Pediatric Laryngopharyngeal Reflux in the Last Decade: What Is New and Where to Next?
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. LPR Versus GER
3.2. Epidemiology
3.3. Pathophysiology
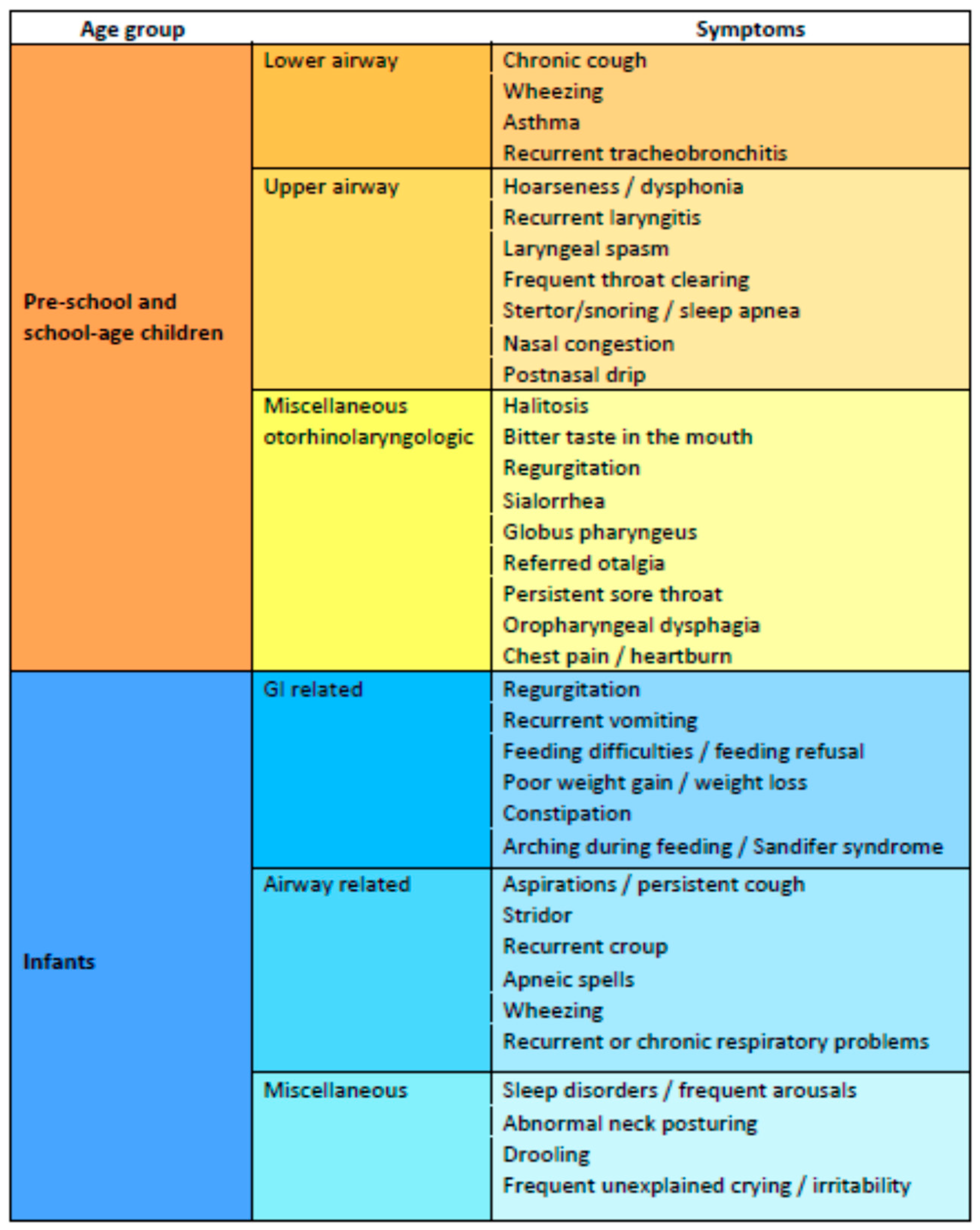
3.4. Symptoms
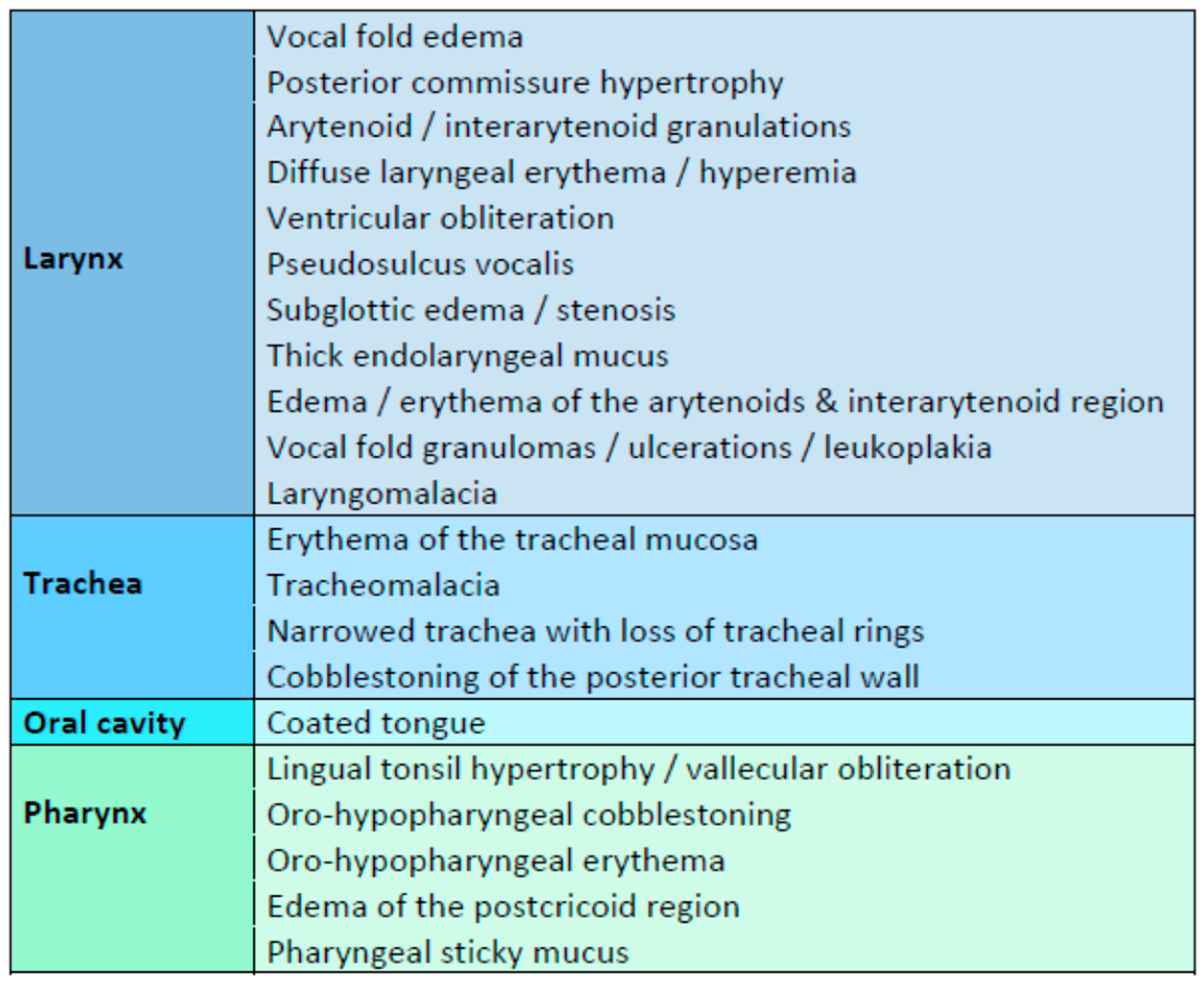
3.5. Endoscopic Findings
3.6. Assessment—Diagnostic Tools
3.6.1. Reflux Symptom Index (RSI), Reflux Finding Score (RFS), and Other Clinical Scoring Systems
3.6.2. Multichannel Intraluminal Impedance-pH Monitoring (MII-pH)
3.6.3. 24-h Pharyngeal pH Monitoring
3.6.4. Pepsin A as a Biomarker
3.7. Management
3.7.1. Behavioral Modifications
- Altering food composition. Thickening of milk and feedings with starch, cereals, carob bean thickeners, or xantham gum improves laryngeal sensation and overall swallowing function and may help in reducing regurgitations and the signs and symptoms of reflux disease as suggested by two systematic reviews of the relevant literature [103,104].
