The Rate of Avoidable Pancreatic Resections at a High-Volume Center: An Internal Quality Control and Critical Review
Abstract
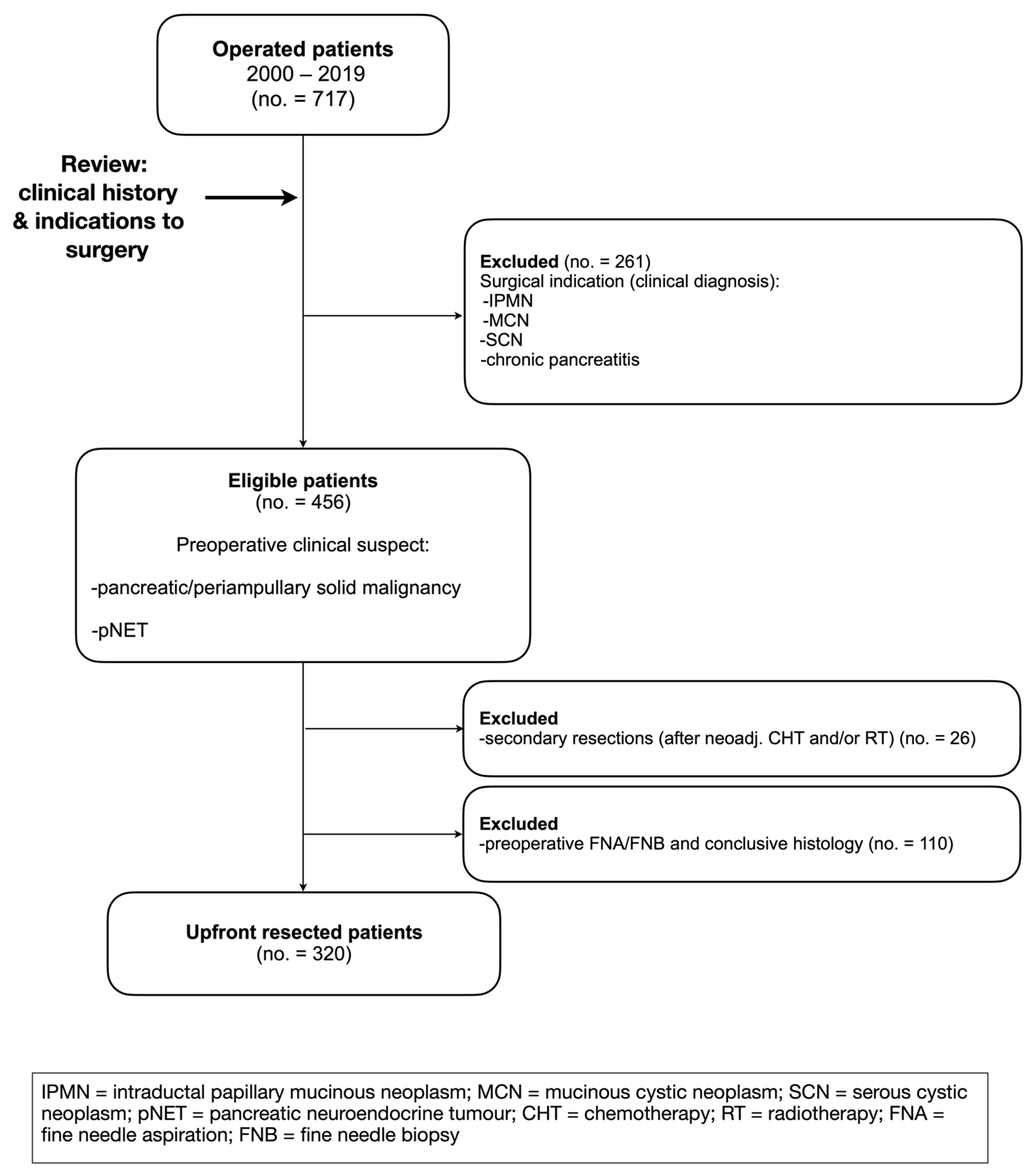
1. Introduction
2. Materials and Methods
2.1. Preoperative Workup
2.2. Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manzia, T.M.; Toti, L.; Lenci, I.; Attia, M.; Tariciotti, L.; Bramhall, S.R.; Buckels, J.A.; Mirza, D.F. Benign disease and unexpected histological findings after pancreaticoduodenectomy: The role of endoscopic ultrasound fine needle aspiration. Ind. Mark. Manag. 2010, 92, 295–301. [Google Scholar] [CrossRef]
- Larghi, A.; Rimbaş, M.; Rizzatti, G.; Quero, G.; Gasbarrini, A.; Costamagna, G.; Alfieri, S. Resectable pancreatic solid lesions: Time to move from surgical diagnosis? Endosc. Ultrasound 2020, 9, 76–82. [Google Scholar] [CrossRef]
- Zamboni, G.; Capelli, P.; Scarpa, A.; Bogina, G.; Pesci, A.; Brunello, E.; Klöppel, G. Nonneoplastic Mimickers of Pancreatic Neoplasms. Arch. Pathol. Lab. Med. 2009, 133, 439–453. [Google Scholar] [CrossRef]
- Schima, W.; Böhm, G.; Rösch, C.S.; Klaus, A.; Függer, R.; Kopf, H. Mass-forming pancreatitis versus pancreatic ductal adenocarcinoma: CT and MR imaging for differentiation. Cancer Imaging 2020, 20, 52. [Google Scholar] [CrossRef]
- Luchini, C.; Fassan, M.; Doglioni, C.; Capelli, P.; Ingravallo, G.; Renzulli, G.; Pecori, S.; Paolino, G.; Florena, A.M.; Scarpa, A.; et al. Inflammatory and tumor-like lesions of the pancreas. Pathologica 2020, 112, 197–209. [Google Scholar] [CrossRef]
- Hartwig, W.; Schneider, L.; Diener, M.K.; Bergmann, F.; Büchler, M.W.; Werner, J. Preoperative tissue diagnosis for tumours of the pancreas. Br. J. Surg. 2009, 96, 5–20. [Google Scholar] [CrossRef]
- Asbun, H.J.; Conlon, K.; Fernandez-Cruz, L.; Friess, H.; Shrikhande, S.V.; Adham, M.; Bassi, C.; Bockhorn, M.; Büchler, M.; Charnley, R.M.; et al. When to perform a pancreatoduodenectomy in the absence of positive histology? A consensus statement by the International Study Group of Pancreatic Surgery. Surgery 2014, 155, 887–892. [Google Scholar] [CrossRef]
- Pancreatic cancer in 2021: What you need to know to win. World J. Gastroenterol. 2021, 27, 5851–5889. [CrossRef]
- Ho, C.; Kleeff, J.; Friess, H.; Büchler, M. Complications of pancreatic surgery. HPB 2005, 7, 99–108. [Google Scholar] [CrossRef]
- Ahola, R.; Sand, J.; Laukkarinen, J. Centralization of Pancreatic Surgery Improves Results: Review. Scand. J. Surg. 2020, 109, 4–10. [Google Scholar] [CrossRef]
- Függer, R.; Gangl, O.; Fröschl, U. Clinical approach to the patient with a solid pancreatic mass. Wien. Med. Wochenschr. 2014, 164, 73–79. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Hilal, M.A.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy hemorrhage (PPH)—An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [Google Scholar] [CrossRef]
