Direct Connection to the ECMO Circuit versus a Hemodialysis Catheter Is Associated with Improved Urea Nitrogen Ultrafiltration during Continuous Renal Replacement Therapy for Patients on Extracorporeal Membrane Oxygenation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Covariates and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Differences in Serum Creatinine
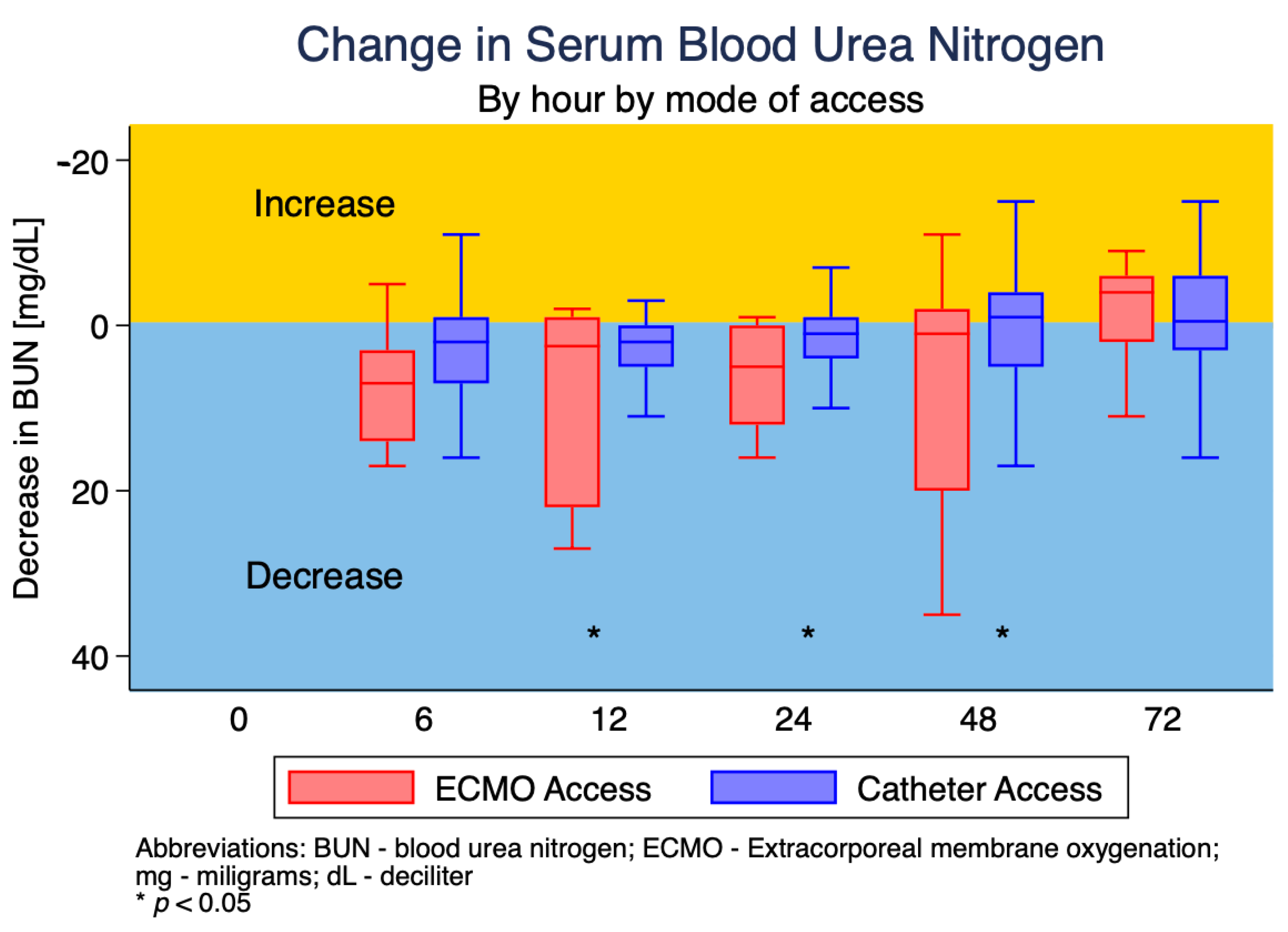
3.3. Changes in Serum Blood Urea Nitrogen (BUN)
3.4. Differences in Platelet Count
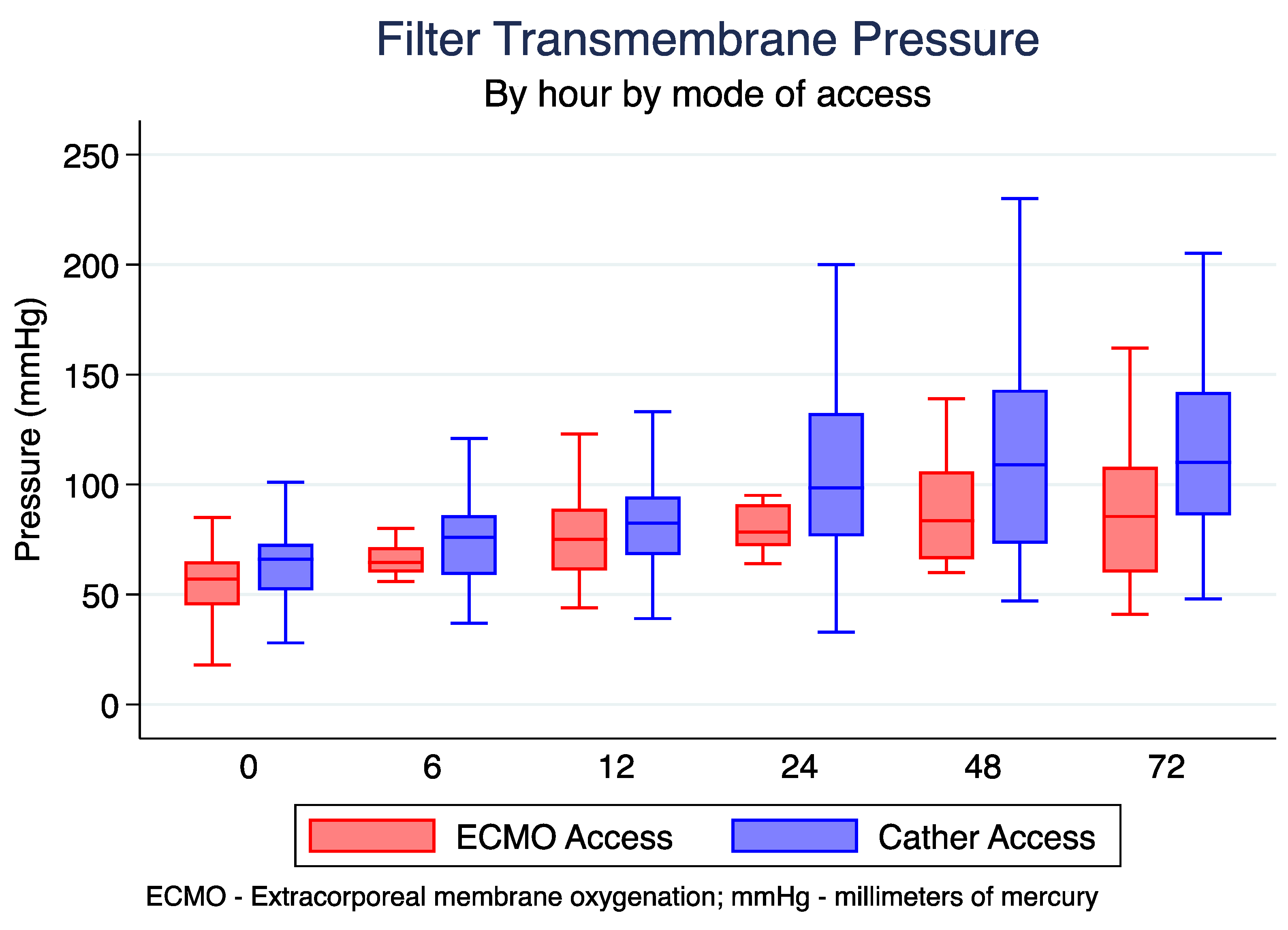
3.5. Differences in Transmembrane Pressure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huriaux, L.; Costille, P.; Quintard, H.; Journois, D.; Kellum, J.A.; Rimmele, T. Haemodialysis catheters in the intensive care unit. Anaesth. Crit. Care Pain Med. 2017, 36, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Tandukar, S.; Palevsky, P.M. Continuous Renal Replacement Therapy: Who, When, Why, and How. Chest 2019, 155, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Singbartl, K.; Kellum, J.A. AKI in the ICU: Definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012, 81, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, D.J.; Shekar, K.; Fraser, J.F. The Complex Relationship of Extracorporeal Membrane Oxygenation and Acute Kidney Injury: Causation or Association? Biomed. Res. Int. 2016, 2016, 1094296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Tsai, F.C.; Fang, J.T.; Yang, C.W. Acute kidney injury in adults receiving extracorporeal membrane oxygenation. J. Formos Med. Assoc. 2014, 113, 778–785. [Google Scholar] [CrossRef]
- Adwaney, A.; Lim, C.; Blakey, S.; Duncan, N.; Ashby, D.R. Central Venous Stenosis, Access Outcome and Survival in Patients undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 378–384. [Google Scholar] [CrossRef]
- Yevzlin, A.S. Hemodialysis catheter-associated central venous stenosis. Semin. Dial. 2008, 21, 522–527. [Google Scholar] [CrossRef]
