Migraine and Hormonal Contraception in Gynecological Outpatient Care—Cross-Sectional Study among Practicing Gynecologists in Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Procedure
2.2. Survey
2.3. Statistical Analysis
3. Results
3.1. Demographics
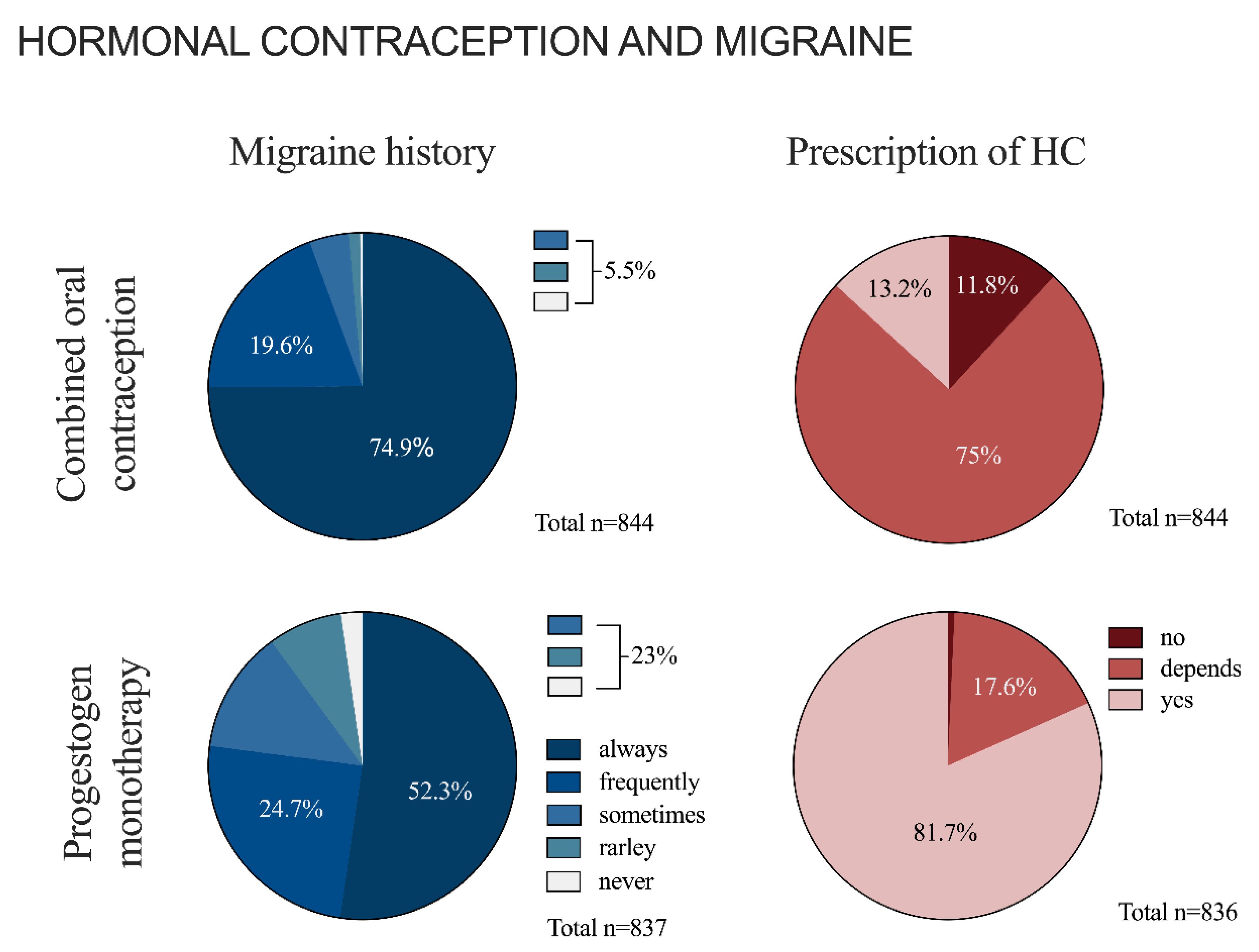
3.2. Hormonal Contraception and Migraine in General
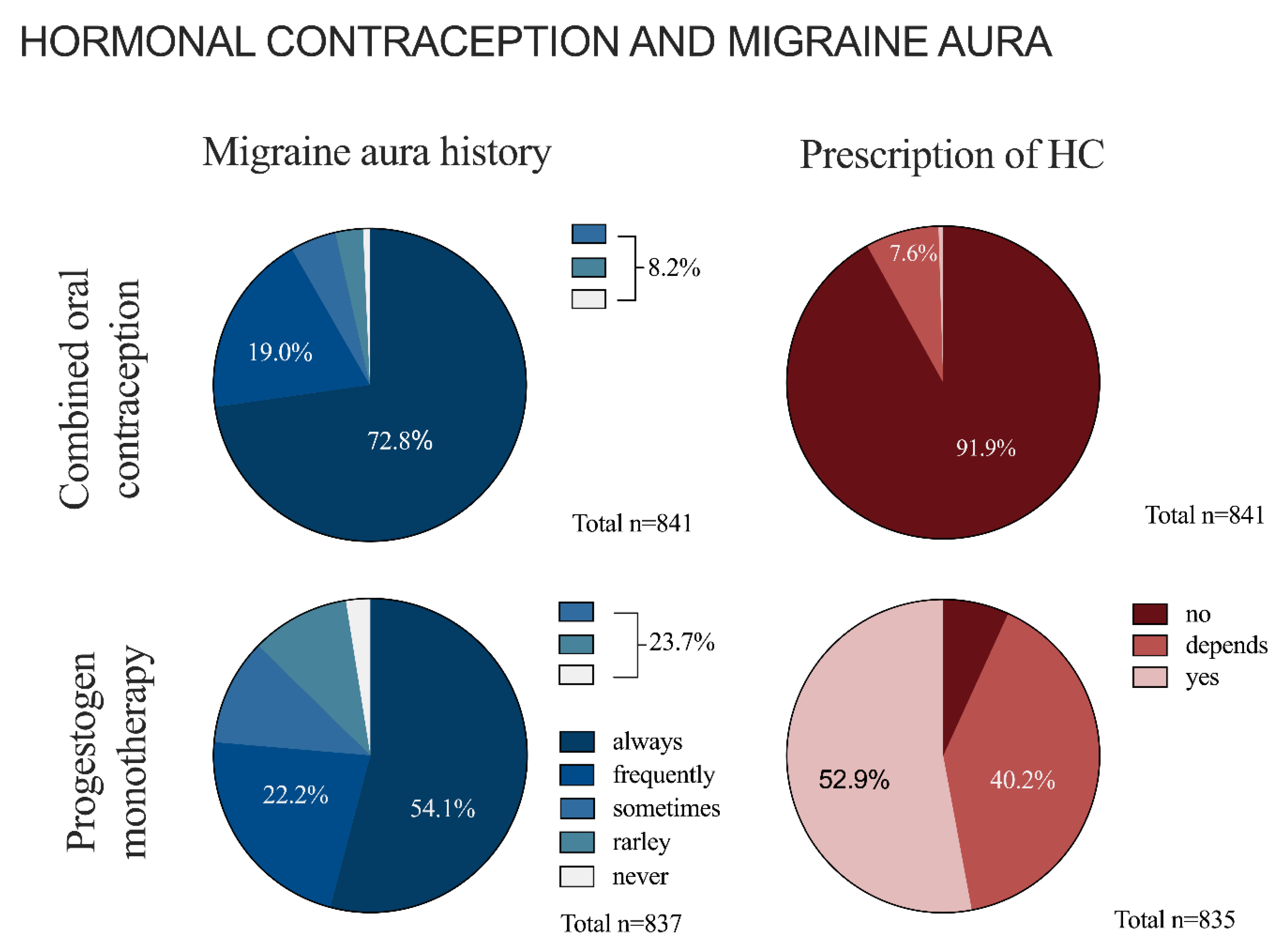
3.3. Hormonal Contraception and Migraine with Aura
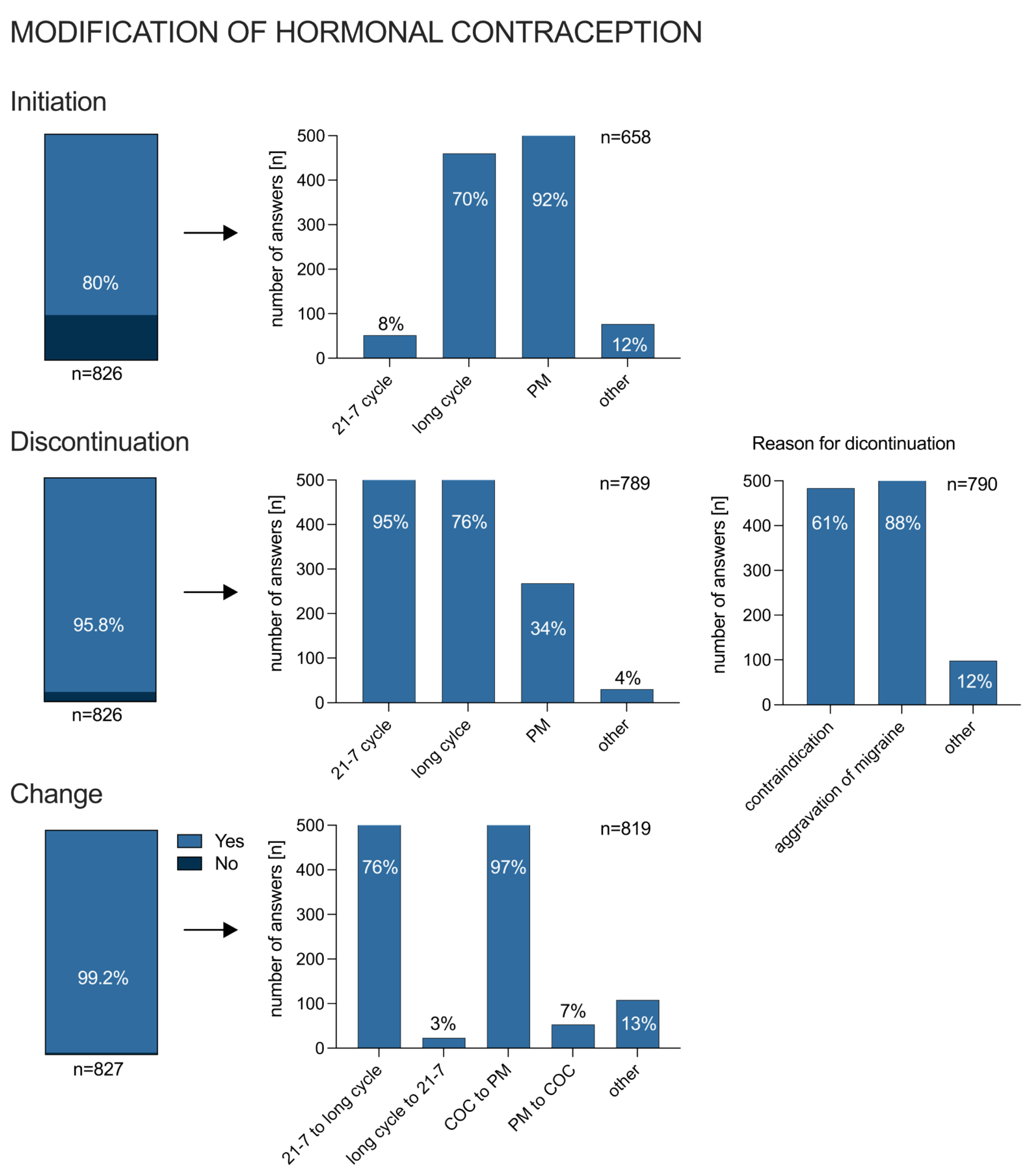
3.4. Modification of Hormonal Treatment due to Migraine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stovner, L.J.; Hagen, K.; Linde, M.; Steiner, T.J. The global prevalence of headache: An update, with analysis of the influences of methodological factors on prevalence estimates. J. Headache Pain 2022, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Allais, G.; Chiarle, G.; Sinigaglia, S.; Airola, G.; Schiapparelli, P.; Benedetto, C. Gender-related differences in migraine. Neurol. Sci. 2020, 41 (Suppl. 2), 429–436. [Google Scholar] [CrossRef]
- Steiner, T.J.; Stovner, L.J.; Jensen, R.; Uluduz, D.; Katsarava, Z.; on behalf of Lifting The Burden: The Global Campaign against Headache. Migraine remains second among the world’s causes of disability, and first among young women: Findings from GBD2019. J. Headache Pain 2020, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Tonini, M.C.; Fiorencis, A.; Iannacchero, R.; Zampolini, M.; Cappuccio, A.; Raddino, R.; Grillo, E.; Albanese, M.; Allais, G.; Bassano, M.A.; et al. Narrative Medicine to integrate patients’, caregivers’ and clinicians’ migraine experiences: The DRONE multicentre project. Neurol. Sci. 2021, 42, 5277–5288. [Google Scholar] [CrossRef]
- Daniels, K. Current Contraceptive Status Among Women Aged 15–49: United States, 2017–2019. NCHS Data Brief 2020, 388, 8. [Google Scholar]