- Maharaj, A.D.; Ioannou, L.; Croagh, D.; Zalcberg, J.; Neale, R.E.; Goldstein, D.; Merrett, N.; Kench, J.G.; White, K.; Pilgrim, C.H.; et al. Monitoring quality of care for patients with pancreatic cancer: A modified Delphi consensus. HPB 2019, 21, 444–455. [Google Scholar] [CrossRef]
- Hurtuk, M.G.; Shoup, M.; Oshima, K.; Yong, S.; Aranha, G.V. Pancreaticoduodenectomies in patients without periampullary neoplasms: Lesions that masquerade as cancer. Am. J. Surg. 2010, 199, 372–376. [Google Scholar] [CrossRef]
- Kennedy, T.; Preczewski, L.; Stocker, S.J.; Rao, S.M.; Parsons, W.G.; Wayne, J.D.; Bell, R.H.; Talamonti, M.S. Incidence of benign inflammatory disease in patients undergoing Whipple procedure for clinically suspected carcinoma: A single-institution experience. Am. J. Surg. 2006, 191, 437–441. [Google Scholar] [CrossRef]
- Schwetz, V.; Horvath, K.; Kump, P.; Lackner, C.; Perren, A.; Forrer, F.; Pieber, T.R.; Treiber, G.; Sourij, H.; Mader, J.K. Successful Medical Treatment of Adult Nesidioblastosis with Pasireotide over 3 Years. Medicine 2016, 95, e3272. [Google Scholar] [CrossRef]
- Gouveia, C.; Fidalgo, C.; Loureiro, R.; Oliveira, H.; Maio, R.; Cravo, M. Adenomyomatosis of the Common Bile Duct and Ampulla of Vater. GE-Port. J. Gastroenterol. 2021, 28, 121–133. [Google Scholar] [CrossRef]
- Miyazaki, M.; Takada, T.; Miyakawa, S.; Tsukada, K.; Nagino, M.; Kondo, S.; Furuse, J.; Saito, H.; Tsuyuguchi, T.; Chijiiwa, K.; et al. Risk factors for biliary tract and ampullary carcinomas and prophylactic surgery for these factors. J. Hepato-Biliary-Pancreat. Surg. 2008, 15, 15–24. [Google Scholar] [CrossRef]
- Lanzillotta, M.; Vinge-Holmquist, O.; Overbeek, K.A.; Poulsen, J.L.; Demirci, A.F.; Macinga, P.; Löhr, M.; Rosendahl, J. PrescrAIP: A Pan-European Study on Current Treatment Regimens of Auto-Immune Pancreatitis. Front. Med. 2020, 7, 408. [Google Scholar] [CrossRef]
- Kamisawa, T.; Imai, M.; Chen, P.Y.; Tu, Y.; Egawa, N.; Tsuruta, K.; Okamoto, A.; Suzuki, M.; Kamata, N. Strategy for Differentiating Autoimmune Pancreatitis From Pancreatic Cancer. Pancreas 2008, 37, e62–e67. [Google Scholar] [CrossRef]
- Notohara, K.; Kamisawa, T.; Furukawa, T.; Fukushima, N.; Uehara, T.; Kasashima, S.; Iwasaki, E.; Kanno, A.; Kawashima, A.; Kubota, K.; et al. Concordance of the histological diagnosis of type 1 autoimmune pancreatitis and its distinction from pancreatic ductal adenocarcinoma with endoscopic ultrasound-guided fine needle biopsy specimens: An interobserver agreement study. Virchows Arch. 2022, 480, 565–575. [Google Scholar] [CrossRef]