- Suga, N.; Matsumura, Y.; Abe, R.; Hattori, N.; Nakada, T.A.; Oda, S. A safe procedure for connecting a continuous renal replacement therapy device into an extracorporeal membrane oxygenation circuit. J. Artif. Organs 2017, 20, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.J.; Sanchez, A.; Lopez-Herce, J.; Perez, R.; del Castillo, J.; Urbano, J.; Carrillo, A. The use of continuous renal replacement therapy in series with extracorporeal membrane oxygenation. Kidney Int. 2009, 76, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, R.G.; Yin, N.N.; Zhou, J.X. Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: A systematic review. Crit. Care 2014, 18, 675. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Connor, M., Jr.; Kashani, K. Continuous renal replacement therapy during extracorporeal membrane oxygenation: Why, when and how? Curr. Opin. Crit. Care 2018, 24, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, C.; Brodie, D.; Strassmann, S.; Stoelben, E.; Philipp, A.; Bein, T.; Muller, T.; Windisch, W. Extracorporeal membrane oxygenation: Evolving epidemiology and mortality. Intensive Care Med. 2016, 42, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Crosswell, A.; Brain, M.J.; Roodenburg, O. Vascular access site influences circuit life in continuous renal replacement therapy. Crit. Care Resusc. 2014, 16, 127–130. [Google Scholar] [PubMed]
- de Tymowski, C.; Desmard, M.; Lortat-Jacob, B.; Pellenc, Q.; Alkhoder, S.; Alouache, A.; Fritz, B.; Montravers, P.; Augustin, P. Impact of connecting continuous renal replacement therapy to the extracorporeal membrane oxygenation circuit. Anaesth. Crit. Care Pain Med. 2018, 37, 557–564. [Google Scholar] [CrossRef] [PubMed]

| Variable 1 | All Admissions (n = 33) | ECMO Access (n = 7) | HD Catheter Access (n = 26) | p-Value |
|---|---|---|---|---|
| Baseline Variables | ||||
| Age, mean (IQR) | 57 (50–64) | 54 (33–61) | 57.5 (50–65) | 0.338 |
| Female, n (%) | 12 (36.4%) | 4 (57.14%) | 8 (30.77%) | 0.198 |
| Male, n (%) | 21 (63.6%) | 3 (42.86%) | 18 (69.23%) | |
| Characteristics at the start of CRRT | ||||
| SOFA score | 12 (10, 14) | 13 (7, 15) | 12 (10, 14) | 0.695 |
| Serum creatinine (mg/dL) | 2.53 (2.09, 327) | 1.91 (1.18, 3.38) | 2.58 (2.29, 3.27) | 0.385 |
