Reintervention after Thoracic Endovascular Aortic Repair of Uncomplicated Type B Aortic Dissection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. TEVAR Procedure
2.3. Data Collection and Follow-Up
2.4. Endpoints and Definitions
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Anatomical Features and TEVAR Procedure
3.3. In-Hospital and 30-Day Outcomes
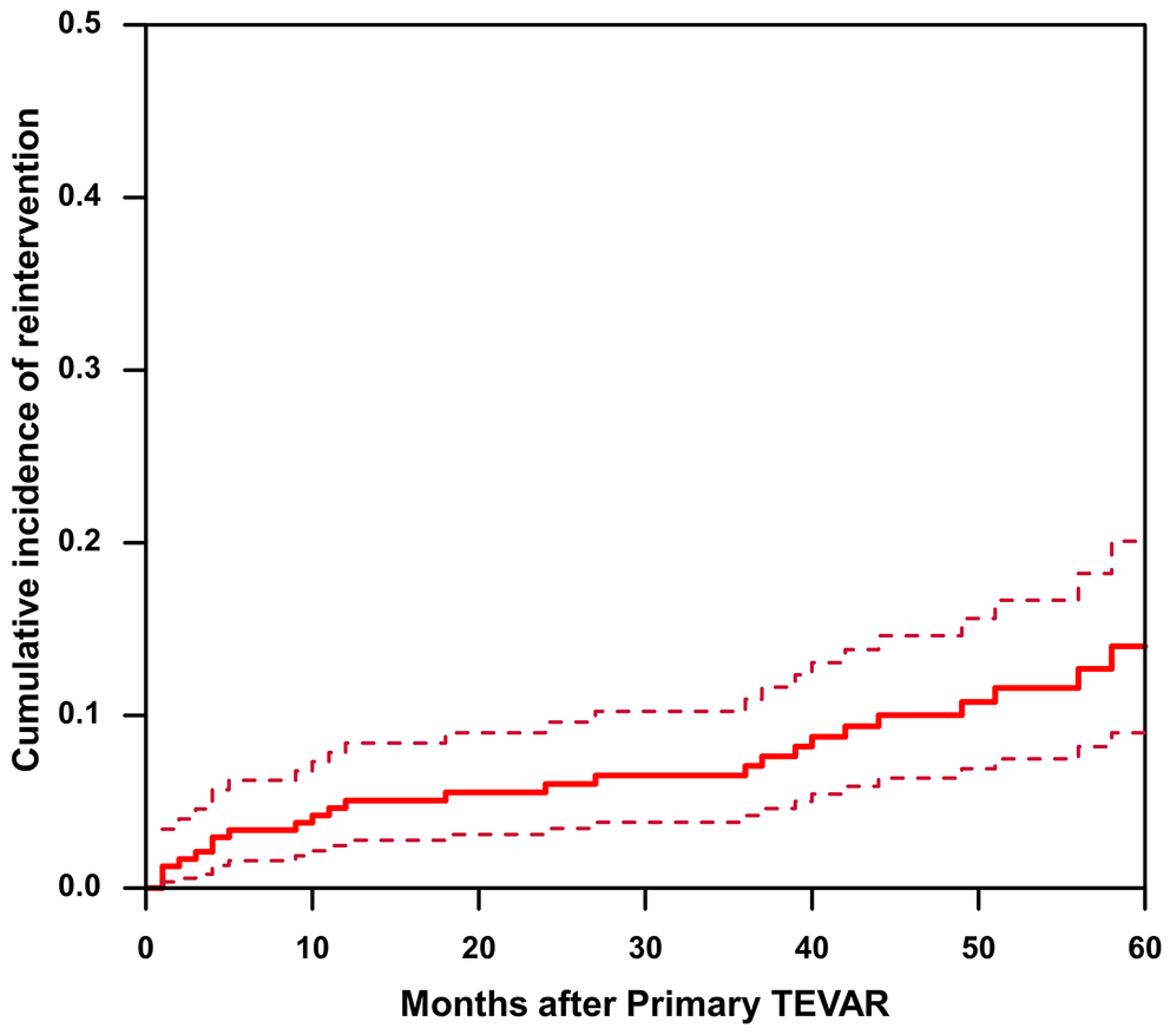
3.4. Incidence and Reasons
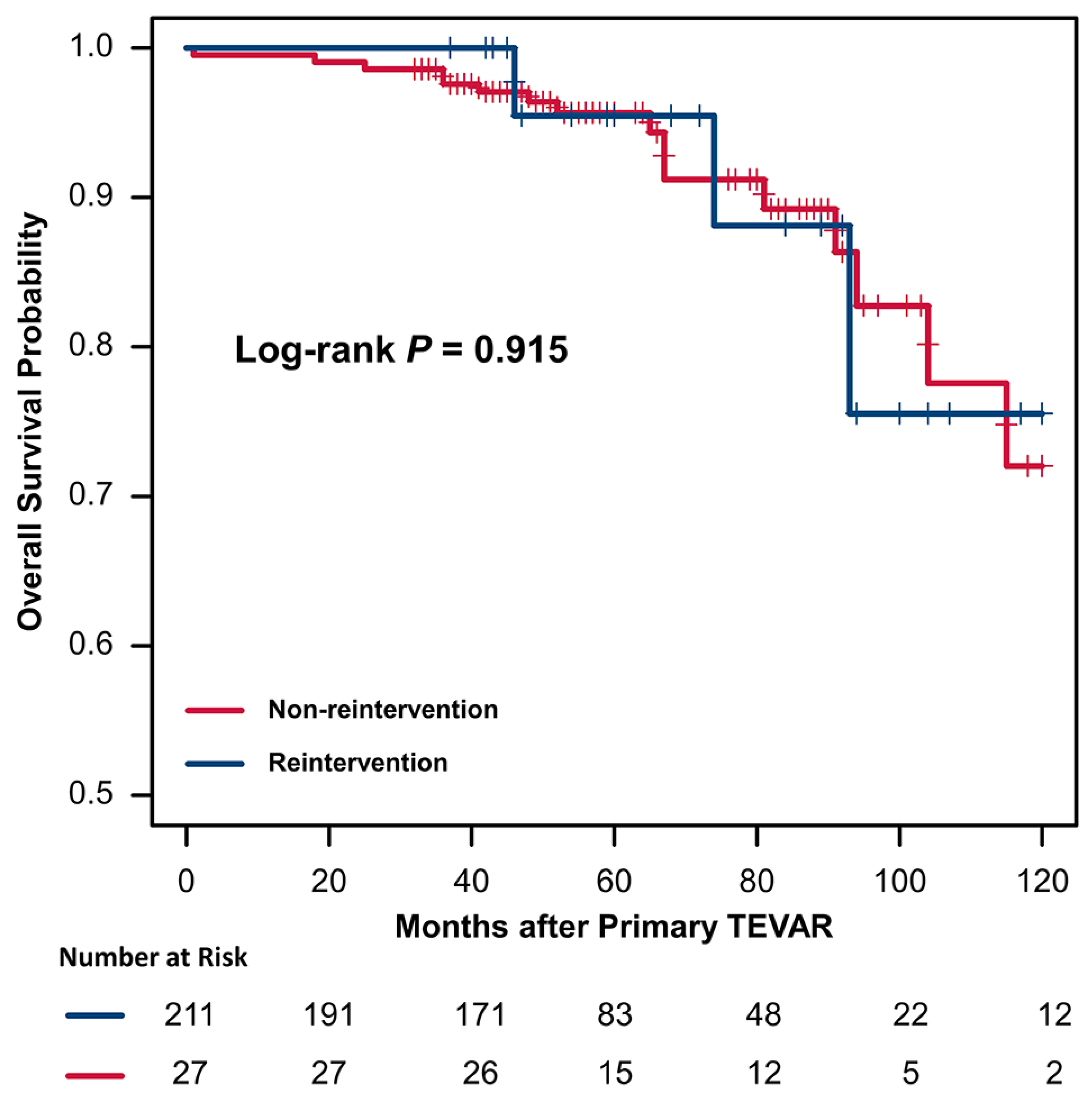
3.5. Risk Factors and Outcome of Reintervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dake, M.D.; Miller, D.C.; Semba, C.P.; Mitchell, R.S.; Walker, P.J.; Liddell, R.P. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N. Engl. J. Med. 1994, 331, 1729–1734. [Google Scholar] [CrossRef]
- Riambau, V.; Bockler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G.; Czerny, M.; Fraedrich, G.; Haulon, S.; Jacobs, M.J.; et al. Editor’s choice—Management of descending thoracic aorta diseases: Clinical practice guidelines of the european society for vascular surgery (esvs). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Di Bartolomeo, R.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 esc guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the european society of cardiology (esc). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [PubMed]
- MacGillivray, T.E.; Gleason, T.G.; Patel, H.J.; Aldea, G.S.; Bavaria, J.E.; Beaver, T.M.; Chen, E.P.; Czerny, M.; Estrera, A.L.; Firestone, S.; et al. The society of thoracic surgeons/american association for thoracic surgery clinical practice guidelines on the management of type b aortic dissection. Ann. Thorac. Surg. 2022, 113, 1073–1092. [Google Scholar] [CrossRef]
- Nienaber, C.A.; Kische, S.; Rousseau, H.; Eggebrecht, H.; Rehders, T.C.; Kundt, G.; Glass, A.; Scheinert, D.; Czerny, M.; Kleinfeldt, T.; et al. Endovascular repair of type b aortic dissection: Long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ. Cardiovasc. Interv. 2013, 6, 407–416. [Google Scholar] [CrossRef]
- Brunkwall, J.; Kasprzak, P.; Verhoeven, E.; Heijmen, R.; Taylor, P.; Alric, P.; Canaud, L.; Janotta, M.; Raithel, D.; Malina, M.; et al. Endovascular repair of acute uncomplicated aortic type b dissection promotes aortic remodelling: 1-year results of the adsorb trial. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 285–291. [Google Scholar] [CrossRef]