- Notohara, K.; Kamisawa, T.; Kanno, A.; Naitoh, I.; Iwasaki, E.; Shimizu, K.; Kuraishi, Y.; Motoya, M.; Kodama, Y.; Kasashima, S.; et al. Efficacy and limitations of the histological diagnosis of type 1 autoimmune pancreatitis with endoscopic ultrasound-guided fine needle biopsy with large tissue amounts. Pancreatology 2020, 20, 834–843. [Google Scholar] [CrossRef]
- van Heerde, M.J.; Biermann, K.; Zondervan, P.E.; Kazemier, G.; van Eijck, C.H.J.; Pek, C.; Kuipers, E.J.; van Buuren, H.R. Prevalence of Autoimmune Pancreatitis and Other Benign Disorders in Pancreatoduodenectomy for Presumed Malignancy of the Pancreatic Head. Dig. Dis. Sci. 2012, 57, 2458–2465. [Google Scholar] [CrossRef]
- Qureshi, A.; Ghobrial, Y.; De Castro, J.; Siami-Namini, K.; Newman, K.A. Autoimmune pancreatitis—What we know and what do we have to know? Autoimmun. Rev. 2021, 20, 102912. [Google Scholar] [CrossRef]
- Shimosegawa, T.; Chari, S.T.; Frulloni, L.; Kamisawa, T.; Kawa, S.; Mino-Kenudson, M.; Kim, M.-H.; Klöppel, G.; Lerch, M.M.; Löhr, M.; et al. International Consensus Diagnostic Criteria for Autoimmune Pancreatitis: Guidelines of the International Association of Pancreatology. Pancreas 2011, 40, 352–358. [Google Scholar] [CrossRef]
- Rahbar, H.; Bhargava, P.; Vaidya, S.; Medverd, J.R. Intrapancreatic accessory spleen. Radiol. Case Rep. 2010, 5, 386. [Google Scholar] [CrossRef]
- Tozbikian, G.; Bloomston, M.; Stevens, R.; Ellison, E.C.; Frankel, W.L. Accessory spleen presenting as a mass in the tail of the pancreas. Ann. Diagn. Pathol. 2007, 11, 277–281. [Google Scholar] [CrossRef]
- Bhutiani, N.; Egger, M.E.; Doughtie, C.A.; Burkardt, E.S.; Scoggins, C.R.; Martin, R.C., II; McMasters, K.M. Intrapancreatic accessory spleen (IPAS): A single-institution experience and review of the literature. Am. J. Surg. 2017, 213, 816–820. [Google Scholar] [CrossRef]
- Baugh, K.A.; Villafane, N.; Farinas, C.; Dhingra, S.; Silberfein, E.J.; Massarweh, N.N.; Cao, H.T.; Fisher, W.E.; Van Buren, G. Pancreatic Incidentalomas: A Management Algorithm for Identifying Ectopic Spleens. J. Surg. Res. 2019, 236, 144–152. [Google Scholar] [CrossRef]
- Herreros-Villanueva, M.; Hijona, E.; Cosme, A.; Bujanda, L. Spontaneous regression of pancreatic cancer: Real or a misdiagnosis? World J. Gastroenterol. 2012, 18, 2902–2908. [Google Scholar] [CrossRef]
- Fleissig, A.; Jenkins, V.; Catt, S.; Fallowfield, L. Multidisciplinary teams in cancer care: Are they effective in the UK? Lancet Oncol. 2006, 7, 935–943. [Google Scholar] [CrossRef]
- Vinod, S.K.; Sidhom, M.A.; Delaney, G.P. Do Multidisciplinary Meetings Follow Guideline-Based Care? J. Oncol. Pract. 2010, 6, 276–281. [Google Scholar] [CrossRef]
- Basta, Y.L.; Bolle, S.; Fockens, P.; Tytgat, K.M.A.J. The Value of Multidisciplinary Team Meetings for Patients with Gastrointestinal Malignancies: A Systematic Review. Ann. Surg. Oncol. 2017, 24, 2669–2678. [Google Scholar] [CrossRef]