| Serum BUN (mg/dL) | 52 (37, 76) | 55 (39, 959) | 50 (36, 83) | 0.832 |
| Hemoglobin (g/dL) | 9.1 (8.1, 9.8) | 8.2 (7.8, 8.3) | 9.2 (8.2, 10) | 0.109 |
| Platelet count (k/uL) | 69.5 (51, 96) | 63 (25, 82) | 72 (51, 122) | 0.229 |
| ICU Variables | ||||
| ICU LOS, days | 22 (15, 43) | 21 (14, 29) | 22 (15, 51) | 0.695 |
| Died in ICU, n (%) | 21 (63.64%) | 4 (66.67%) | 17 (62.96%) | 1.0 |
| Variable 1 | All Admissions (n = 33) | ECMO Access (n = 7) | HD Catheter Access (n = 26) | p-Value 2 |
|---|---|---|---|---|
| Laboratory values at end of each session | ||||
| Serum creatinine (mg/dL) | 1.58 (1.22, 2.15) | 1.31 (0.92, 1.75) | 1.65 (1.26, 2.19) | 0.381 |
| Serum BUN (mg/dL) | 43 (34, 57) | 43 (33, 54) | 44 (34, 58) | 0.879 |
| Hemoglobin (g/dL) | 8.9 (8.3, 9.4) | 8.7 (8.05, 9.55) | 8.9 (8.4, 9.4) | 0.437 |
| Platelet count (1000/microliter) | 71 (51, 104) | 89 (73, 113) | 66 (49, 99) | 0.257 |
| TMP (mmHg) | 78 (63, 101) | 70 (60, 86.5) | 80 (65, 106) | 0.215 |
| Inflow pressure (mmHg) | −42 (−57, −27) | 185 (153–213) | −47 (−60, −36) | <0.001 |
| Outflow pressure (mmHg) | 51 (33–64) | −66 (−81, −48) | 55 (44, 67) | <0.001 |
| Decrease in serum BUN over 6 h (mg/dL) | 1 (−2, 5) | 2.5 (−1, 12.5) | 1 (−2, 5) | 0.054 |
| Decrease in serum creatinine over 6 h (mg/dL) | 0.07 (0, 0.23) | 0.07 (0, 3) | 0.07 (0, 0.22) | 0.137 |
| Average aPTT (seconds) | 50 (41, 69.5) | 49 (42, 75) | 50 (40, 67) | 0.456 |
| Average platelet count (1000/microliter) | 71 (51, 104) | 89 (73, 113) | 66 (49, 99) | 0.257 |
| Average hemoglobin (g/dL) | 8.9 (8.3, 9.4) | 8.7 (8.05, 9.55) | 8.9 (8.4, 9.4) | 0.437 |
| ECMO Flow during Session (L) | 4.45 (3.73, 4.98) | 4.28 (3.85, 4.54) | 4.5 (3.7, 5.05) | 0.430 |
| Variable 1 | All Admissions (n = 33) | ECMO Access (n = 7) | HD Catheter Access (n = 26) | p-Value |
|---|---|---|---|---|
| Average SCr (mg/dL) | ||||
| Hour 0 | 1.91 (1.32, 2.5) | 1.63 (1.19, 2.34) | 2 (1.46, 2.41) | 0.801 |
| Hour 6 | 1.98 (1.35, 2.41) | 1.63 (1.19, 2.34) | 2.00 (1.46, 2.41) | 0.497 |
| Hour 12 | 1.54 (1.22, 2.06) | 1.31 (0.90, 1.73) | 1.65 (1.25, 2.08) | 0.349 |
| Hour 24 | 1.63 (1.22, 2.06) | 1.06 (0.86, 1.36) | 1.68 (1.27, 2.13) | 0.267 |