- Calhoun, A.H.; Batur, P. hormonal contraceptives and migraine: An update on the evidence. Clevel. Clin. J. Med. 2017, 84, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Lidegaard, Ø.; Løkkegaard, E.; Jensen, A.; Skovlund, C.W.; Keiding, N. Thrombotic stroke and myocardial infarction with hormonal contraception. N. Engl. J. Med. 2012, 366, 2257–2266. [Google Scholar] [CrossRef]
- Etminan, M.; Takkouche, B.; Isorna, F.C.; Samii, A. Risk of ischaemic stroke in people with migraine: Systematic review and meta-analysis of observational studies. BMJ 2005, 330, 63. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Y.; Zhao, H.; Peng, C. Migraine and the risk of stroke: An updated meta-analysis of prospective cohort studies. Neurol. Sci. 2017, 38, 33–40. [Google Scholar] [CrossRef]
- Øie, L.R.; Kurt, T.; Gulati, S.; Dodick, D.W. Migraine and risk of stroke. J. Neurol. Neurosurg. Psychiatry 2020, 91, 593–604. [Google Scholar] [CrossRef]
- Sacco, S.; Merki-Feld, G.S.; Ægidius, K.L.; Bitzer, J.; Canonico, M.; Kurth, T.; Lampl, C.; Lidegaard, Ø.; MacGregor, E.A.; MaassenVanDenBrink, A.; et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: A consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J. Headache Pain 2017, 18, 108, Correction in J. Headache Pain 2018, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Schürks, M.; Rist, P.M.; Bigal, M.E.; Buring, J.E.; Lipton, R.B.; Kurth, T. Migraine and cardiovascular disease: Systematic review and meta-analysis. BMJ 2009, 339, b3914. [Google Scholar] [CrossRef] [PubMed]
- Weill, A.; Dalichampt, M.; Raguideau, F.; Ricordeau, P.; Blotière, P.-O.; Rudant, J.; Alla, F.; Zureik, M. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: Cohort study. BMJ 2016, 353, i2002. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, E.A. Contraception and headache. Headache 2013, 53, 247–276. [Google Scholar] [CrossRef]