- Use smaller but more frequent feedings while maintaining an appropriate total daily amount of formula or breastmilk [107].
- Avoidance of feeding just before bedtime.
- Adjusting the child’s or mother’s (in case of breastfeeding) diet to eliminate substances that increase the number of transient relaxations of esophageal sphincters, such as chocolate, caffeine, alcohol, mint, peppermint, fried and fatty foods, etc.
- Avoidance of acidic or spicy meals or beverages, such as citrus fruits and juices or tomatoes and tomato-based foods. Carbonated beverages may cause gastric bloating and should also be avoided. Keeping a diary of food/beverage intake is often helpful.
- Elimination of secondhand smoke. Tobacco is a common cause of GER/LPR in adults, and environmental smoking was also implied to cause reflux in infants and neonates [108].
- Keeping an infant in a relatively vertical position for at least 30 min after feeding.
- Elevating the head of the bed may be used in preschool and school-aged children but is not recommended in infants and children younger than two years of age, as it is uncertain that it helps reduce reflux, but, most importantly, because of the risk of suffocating, as it may cause the baby to slide down to the foot of the cot or under the beddings [3,104,109,110].
- Sleep positioning may provide further benefits to infants; however, the ideal position remains a subject of debate [66,111]. Importantly, because of sudden infant death syndrome (SIDS) concerns, the supine sleeping position is considered safer, as the prone and side sleeping positions increase the risk of SIDS significantly [109,112]. Currently, the American Academy of Pediatrics advises that the supine position is recommended in infants with GER. Alternative positioning during sleep is considered reasonable only exceptionally in infants with specific upper airway disorders in which the risk of death from GERD/LPRD may outweigh the risk of SIDS [109].
3.7.2. Pharmacotherapy
Proton Pump Inhibitors
Alginates
3.7.3. Anti-Reflux Surgery
3.8. Summary and Future Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koufman, J.A.; Aviv, J.E.; Casiano, R.R.; Shaw, G.Y. Laryngopharyngeal Reflux: Position Statement of the Committee on Speech, Voice, and Swallowing Disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol. Head Neck Surg. 2002, 127, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Akst, L.M.; Hamdan, A.L.; Schindler, A.; Karkos, P.D.; Barillari, M.R.; Calvo-Henriquez, C.; Crevier-Buchman, L.; Finck, C.; Eun, Y.G.; et al. Evaluation and Management of Laryngopharyngeal Reflux Disease: State of the Art Review. Otolaryngol. Head Neck Surg. 2019, 160, 762–782. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Vandenplas, Y.; Singendonk, M.; Cabana, M.; Dilorenzo, C.; Gottrand, F.; Gupta, S.; Langendam, M.; Staiano, A.; Thapar, N.; et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 516–554. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Sacré-Smits, L. Continuous 24-Hour Esophageal PH Monitoring in 285 Asymptomatic Infants 0–15 Months Old. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Lightdale, J.R.; Gremse, D.A.; Heitlinger, L.A.; Cabana, M.; Gilger, M.A.; Gugig, R.; Hill, I.D. Gastroesophageal Reflux: Management Guidance for the Pediatrician. Pediatrics 2013, 131, e1684–e1695. [Google Scholar] [CrossRef] [PubMed]