- Qin, Y.L.; Wang, F.; Li, T.X.; Ding, W.; Deng, G.; Xie, B.; Teng, G.J. Endovascular repair compared with medical management of patients with uncomplicated type b acute aortic dissection. J. Am. Coll. Cardiol. 2016, 67, 2835–2842. [Google Scholar] [CrossRef]
- Weissler, E.H.; Osazuwa-Peters, O.L.; Greiner, M.A.; Hardy, N.C.; Kougias, P.; O’Brien, S.M.; Mark, D.B.; Jones, W.S.; Secemsky, E.A.; Vekstein, A.M.; et al. Initial Thoracic Endovascular Aortic Repair vs Medical Therapy for Acute Uncomplicated Type B Aortic Dissection. JAMA Cardiol. 2023, 8, 44–53. [Google Scholar] [CrossRef]
- Dumfarth, J.; Michel, M.; Schmidli, J.; Sodeck, G.; Ehrlich, M.; Grimm, M.; Carrel, T.; Czerny, M. Mechanisms of failure and outcome of secondary surgical interventions after thoracic endovascular aortic repair (tevar). Ann. Thorac. Surg. 2011, 91, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Faure, E.M.; Canaud, L.; Agostini, C.; Shaub, R.; Böge, G.; Marty-ané, C.; Alric, P. Reintervention after thoracic endovascular aortic repair of complicated aortic dissection. J. Vasc. Surg. 2014, 59, 327–333. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; Lu, Q.; Zhao, Z.; Bao, J.; Jing, Z. Potential risk factors of re-intervention after endovascular repair for type b aortic dissections. Catheter. Cardiovasc. Interv. 2015, 86, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Alhussaini, M.; Arnaoutakis, G.J.; Scali, S.T.; Giles, K.A.; Fatima, J.; Back, M.; Arnaoutakis, D.; Jeng, E.I.; Martin, T.D.; Neal, D.; et al. Impact of secondary aortic interventions after thoracic endovascular aortic repair on long-term survival. Ann. Thorac. Surg. 2020, 110, 27–38. [Google Scholar] [CrossRef]
- Trimarchi, S.; Eagle, K.A.; Nienaber, C.A.; Pyeritz, R.E.; Jonker, F.H.; Suzuki, T.; O’Gara, P.T.; Hutchinson, S.J.; Rampoldi, V.; Grassi, V.; et al. Importance of refractory pain and hypertension in acute type b aortic dissection insights from the international registry of acute aortic dissection (irad). Circulation 2010, 122, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Evangelista, A.; Nienaber, C.A.; Myrmel, T.; Meinhardt, G.; Cooper, J.V.; Smith, D.E.; Suzuki, T.; Fattori, R.; Llovet, A.; et al. Partial thrombosis of