- MacGregor, E.A. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004, 3, 354–361. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Frith, A.; Ellis, J.; Aspinall, L.; Hackshaw, A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 2006, 67, 2154–2158. [Google Scholar] [CrossRef] [PubMed]
- Allais, G.; Chiarle, G.; Sinigaglia, S.; Airola, G.; Schiapparelli, P.; Bergandi, F.; Benedetto, C. Treating migraine with contraceptives. Neurol. Sci. 2017, 38, 85–89. [Google Scholar] [CrossRef]
- Baillargeon, J.P.; McClish, D.K.; Essah, P.A.; Nestler, J.E. Association between the current use of low-dose oral contraceptives and cardiovascular arterial disease: A meta-analysis. J. Clin. Endocrinol. Metab. 2005, 90, 3863–3870. [Google Scholar] [CrossRef]
- Massiou, H.; MacGregor, E.A. Evolution and treatment of migraine with oral contraceptives. Cephalalgia 2000, 20, 170–174. [Google Scholar] [CrossRef]
- Edelman, A.; Micks, E.; Gallo, M.F.; Jensen, J.T.; Grimes, D.A. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst. Rev. 2014, 7, CD004695. [Google Scholar] [CrossRef]
- Merki-Feld, G.S.; Imthurn, B.; Seifert, B.; Merki, L.L.; Agosti, R.; Gantenbein, A.R. Desogestrel-only contraception may reduce headache frequency and improve quality of life in women suffering from migraine. Eur. J. Contracept. Reprod. Health Care 2013, 18, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Nappi, R.E.; Sances, G.; Allais, G.; Terreno, E.; Benedetto, C.; Vaccaro, V.; Polatti, F.; Facchinetti, F. Effects of an estrogen-free, desogestrel-containing oral contraceptive in women with migraine with aura: A prospective diary-based pilot study. Contraception 2011, 83, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, A.M.S.; Williamson, A.; Johnson, A.; Murphy, A.; Saidel, M.; Chua, A.L.; Minen, M.; Grosberg, B.M. Migraine diagnosis and treatment: A