- Surci, N.; SPaRo Group; Ramera, M.; Borin, A.; Marchegiani, G.; Salvia, R.; Bassi, C. Implementation of a strategic preoperative surgical meeting to improve the level of care at a high-volume pancreatic center: A before–after analysis of 1000 consecutive cases. Updat. Surg. 2020, 72, 155–161. [Google Scholar] [CrossRef]
- Tempero, M.A.; Behrman, S.; Ben-Josef, E.; Benson, A.B.; Cameron, J.L.; Casper, E.S.; Hoffman, J.P.; Karl, R.C.; Kim, P.; Koh, W.-J.; et al. Pancreatic Adenocarcinoma Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2005, 3, 598–626. [Google Scholar] [CrossRef]
- Bünger, S.; Laubert, T.; Roblick, U.J.; Habermann, J.K. Serum biomarkers for improved diagnostic of pancreatic cancer: A current overview. J. Cancer Res. Clin. Oncol. 2011, 137, 375–389. [Google Scholar] [CrossRef]
- de la Fuente, S.G.; Ceppa, E.P.; Reddy, S.K.; Clary, B.M.; Tyler, D.S.; Pappas, T.N. Incidence of Benign Disease in Patients that Underwent Resection for Presumed Pancreatic Cancer Diagnosed by Endoscopic Ultrasonography (EUS) and Fine-Needle Aspiration (FNA). J. Gastrointest. Surg. 2010, 14, 1139–1142. [Google Scholar] [CrossRef]
- Clarke, D.; Clarke, B.; Thomson, S.; Garden, O.; Lazarus, N. The role of preoperative biopsy in pancreatic cancer. HPB 2004, 6, 144–153. [Google Scholar] [CrossRef]
- Iglesias-Garcia, J.; Lariño-Noia, J.; Dominguez-Munoz, J.E. When to puncture, when not to puncture: Pancreatic masses. Endosc. Ultrasound 2014, 3, 91–97. [Google Scholar] [CrossRef]
- Beger, H.G.; Birk, D.; Bodner, E.; Fritsch, A.; Gall, F.P.; Trede, M. Ist die histologische sicherung des pankreaskarzinoms voraussetzung für die pankreasresektion? Langenbeck’s Arch. Surg. 1995, 380, 62–66. [Google Scholar] [CrossRef]
- Bang, J.Y.; Kirtane, S.; Krall, K.; Navaneethan, U.; Hasan, M.; Hawes, R.; Varadarajulu, S. In memoriam: Fine-needle aspiration, birth: Fine-needle biopsy: The changing trend in endoscopic ultrasound-guided tissue acquisition. Dig. Endosc. 2019, 31, 197–202. [Google Scholar] [CrossRef]
- Chawla, A.; Ferrone, C.R. Neoadjuvant Therapy for Resectable Pancreatic Cancer: An Evolving Paradigm Shift. Front. Oncol. 2019, 9, 1085. [Google Scholar] [CrossRef]
- Oba, A.; Ho, F.; Bao, Q.R.; Al-Musawi, M.H.; Schulick, R.D.; Del Chiaro, M. Neoadjuvant Treatment in Pancreatic Cancer. Front. Oncol. 2020, 10, 245. [Google Scholar] [CrossRef]

| Sex (no., %) | |
| M | 149 (46.6%) |
| F | 171 (53.4%) |
| Age (years, mean ± SD) | 67.2 (±10.2) |
| Preoperative clinical suspicion (no., %) | |
| PDAC | 217 (67.8%) |
| Periampullary malignancy | 59 (18.5%) |
| pNET | 44 (13.7%) |
| Type of surgery (no., %) | |