the false lumen in patients with acute type b aortic dissection. N. Engl. J. Med. 2007, 357, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.; Salas, A.; Ribera, A.; Ferreira-González, I.; Cuellar, H.; Pineda, V.; González-Alujas, T.; Bijnens, B.; Permanyer-Miralda, G.; Garcia-Dorado, D. Long-term outcome of aortic dissection with patent false lumen: Predictive role of entry tear size and location. Circulation 2012, 125, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Kan, X.; Liang, H.; Xiong, B.; Liang, B.; Wang, L.; Zheng, C. Comparison of mid-term outcomes of endovascular repair and medical management in patients with acute uncomplicated type b aortic dissection. J. Thorac. Cardiovasc. Surg. 2021, 162, 26–36.e1. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Chen, Y.; Sun, Y.; Bao, J.; Jing, Z.; Zhou, J. Reintervention after endovascular repair for aortic dissection: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2016, 152, 1279–1288.e3. [Google Scholar] [CrossRef]
- Schwartz, S.I.; Durham, C.; Clouse, W.D.; Patel, V.I.; Lancaster, R.T.; Cambria, R.P.; Conrad, M.F. Predictors of late aortic intervention in patients with medically treated type b aortic dissection. J. Vasc. Surg. 2018, 67, 78–84. [Google Scholar] [CrossRef]
- Nozdrzykowski, M.; Luehr, M.; Garbade, J.; Schmidt, A.; Leontyev, S.; Misfeld, M.; Mohr, F.-W.; Etz, C.D. Outcomes of secondary procedures after primary thoracic endovascular aortic repair. Eur. J. Cardio-Thorac. 2016, 49, 770–777. [Google Scholar] [CrossRef]
- Marrocco-Trischitta, M.M.; de Beaufort, H.W.; Secchi, F.; van Bakel, T.M.; Ranucci, M.; Van Herwaarden, J.A.; Moll, F.L.; Trimarchi, S. A geometric reappraisal of proximal landing zones for thoracic endovascular aortic repair according to aortic arch types. J. Vasc. Surg. 2017, 65, 1584–1590. [Google Scholar] [CrossRef]
- Lou, X.; Duwayri, Y.M.; Jordan, W.D., Jr.; Chen, E.P.; Veeraswamy, R.K.; Leshnower, B.G. The safety and efficacy of extended tevar in acute type b aortic dissection. Ann. Thorac. Surg. 2020, 110, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Leshnower, B.G. Permissive hypertension and selective cerebrospinal fluid drainage for extended tevar for acute type b dissection: Reply. Ann. Thorac. Surg. 2020, 110, 1435–1436. [Google Scholar] [CrossRef]
