopy 2022, 54, 384–388. [Google Scholar] [CrossRef] [PubMed]
| Indications |
|---|
| Native Anatomy (usually after failed ERP) |
| Chronic pancreatitis and main pancreatic duct (MPD) obstruction |
| Symptomatic pancreatic stones |
| Disconnected Pancreatic Duct Syndrome |
| Surgically Altered Anatomy/Inaccessible PD |
| Pancreatico-jejunostomy anastomosis stricture post Whipple operation |
| Standard ERP indications with history of previous billroth II/Roux-en-Y Gastrectomy |
| Inaccessible papilla due to malignant/benign duodenal strictures |
| Contraindications |
| Technical Factors |
| Inability to locate the MPD on EUS |
| Insufficient dilatation of MPD (MPD size < 4 mm) |
| Intervening vessels at the puncture site |
| Long distance between bowel and pancreatic duct |
| Multi-level strictures |
| Patient factors |
| Hemodynamic instability |
| Uncorrected coagulopathy, thrombocytopenia |
| Author | Type of Study | Patients | Indications | Technical Success, n (%) | Clinical Success, n (%) | Adverse Events, n (%) | Comments |
|---|---|---|---|---|---|---|---|
| Kahaleh et al. (2007) [4] | Prospective | 13 [EUS- TMD] | SAA, strictures secondary to pancreatitis, IPMN | 10/13 (77%) | NR | 2/13 (15.3%) Bleeding (1), perforation (1) | Improvement in MPD diameter, pain score, and weight on long term follow up |
| Tessier et al. (2007) [37] | Retrospective | 36 [EUS- TMD] | SAA, chronic pancreatitis, PJAS | 33/36 (92%) | Pain relief: 25/36 (69%) Stent dysfunction: 20/36 (55%) | 5/35 (13.8%) 2 severe, 3 mild | |
| Barkay et al. (2010) [38] | Retrospective | 21 [EUS- RV] | Failed ERP | 10/21 (48%) | NR | 2/20 (10%) 1 case of pancreatitis 1 case of peripancreatic abscess) | Dilated PD was associated with greater likelihood of EUS-guided pancreatography |
| Ergun et al. (2011) [39] | Retrospective | 20 [total]/ 24 procedures 5 [EUS- RV] 19 [EUS- TMD] | CP, PJAS | 18/20 (90%) 5/5 (100%) 15/19 (79%) | Pain term pain resolution: 13/18 (72%) | 2/20 (10%) including bleeding and perigatric collection. 9/18 (50%) developed stent dysfunction | Significant decrease in pain scores and MPD size after long-term follow-up. |
| Shah et al. (2012) [40] | Retrospective | 24 [total]/30 procedures 16 [EUS- RV] 14 [EUS- TMD] | CP, pancreatic duct leak, PJAS | 19/30 (63%) 9/16 (56%) 10/14 (71%) | NR | 4/ 22 (18%) | |
| Kurihara et al. (2013) [20] | Retrospective | 14 [total]/17 procedures 11 [EUS- RV] 5 [EUS- TMD] | PJAS, CP | 14/17 (82.3%) 11/17 (64.7%) 3/5 (60%) | NR | 1/17 (5.8%) 1 case developed pancreatic pseudocyst with aneurysm | Patients underwent EUS- PD after failed EUS- RV. |
| Fujii et al. (2013) [12] | Retrospective | 45 [total] | SAA, failed ERP | 32/43 (74%) 14 [EUS- RV] 18 [EUS- TMD] | Long-term symptom resolution: 24/29 (83%) | 3/45 (6.6%) with severe complications 16/35 (35.5%) developed abdominal pain | EUS- RV significantly longer than EUS- TMD (130 vs. 125 min, p = 0.05) |