| Hour 48 | 1.32 (1.05, 1.79) | 1.02 (0.91, 1.24) | 1.49 (1.19, 1.93) | 0.260 |
| Hour 72 | 1.23 (0.97, 1.60) | 1.00 (0.93, 1.42) | 1.29 (1.07, 1.63) | 0.556 |
| Average BUN (mg/dL) | ||||
| Hour 0 | 46 (37, 62) | 48 (31.5, 62) | 50 (36, 62) | 0.490 |
| Hour 6 | 49 (36, 62) | 48 (31.5, 62) | 50 (36, 62) | 0.801 |
| Hour 12 | 42 (34, 55) | 45 (33, 60) | 42 (34, 55) | 0.765 |
| Hour 24 | 42 (33, 54) | 41 (31, 50) | 42.5 (34, 57) | 0.685 |
| Hour 48 | 43 (30, 50) | 34 (29, 43) | 45 (30, 52) | 0.458 |
| Hour 72 | 41 (33, 48) | 39 (31.5, 45) | 42 (33, 51) | 0.459 |
| Average TMP (mmHg) | ||||
| Hour 0 | 64.5 (50.5, 72) | 64.5 (60, 71.5) | 76 (59, 86) | 0.154 |
| Hour 6 | 72.5 (60, 84) | 64.5 (60, 71.5) | 76 (59, 86) | 0.248 |
| Hour 12 | 81 (67, 93) | 75 (61, 89) | 82.5 (68, 94.5) | 0.451 |
| Hour 24 | 91.5 (73.5, 125) | 78.5 (72, 91) | 98.5 (76.5, 132.5) | 0.416 |
| Hour 48 | 106 (71, 141) | 83.5 (66, 106) | 109 (73, 143) | 0185 |
| Hour 72 | 101 (70, 136) | 85.5 (60, 108) | 110 (86, 142) | 0.193 |
| Average PTT (seconds) | ||||
| Hour 0 | 49.5 (39.5, 69.5) | 54.4 (44.5, 69) | 47 (38, 63) | 0.344 |
| Hour 6 | 47 (40, 63) | 54.5 (44.5, 69) | 47 (38, 63) | 0.476 |
| Hour 12 | 50 (41,70) | 61.5 (44, 79) | 50 (41, 66) | 0.625 |
| Hour 24 | 49 (40, 70) | 46 (39, 73) | 50 (40, 70) | 0.309 |
| Hour 48 | 53 (41, 69) | 48.5 (41, 79) | 54 (42, 66) | 0.908 |
| Hour 72 | 54 (42, 73) | 49 (42.5, 75) | 57.5 (42, 72) | 0.293 |
| Average PLT (k/uL) | ||||
| Hour 0 | 69.5 (51, 96) | 75.5 (66, 92) | 65 (51, 113) | 0.152 |
| Hour 6 | 67 (54, 102) | 75.5 (66, 92) | 65 (51, 113) | 0.876 |
| Hour 12 | 67 (49, 107.5) | 86 (74.5, 130.5) | 61.5 (45, 102) | 0.819 |
| Hour 24 | 77 (51, 108) | 106 (84, 122.5) | 69 (48, 94) | 0.985 |
| Hour 48 | 69.5 (48, 111) | 108 (76, 127) | 65.5 (45.5, 98) | 0.734 |
| Hour 72 | 78 (52, 102) | 94.5 (75.5, 126) | 71 (50, 100) | 0.008 |
| Change in SCr (mg/dL) | ||||
| Hour 6 | 0.18 (0, 0.38) | 0.27 (0, 0.48) | 0.17 (0, 0.36) | 0.318 |
| Hour 12 | 0.11 (0.04, 0.22) | 0.08 (0.02, 0.62) | 0.12 (0.05, 0.22) | 0.069 |