- Dong, Z.H.; Fu, W.G.; Wang, Y.Q.; Guo, D.Q.; Xu, X.; Ji, Y.; Chen, B.; Jiang, J.H.; Yang, J.; Shi, Z.Y.; et al. Retrograde type a aortic dissection after endovascular stent graft placement for treatment of type b dissection. Circulation 2009, 119, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.B.; Andersen, N.D.; Bhattacharya, S.D.; Scheer, E.; Piccini, J.P.; McCann, R.L.; Hughes, G.C. Retrograde ascending aortic dissection as an early complication of thoracic endovascular aortic repair. J. Vasc. Surg. 2012, 55, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Canaud, L.; Ozdemir, B.A.; Patterson, B.O.; Holt, P.J.; Loftus, I.M.; Thompson, M.M. Retrograde aortic dissection after thoracic endovascular aortic repair. Ann. Surg. 2014, 260, 389–395. [Google Scholar] [CrossRef]
- Giles, K.A.; Beck, A.W.; Lala, S.; Patterson, S.; Back, M.; Fatima, J.; Arnaoutakis, D.J.; Arnaoutakis, G.J.; Beaver, T.M.; Berceli, S.A.; et al. Implications of secondary aortic intervention after thoracic endovascular aortic repair for acute and chronic type b dissection. J. Vasc. Surg. 2019, 69, 1367–1378. [Google Scholar] [CrossRef]
- Jang, H.; Kim, M.D.; Kim, G.M.; Won, J.Y.; Ko, Y.G.; Choi, D.; Joo, H.-C.; Lee, D.Y. Risk factors for stent graft-induced new entry after thoracic endovascular aortic repair for stanford type b aortic dissection. J. Vasc. Surg. 2017, 65, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Kasirajan, K.; Dake, M.D.; Lumsden, A.; Bavaria, J.; Makaroun, M.S. Incidence and outcomes after infolding or collapse of thoracic stent grafts. J. Vasc. Surg. 2012, 55, 652–658. [Google Scholar] [CrossRef]

| Variable | Overall (n = 238) | Non-Reintervention (n = 211) | Reintervention (n = 27) | p Value |
|---|---|---|---|---|
| Age (y) | 52.8 ± 10.7 | 52.6 ± 10.9 | 54.4 ± 9.1 | 0.397 |
| Male | 199 (83.6) | 176 (83.4) | 23 (85.2) | 0.815 |
| Hypertension | 156 (65.5) | 138 (65.4) | 18 (66.7) | 0.896 |
| Smoking | 111 (46.6) | 97 (46.0) | 14 (51.9) | 0.564 |
| Drinking | 74 (31.1) | 62 (29.4) | 12 (44.4) | 0.111 |
| Diabetes mellitus | 12 (5.0) | 11 (5.2) | 1 (3.7) | 0.736 |
| COPD | 27 (11.3) | 24 (11.4) | 3 (11.1) | 1.000 |
| Renal insufficiency | 28 (11.8) | 27 (12.8) | 1 (3.7) | 0.218 |
| Coronary artery disease | 18 (7.6) | 15 (7.1) | 3 (11.1) | 0.439 |
| Hyperlipidemia | 6 (2.5) | 6 (2.8) | 0 (0.0) | 1.000 |