| Will et al. (2015) [41] | Retrospective | 94 [total]/111 procedures | CP, pancreatic divisum, DPDS, POPF | 47/83 (56.6%) 21 [EUS- RV] 26 [EUS- TMD] | 68/83 (81.9%) | 24/111 (21.6%) (2 severe, 20 intermediate, 2 minor AEs) | |
| Chen et al. (2017) [42] | Retrospective | 40 [Total] 37 [EUS- TMD] 3 [EUS- RV] | Pancreatic intervention post-Whipple operation | 37/40 (92.5%) 34/ 37 (91.8%) 3/3 (100%) | 32/40 (87.5%) 29/37 (78.3%) 3/3 (100%) | 14/40 (35.0%) | |
| Tyberg et al. (2018) [5] | Retrospective | 80 [total] 66 [EUS- RV] 14 [EUS- TMD] | Malignancy, chronic pancreatitis | 71/ 80 (89%) | 65/80 (81%) | Immediate 16/80 (20%); Delayed 9/80 (11%) | Comparative study of EUS- PDD and e-ERP. EUS- PDD had higher clinical and technical success. |
| Uchida et al. (2018) [25] | Retrospective | 15 [total] 2 [EUS- RV] 13 [EUS- TMD] | Pancreatic strictures (8 benign, 7 malignant) | 13/15 (86%) Benign 75% (6/8) malignant 100% (7/7) | 12/13 (92.3%) Benign 100% (6/6), malignant 87.5% (6/7) | 4/15 (26.7%)–peritonitis, stent migration, bleeding | |
| Tellez-Avina et al. (2018) [43] | Retrospective | 21 [EUS- TMD] | DPDS | 21/21 (100%) | 17/21 (80.9%) | 5/21 (23.8%) | |
| Matsunami et al. (2019) [44] | Retrospective | 30 [EUS- TMD] | Acute recurrent pancreatitis with stricture | 30/30 (100%) | 23/30 (76%) | 7/30 (23%): mild abdominal pain/bleeding/pancreatitis 6/25 (24%): stent dislodgement | |
| Oh et al. (2019) [33] | Retrospective | 23 [total] 3 patients underwent plastic stenting 20 patients underwent FCSEMS | PJAS | 23/23 (100%) | 23/23 (100%) | Early adverse events: 4/23 (17.4%) Late adverse events: 5/23 (21.7%) | Utilized FCSEMS. |
| Krafft et al. (2020) [11] | Retrospective | 28 [EUS- TMD] | CP, PJS | 23/28 (82%) | 21/28(75%) | 4/28 (14.2%) | Long-term outcomes: 52% developed DM, 14.2% developed exocrine insufficiency, 83% had stents in situ after 12 months |
| Dalal et al. (2020) [16] | Retrospective | 44 [total] 23/44 [EUS- RV] 21/44 [EUS- TMD] | Failed ERP, SAA | 39/44 (88.6%) 22/23 (95.6%) 17/21 (80.9%) | 35/44 (79.5%) 19/23 (82.6%) 16/21 (76.1%) | 10/44 (22.7%) | 2/28 patients underwent gastropancreaticoenterostomy “ring drainage” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teh, J.L.; Teoh, A.Y.B. Techniques and Outcomes of Endoscopic Ultrasound Guided—Pancreatic Duct Drainage (EUS- PDD). J. Clin. Med. 2023, 12, 1626. https://doi.org/10.3390/jcm12041626
Teh JL, Teoh AYB. Techniques and Outcomes of Endoscopic Ultrasound Guided—Pancreatic Duct Drainage (EUS- PDD). Journal of Clinical Medicine. 2023; 12(4):1626. https://doi.org/10.3390/jcm12041626
Chicago/Turabian StyleTeh, Jun Liang, and Anthony Yuen Bun Teoh. 2023. "Techniques and Outcomes of Endoscopic Ultrasound Guided—Pancreatic Duct Drainage (EUS- PDD)" Journal of Clinical Medicine 12, no. 4: 1626. https://doi.org/10.3390/jcm12041626
APA StyleTeh, J. L., & Teoh, A. Y. B. (2023). Techniques and Outcomes of Endoscopic Ultrasound Guided—Pancreatic Duct Drainage (EUS- PDD). Journal of Clinical Medicine, 12(4), 1626. https://doi.org/10.3390/jcm12041626