- Górecka-Tuteja, A.; Jastrzebska, I.; Składzien, J.; Fyderek, K. Laryngopharyngeal Reflux in Children with Chronic Otitis Media with Effusion. J. Neurogastroenterol. Motil. 2016, 22, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Hans, S.; Simon, F.; Horoi, M.; Calvo-Henriquez, C.; Chiesa-Estomba, C.M.; Mayo-Yáñez, M.; Bartel, R.; Piersiala, K.; Nguyen, Y.; et al. Association Between Laryngopharyngeal Reflux and Media Otitis: A Systematic Review. Otol. Neurotol. 2021, 42, e801–e814. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Henriquez, C.C.; Mouawad, F.; Ristagno, C.; Barillari, M.R.; Schindler, A.; Nacci, A.; Bouland, C.; Laino, L.; et al. Laryngopharyngeal Reflux, Gastroesophageal Reflux and Dental Disorders: A Systematic Review. PLoS ONE 2020, 15, e0237581. [Google Scholar] [CrossRef]
- Abdel-aziz, M.M.; El-Fattah, A.M.A.; Abdalla, A.F. Clinical Evaluation of Pepsin for Laryngopharyngeal Reflux in Children with Otitis Media with Effusion. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1765–1770. [Google Scholar] [CrossRef]
- Nation, J.; Kaufman, M.; Allen, M.; Sheyn, A.; Coticchia, J. Incidence of Gastroesophageal Reflux Disease and Positive Maxillary Antral Cultures in Children with Symptoms of Chronic Rhinosinusitis. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 218–222. [Google Scholar] [CrossRef]
- Iannella, G.; Di Nardo, G.; Plateroti, R.; Rossi, P.; Plateroti, A.M.; Mariani, P.; Magliulo, G. Investigation of Pepsin in Tears of Children with Laryngopharyngeal Reflux Disease. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 2312–2315. [Google Scholar] [CrossRef]
- Önal, Z.; Çullu-Çokuğraş, F.; Işıldak, H.; Kaytaz, A.; Kutlu, T.; Erkan, T.; Doğusoy, G. Evaluation of the Likelihood of Reflux Developing in Patients with Recurrent Upper Respiratory Infections, Recurrent Sinusitis or Recurrent Otitis Seen in Ear-Nose-Throat Outpatient Clinics. Turk. J. Pediatr. 2015, 57, 258–265. [Google Scholar] [PubMed]
- Luebke, K.; Samuels, T.L.; Chelius, T.H.; Sulman, C.G.; McCormick, M.E.; Kerschner, J.E.; Johnston, N.; Chun, R.H. Pepsin as a Biomarker for Laryngopharyngeal Reflux in Children with Laryngomalacia. Laryngoscope 2017, 127, 2413–2417. [Google Scholar] [CrossRef]
- Formánek, M.; Komínek, P.; Jančatová, D.; Staníková, L.; Tomanová, R.; Vaculová, J.; Urík, M.; Šlapák, I.; Zeleník, K. Laryngopharyngeal Reflux Is a Potential Risk Factor for Juvenile-Onset Recurrent Respiratory Papillomatosis. BioMed Res. Int. 2019, 2019, 1463896. [Google Scholar] [CrossRef] [PubMed]