| History of stroke | 13 (5.5) | 13 (6.2) | 0 (0.0) | 0.372 |
| Atherosclerosis | 83 (34.9) | 70 (33.2) | 13 (48.1) | 0.124 |
| Pleural effusion | 45 (18.9) | 38 (18.0) | 7 (25.9) | 0.323 |
| TBAD duration | 0.429 | |||
| Acute aortic dissection | 144 (60.5) | 130 (61.6) | 14 (51.9) | |
| Subacute aortic dissection | 78 (32.8) | 66 (31.3) | 12 (44.4) | |
| Chronic aortic dissection | 16 (6.7) | 15 (7.1) | 1 (3.7) | |
| Dissection morphology | 0.263 | |||
| Confined in thoracic aorta | 36 (15.1) | 30 (14.2) | 6 (22.2) | |
| Extended to abdominal aorta | 202 (84.9) | 181 (85.8) | 21 (77.8) | |
| False lumen patency | 0.778 | |||
| Patent false lumen | 135 (56.7) | 119 (56.4) | 16 (59.3) | |
| Partial thrombosis | 103 (43.3) | 92 (43.6) | 11 (40.7) | |
| Complete thrombosis | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| SBP on admission (mmHg) | 142 (127, 159) | 142 (126, 159) | 140 (127, 160) | 0.950 |
| DBP on admission (mmHg) | 83 (75, 93) | 83 (74, 91) | 84 (78, 97) | 0.335 |
| Creatinine (μmol/L) | 71.1 (62.5, 80.3) | 71.0 (61.4, 81.1) | 71.8 (65.1, 77.3) | 0.878 |
| ALT (U/L) | 23.0 (16.0, 35.0) | 24.0 (16.0, 35.0) | 20.0 (16.0, 44.0) | 0.833 |
| AST (U/L) | 20.0 (14.8, 27.0) | 20.0 (14.0, 27.0) | 20.0 (15.0, 27.0) | 0.763 |
| Symptoms at presentation | 0.488 | |||
| Chest and back pain | 204 (85.7) | 182 (86.3) | 22 (81.5) | |
| Abdominal pain | 22 (9.2) | 18 (8.5) | 4 (14.8) | |
| Other symptoms | 12 (5.0) | 11 (5.2) | 1 (3.7) |
| Variable | Non-Reintervention (n = 211) | Reintervention (n = 27) | p Value |
|---|---|---|---|
| Aortic arch classification | 0.242 | ||
| Type I | 106 (50.2) | 9 (33.3) | |
| Type II | 63 (29.9) | 10 (37.0) | |
| Type III | 42 (19.9) | 8 (29.6) | |
| Maximal aortic diameter (cm) | 3.5 (3.2, 3.8) | 3.6 (3.3, 4.5) | 0.142 |
| Proximal landing zone oversizing (%) | 4.57 ± 4.26 | 6.68 ± 6.20 | 0.067 |
| Proximal landing zone diameter (mm) | 29.81 ± 2.68 | 29.94 ± 2.93 | 0.803 |
| Main stent graft length (mm) | 175.37 ± 24.43 | 177.04 ± 22.50 | 0.737 |
| Length of covered thoracic aorta (mm) | 177.68 ± 25.69 | 182.59 ± 26.11 | 0.351 |
| Coverage ratio of thoracic aorta (%) | 62.11 ± 9.07 | 63.22 ± 9.98 | 0.555 |
| Stent graft proximal diameter (mm) | 30.88 ± 2.73 | 31.44 ± 3.19 | 0.324 |