| Hour 24 | 0.045 (0.005, 0.14) | 0.06 (0.03, 0.25) | 0.04 (−0.03, 0.14) | 0.171 |
| Hour 48 | 0.05 (−0.03, 0.22) | 0.045 (−0.035, 0.335) | 0.05 (−0.02, 0.22) | 0.53 |
| Hour 72 | 0.01 (−0.06, 0.18) | −0.01 (−0.17, 0.07) | 0.025 (−0.035, 0.215) | 0.167 |
| Change in BUN (mg/dL) | ||||
| Hour 6 | 3 (−1, 8) | 7 (3, 14) | 2 (−1, 7) | 0.360 |
| Hour 12 | 2 (0, 5) | 2.5 (−1, 22) | 2 (0, 5) | 0.035 |
| Hour 24 | 1.5 (−1, 5) | 5 (0, 12) | 1 (−1, 4) | 0.039 |
| Hour 48 | 0 (−3, 6) | 1 (−2, 20) | −1 (−4, 5) | 0.009 |
| Hour 72 | −1 (−6, 3) | −4 (−6, 2) | −0.5 (−6, 3) | 0.744 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciullo, A.L.; Knecht, R.; Levin, N.M.; Mitchell, N.; Tonna, J.E. Direct Connection to the ECMO Circuit versus a Hemodialysis Catheter Is Associated with Improved Urea Nitrogen Ultrafiltration during Continuous Renal Replacement Therapy for Patients on Extracorporeal Membrane Oxygenation. J. Clin. Med. 2023, 12, 1488. https://doi.org/10.3390/jcm12041488
Ciullo AL, Knecht R, Levin NM, Mitchell N, Tonna JE. Direct Connection to the ECMO Circuit versus a Hemodialysis Catheter Is Associated with Improved Urea Nitrogen Ultrafiltration during Continuous Renal Replacement Therapy for Patients on Extracorporeal Membrane Oxygenation. Journal of Clinical Medicine. 2023; 12(4):1488. https://doi.org/10.3390/jcm12041488
Chicago/Turabian StyleCiullo, Anna L., Richard Knecht, Nicholas M. Levin, Nathan Mitchell, and Joseph E. Tonna. 2023. "Direct Connection to the ECMO Circuit versus a Hemodialysis Catheter Is Associated with Improved Urea Nitrogen Ultrafiltration during Continuous Renal Replacement Therapy for Patients on Extracorporeal Membrane Oxygenation" Journal of Clinical Medicine 12, no. 4: 1488. https://doi.org/10.3390/jcm12041488
APA StyleCiullo, A. L., Knecht, R., Levin, N. M., Mitchell, N., & Tonna, J. E. (2023). Direct Connection to the ECMO Circuit versus a Hemodialysis Catheter Is Associated with Improved Urea Nitrogen Ultrafiltration during Continuous Renal Replacement Therapy for Patients on Extracorporeal Membrane Oxygenation. Journal of Clinical Medicine, 12(4), 1488. https://doi.org/10.3390/jcm12041488