knowledge and needs assessment of women’s healthcare providers. Headache 2021, 61, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Jensen, I.; Bretschneider, A.; Stiel, S.; Wegner, F.; Höglinger, G.U.; Klietz, M. Analysis of Parkinson’s Disease Outpatient Counselling for Advance Directive Creation: A Cross-Sectional Questionnaire-Based Survey of German General Practitioners and Neurologists. Brain Sci. 2022, 12, 749. [Google Scholar] [CrossRef]
- Lohmann, L.; Lammerskitten, A.; Korsen, M.; Dodel, R.; Gaul, C.; Hamer, H.M.; Kleineberg, N.N.; Ludolph, A.C.; Mayer, G.; Poli, S.; et al. Status of clinical research in neurology in Germany—A national survey. Eur. J. Neurol. 2021, 28, 1446–1452. [Google Scholar] [CrossRef]

| Age in Years, Mean ± SD (Min–Max; n) | 52 ± 8 (33–78; 775) |
|---|---|
| Sex, n (%) | 845 |
| Male Female Divers | 175 (21) 669 (79) 1 (0.1) |
| Work location, n (%) Rural Small town (<100,000 residents) City (100,000–500,000 residents) Large city (>500,000 residents) | 846 170 (20) 317 (37) 185 (22) 174 (21) |
| Work experience in years, n (%) <5 5–10 11–20 >20 | 847 144 (17) 161 (19) 276 (33) 266 (31) |
| Clinical focus, n (MC) General gynecology, n (% of cases) Oncology, n (% of cases) Endocrinology, n (% of cases) Reproductive medicine, n (% of cases) Other focus, n (% of cases) | 842 811 (96) 34 (4) 37 (4) 14 (2) 31 (4) |
| Weekly number of patients, n (%) (MC) <50 50–100 101–150 151–200 >200 | 844 41 (5) 256 (30) 338 (40) 149 (18) 60 (7) |
| Influencing Factors | HC with COC % (n/Total *) | HC with PM % (n/Total *) |
|---|---|---|
| Cardiovascular risk | 78 (494/633) | 66 (97/147) |
| Migraine severity | 60 (373/633) | 53 (78/147) |
| Migraine frequency | 61 (383/633) | 50 (73/147) |
| Other comorbidities | 71 (447/633) | N/A |
| Migraine treatment | 26 (163/633) | 29 (43/147) |
| others | 23 (143/633) | 39 (57/147) |