| Proximal bare stent | 205 (97.2) | 25 (92.6) | 0.226 |
| Proximal landing zone | 0.509 | ||
| Zone 1 | 5 (2.4) | 1 (3.7) | |
| Zone 2 | 147 (69.7) | 17 (63.0) | |
| Zone 3 | 59 (28.0) | 9 (33.3) | |
| Distance between LSA and PET (mm) | 20.72 ± 13.27 | 23.31 ± 13.61 | 0.342 |
| Intentional coverage of the LSA | 48 (22.7) | 8 (29.6) | 0.427 |
| Chimney graft of the LSA | 8 (3.8) | 1 (3.7) | 0.982 |
| Chimney graft of the LCCA | 5 (2.4) | 1 (3.7) | 0.518 |
| Thoracic stent graft brand | 235 | 34 | |
| Valiant (Medtronic, Inc, Minneapolis, MN, USA) | 109 (46.4) | 15 (44.2) | 0.804 |
| E-vita (JOTEC GmbH, Hechingen, Germany) | 24 (10.2) | 2 (5.9) | 0.752 |
| Zenith TX2(Cook, Bloomington, IN, USA) | 7 (3.0) | 1 (2.9) | 1.000 |
| Hercules (Microport, Shanghai, China) | 37 (15.7) | 8 (23.5) | 0.256 |
| Ankura (Lifetech Scientific, Shenzhen, China) | 58 (24.7) | 8 (23.5) | 0.884 |
| Variable | Non-Reintervention (n = 211) | Reintervention (n = 27) | p Value |
|---|---|---|---|
| Hospital stay (days) | 10.1 ± 3.3 | 10.4 ± 3.3 | 0.602 |
| 30-day mortality | 1 (0.5) | 0 (0.0) | 1.000 |
| Adverse events | 16 (7.6) | 5 (18.5) | 0.072 |
| Acute renal failure | 2 (0.9) | 0 (0.0) | 1.000 |
| Type I endoleak | 9 (4.3) | 2 (7.4) | 0.361 |
| Retrograde type A aortic dissection | 1 (0.5) | 2 (7.4) | 0.035 |
| Stroke | 1 (0.5) | 0 (0.0) | 1.000 |
| Organ failure | 3 (1.4) | 1 (3.7) | 0.384 |
| Indications | Number (%) | Type of Reintervention | Time to Reintervention (Months after Initial TEVAR) |
|---|---|---|---|
| Endoleaks | 7 (25.9%) | 7 endovascular | 10.0 (3.0, 42.0) |
| Type I endoleak | 6 (22.2%) | 6 endovascular | 20.0 (2.5, 46.0) |
| Type III endoleak | 1 (3.7%) | 1 endovascular | 10.0 |
| RTAD | 5 (18.5%) | 5 open surgery | 9.0 (1.0, 68.0) |
| dSINE and false lumen expansion | 5 (18.5%) | 5 endovascular | 5.0 (3.0, 20.0) |
| Aneurysmal dilation (non-dSINE causes) | 6 (22.2%) | 5 endovascular; 1 open surgery | 47.5 (39.3, 64.5) |
| Dissection progression and/or malperfusion | 4 (14.8%) | 3 endovascular; 1 open surgery | 26.5 (11.8, 46.5) |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (y) | 1.02 (0.98–1.06) | 0.347 | ||
| Male | 1.05 (0.36–3.04) | 0.932 | ||
| Hypertension | 0.97 (0.44–2.17) | 0.946 | ||
| TBAD duration | ||||
| Acute aortic dissection | Ref | |||
| Subacute aortic dissection | 1.62 (0.75–3.49) | 0.222 | ||