- Dziekiewicz, M.; Cudejko, R.; Banasiuk, M.; Dembiński, Ł.; Skarżyński, H.; Radzikowski, A.; Banaszkiewicz, A. Frequency of Gastroesophageal Reflux Disease in Children with Adenoid Hypertrophy. Int. J. Pediatr. Otorhinolaryngol. 2020, 138, 110304. [Google Scholar] [CrossRef] [PubMed]
- Tumgor, G.; Midilli, R.; Doganavsargil, B.; Ozgenc, F.; Arikan, C.; Kirazli, T.; Yagci, R.V. Gastroesophageal Reflux with Children Requiring Adenotonsillectomy. Minerva Pediatr. 2021, 73, 256–262. [Google Scholar] [CrossRef]
- Salturk, Z.; Kumral, T.L.; Arslanoglu, A.; Aydogdu, I.; Yildirim, G.; Berkiten, G.; Uyar, Y. Role of Laryngopharyngeal Reflux in Complications of Tonsillectomy in Pediatric Patients. Indian J. Otolaryngol. Head Neck Surg. 2017, 69, 392–396. [Google Scholar] [CrossRef]
- Lee, J.A.; Schaffer, C.E.; Mehta, C.H.; Close, M.F.; Nguyen, S.A.; Meyer, T.A. Impact of Early Gastroesophageal Reflux Disease on Childhood Otologic Outcomes. Int. J. Pediatr. Otorhinolaryngol. 2020, 134, 110069. [Google Scholar] [CrossRef]
- Zoizner-Agar, G.; Rotsides, J.M.; Shao, Q.; Rickert, S.; Ward, R.; Greifer, M.; April, M. Proton Pump Inhibitor Administration in Neonates and Infants. Lack of Consensus—An ASPO Survey. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110200. [Google Scholar] [CrossRef]
- Mahoney, L.B.; Esther, C.R.; May, K.; Rosen, R. Metabolomic Profiling of Extraesophageal Reflux Disease in Children. Clin. Transl. Sci. 2021, 14, 2025–2033. [Google Scholar] [CrossRef]
- Samuels, T.L.; Khampang, P.; Espahbodi, M.; McCormick, C.A.; Chun, R.H.; McCormick, M.E.; Yan, K.; Kerschner, J.E.; Johnston, N. Association of Pepsin With Inflammatory Signaling and Effusion Viscosity in Pediatric Otitis Media. Laryngoscope 2022, 132, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Ugras, M.K.; Dogan, M.; Pata, D.Y.S.; Ozkan, F. Can the Reflux Finding Score and Reflux Symptom Index Be Used to Evaluate the Severity of Esophagitis in Children? J. Voice 2021, 35, e7–e157. [Google Scholar] [CrossRef]
- Lei, L.; Yu, Z.; Yu, R.; Yang, H.; Zou, J.; Ren, J.; Zhang, J.; Zhong, D. Correlation of Pathogenic Effects of Laryngopharyngeal Reflux and Bacterial Infection in COME of Children. Acta Otolaryngol. 2021, 141, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.B.; Yvette War, G.; Shukla, S.; Kaur, R.; Malhotra, S.; Kumar, S. The Role of Helicobacter Pylori and Laryngopharyngeal Reflux in Recurrent Tonsillitis. Int. J. Pediatr. Otorhinolaryngol. 2020, 138, 110376. [Google Scholar] [CrossRef]
- Mantegazza, C.; Mallardo, S.; Rossano, M.; Meneghin, F.; Ricci, M.; Rossi, P.; Capra, G.; Latorre, P.; Schindler, A.; Isoldi, S.; et al. Laryngeal Signs and PH-Multichannel Intraluminal Impedance in Infants and Children: The Missing Ring: LPR and MII-PH in Children. Dig. Liver Dis. 2020, 52, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Upendran, Y.; Leach, S.T.; Singh, H.; McBride, J.; Thomas, P.S.; Belessis, Y.; Krishnan, U. Pepsin as a Marker of Reflux Aspiration in Children With Esophageal Atresia: A Pilot Study. Front. Pediatr. 2020, 8, 94. [Google Scholar] [CrossRef]