| pancreatoduodenectomy (—PP or Kausch-Whipple) | 198 (61.9%) |
| distal pancreatectomy | 75 (23.4%) |
| total pancreatectomy | 47 (14.7%) |
| Complications (Clavien-Dindo Grading System) (no., %) | |
| 0 | 188 (58.8%) |
| I | 32 (10%) |
| II | 50 (15.6%) |
| IIIa | 24 (7.5%) |
| IIIb | 10 (3.1%) |
| IVa | 6 (1.9%) |
| IVb | 2 (0.6%) |
| V | 8 (2.5%) |
| Organ-specific complications (no., %) | |
| POPF | 45 (14.1%) |
| DGE | 15 (4.7%) |
| PPH | 11 (3.4%) |
| Biliary fistula | 2 (0.6%) |
| Reintervention | 26 (8.1%) |
| Hospital stay (median, IQR, days) | 14 (11–20) |
| 30-days mortality (no., %) | 8 (2.5%) |
| Pathology report (no., %) Malignant or pNET PDAC Papilla/Ampulla Ca. Choledochus Ca. Duodenal Ca. pNET Benign no lesion, Pan-In (MAJ-M) accessory spleen (MAJ-M) autoimmune pancreatitis (MAJ-M) adenomyoma choledochus (MIN-M) flogistic Vater’s papilla (MAJ-M) adenomyomatous hyperplasia Vater’s papilla (MIN-M) lymphoepithelial cyst (MAJ-M) nesidioblastosis (MIN-M) | 220 (68.8%) 22 (6.9%) 18 (5.6%) 10 (3.1%) 37 (11.6%) 1 (0.3%) 2 (0.6%) 4 (1.3%) 1 (0.3%) 1 (0.3%) 2 (0.6%) 1 (0.3%) 1 (0.3%) |
| 2000–2009 | 2010–2014 | 2015–2019 | |
|---|---|---|---|
| Total mismatches | 2 (1.9%) A | 7 (7.1%) B | 4 (3.4%) C |
| MIN-M | 0 (0%) | 2 (2.0%) | 2 (1.7%) |
| MAJ-M | 2 (1.9%) | 5 (5.1%) | 2 (1.7%) |
| Total resections | 101 | 99 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surci, N.; Rösch, C.S.; Kirchweger, P.; Havranek, L.; von Boetticher, P.; Fischer, I.; Wundsam, H.V.; Biebl, M.; Függer, R. The Rate of Avoidable Pancreatic Resections at a High-Volume Center: An Internal Quality Control and Critical Review. J. Clin. Med. 2023, 12, 1625. https://doi.org/10.3390/jcm12041625
Surci N, Rösch CS, Kirchweger P, Havranek L, von Boetticher P, Fischer I, Wundsam HV, Biebl M, Függer R. The Rate of Avoidable Pancreatic Resections at a High-Volume Center: An Internal Quality Control and Critical Review. Journal of Clinical Medicine. 2023; 12(4):1625. https://doi.org/10.3390/jcm12041625
Chicago/Turabian StyleSurci, Niccolò, Christiane Sophie Rösch, Patrick Kirchweger, Lukas Havranek, Paul von Boetticher, Ines Fischer, Helwig Valentin Wundsam, Matthias Biebl, and Reinhold Függer. 2023. "The Rate of Avoidable Pancreatic Resections at a High-Volume Center: An Internal Quality Control and Critical Review" Journal of Clinical Medicine 12, no. 4: 1625. https://doi.org/10.3390/jcm12041625
APA StyleSurci, N., Rösch, C. S., Kirchweger, P., Havranek, L., von Boetticher, P., Fischer, I., Wundsam, H. V., Biebl, M., & Függer, R. (2023). The Rate of Avoidable Pancreatic Resections at a High-Volume Center: An Internal Quality Control and Critical Review. Journal of Clinical Medicine, 12(4), 1625. https://doi.org/10.3390/jcm12041625