| Chronic aortic dissection | 0.52 (0.07–3.96) | 0.528 | ||
| Dissection morphology | 1.80 (0.72–4.49) | 0.209 | ||
| Patent false lumen | 1.16 (0.54–2.51) | 0.700 | ||
| Aortic arch classification | ||||
| Type I | Ref | |||
| Type II | 1.65 (0.69–4.07) | 0.278 | ||
| Type III | 2.13 (0.81–5.52) | 0.121 | ||
| Maximal aortic diameter (mm) | 1.65 (1.08–2.52) | 0.022 | 1.75 (1.13–2.69) | 0.011 |
| Proximal landing zone diameter (mm) | 1.01 (0.87–1.16) | 0.922 | ||
| Proximal landing zone oversizing (%) | 1.06 (0.99–1.13) | 0.063 | 1.07 (1.01–1.47) | 0.033 |
| Main stent graft length (mm) | 1.01 (0.99–1.02) | 0.534 | ||
| Length of covered thoracic aorta (mm) | 1.10 (0.96–1.27) | 0.169 | ||
| Coverage ratio of thoracic aorta (%) | 1.02 (0.98–1.06) | 0.363 | ||
| Stent graft proximal diameter (mm) | 1.05 (0.91–1.20) | 0.516 | ||
| Proximal bare stent | 1.83 (0.42–8.04) | 0.425 | ||
| Proximal landing zone | ||||
| Zone 1 | Ref | |||
| Zone 2 | 0.85 (0.11–6.56) | 0.878 | ||
| Zone 3 | 0.99 (0.12–8.01) | 0.996 | ||
| Distance between LSA and PET (mm) | 1.02 (0.99–1.04) | 0.250 | ||
| Intentional coverage of the LSA | 0.83 (0.36–1.89) | 0.652 | ||
| Chimney graft of the LSA | 0.77 (0.10–5.69) | 0.795 | ||
| Chimney graft of the LCCA | 0.56 (0.08–4.15) | 0.570 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Xiang, D.; Zhang, S.; Zheng, C.; Wu, X. Reintervention after Thoracic Endovascular Aortic Repair of Uncomplicated Type B Aortic Dissection. J. Clin. Med. 2023, 12, 1418. https://doi.org/10.3390/jcm12041418
Cheng L, Xiang D, Zhang S, Zheng C, Wu X. Reintervention after Thoracic Endovascular Aortic Repair of Uncomplicated Type B Aortic Dissection. Journal of Clinical Medicine. 2023; 12(4):1418. https://doi.org/10.3390/jcm12041418
Chicago/Turabian StyleCheng, Li, Dongqiao Xiang, Shan Zhang, Chuansheng Zheng, and Xiaoyan Wu. 2023. "Reintervention after Thoracic Endovascular Aortic Repair of Uncomplicated Type B Aortic Dissection" Journal of Clinical Medicine 12, no. 4: 1418. https://doi.org/10.3390/jcm12041418
APA StyleCheng, L., Xiang, D., Zhang, S., Zheng, C., & Wu, X. (2023). Reintervention after Thoracic Endovascular Aortic Repair of Uncomplicated Type B Aortic Dissection. Journal of Clinical Medicine, 12(4), 1418. https://doi.org/10.3390/jcm12041418