| Influencing Factors | HC with COC % (n/Total *) | HC with PM % (n/Total *) |
|---|---|---|
| Cardiovascular risk | 57 (36/63) | 68 (224/332) |
| Migraine severity | 57 (36/63) | 51 (69/332) |
| Migraine frequency | 56 (35/63) | 49 (161/332) |
| Migraine aura severity | 46 (29/63) | 42 (140/332) |
| Migraine aura frequency | 46 (29/63) | 44 (147/332) |
| Other comorbidities | 54 (34/63) | N/A |
| Migraine treatment | 41 (26/63) | 39 (129/332) |
| Others | 38 (24/63) | 28 (93/332) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzek, M.P.; Storch, E.; Overeem, L.H.; Kull, P.; Terhart, M.; Lange, K.S.; Reuter, U.; Raffaelli, B. Migraine and Hormonal Contraception in Gynecological Outpatient Care—Cross-Sectional Study among Practicing Gynecologists in Germany. J. Clin. Med. 2023, 12, 1434. https://doi.org/10.3390/jcm12041434
Fitzek MP, Storch E, Overeem LH, Kull P, Terhart M, Lange KS, Reuter U, Raffaelli B. Migraine and Hormonal Contraception in Gynecological Outpatient Care—Cross-Sectional Study among Practicing Gynecologists in Germany. Journal of Clinical Medicine. 2023; 12(4):1434. https://doi.org/10.3390/jcm12041434
Chicago/Turabian StyleFitzek, Mira P., Elisabeth Storch, Lucas H. Overeem, Pia Kull, Maria Terhart, Kristin S. Lange, Uwe Reuter, and Bianca Raffaelli. 2023. "Migraine and Hormonal Contraception in Gynecological Outpatient Care—Cross-Sectional Study among Practicing Gynecologists in Germany" Journal of Clinical Medicine 12, no. 4: 1434. https://doi.org/10.3390/jcm12041434
APA StyleFitzek, M. P., Storch, E., Overeem, L. H., Kull, P., Terhart, M., Lange, K. S., Reuter, U., & Raffaelli, B. (2023). Migraine and Hormonal Contraception in Gynecological Outpatient Care—Cross-Sectional Study among Practicing Gynecologists in Germany. Journal of Clinical Medicine, 12(4), 1434. https://doi.org/10.3390/jcm12041434