- Wertz, A.; Carroll, L.M.; Zur, K.B. Pediatric Laryngopharyngeal Reflux: Perceptual, Acoustic, and Laryngeal Findings. Int. J. Pediatr. Otorhinolaryngol. 2020, 133, 109974. [Google Scholar] [CrossRef] [PubMed]
- Košec, A.; Žaja, O.; Matovinović, F.; Jelavić, B.; Baudoin, T. Significance of Extra-Esophageal Symptoms in Pediatric Gastroesophageal Reflux Disease. Int. Arch. Otorhinolaryngol. 2020, 24, 472–476. [Google Scholar] [CrossRef]
- Maholarnkij, S.; Sanpavat, A.; Decharun, K.; Dumrisilp, T.; Tubjareon, C.; Kanghom, B.; Patcharatrakul, T.; Chaijitraruch, N.; Chongsrisawat, V.; Sintusek, P. Detection of Reflux-Symptom Association in Children with Esophageal Atresia by Video-PH-Impedance Study. World J. Gastroenterol. 2020, 26, 4159–4169. [Google Scholar] [CrossRef]
- Plocek, A.; Gębora-Kowalska, B.; Białek, J.; Fendler, W.; Toporowska-Kowalska, E. Esophageal Impedance-PH Monitoring and Pharyngeal PH Monitoring in the Diagnosis of Extraesophageal Reflux in Children. Gastroenterol. Res. Pract. 2019, 2019, 6271910. [Google Scholar] [CrossRef]
- Galli, J.; Meucci, D.; Salonna, G.; Anzivino, R.; Giorgio, V.; Trozzi, M.; Settimi, S.; Tropiano, M.L.; Paludetti, G.; Bottero, S. Use OF NBI for the Assessment of Clinical Signs of Rhino-Pharyngo-Laryngeal Reflux in Pediatric Age: Preliminary Results. Int. J. Pediatr. Otorhinolaryngol. 2020, 128, 109733. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, E.; Jetka, T.; Raj-Koziak, D.; Panasiewicz, A.; Szkiełkowska, A.; Skarżyński, P.H.; Skarżyński, H. Diagnosis of Laryngopharyngeal Reflux in Children with Voice Disorders Using 24-Hour Pharyngeal PH Monitoring. Int. J. Pediatr. Otorhinolaryngol. 2019, 121, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Klimara, M.J.; Johnston, N.; Samuels, T.L.; Visotcky, A.M.; Poetker, D.M.; Loehrl, T.A.; Blumin, J.H.; Bock, J.M. Correlation of Salivary and Nasal Lavage Pepsin with MII-PH Testing. Laryngoscope 2020, 130, 961–966. [Google Scholar] [CrossRef]
- Baran, M.; Cagan Appak, Y.; Karakoyun, M.; Yalcinkaya, S.; Eliacik, K.; Dundar, B.N. The Overlap of Gastroesophageal Reflux Disease and Functional Constipation in Children: The Efficacy of Constipation Treatment. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Siupsinskiene, N.; Katutiene, I.; Jonikiene, V.; Janciauskas, D.; Vaitkus, S. Helicobacter Pylori in the Tonsillar Tissue: A Possible Association with Chronic Tonsillitis and Laryngopharyngeal Reflux. J. Laryngol. Otol. 2017, 131, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Pavić, I.; Babić, I.; Čepin Bogović, J.; Hojsak, I. The Importance of Combined 24-Hour Multichannel Intraluminal Impedance–PH Monitoring in the Evaluation of Children with Suspected Laryngopharyngeal Reflux. Clin. Otolaryngol. 2017, 42, 544–549. [Google Scholar] [CrossRef]
- Mesallam, T.A. Oropharyngeal 24-Hour PH Monitoring in Children with Airway-Related Problems. Clin. Exp. Otorhinolaryngol. 2016, 9, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Singendonk, M.M.J.; Pullens, B.; van Heteren, J.A.A.; de Gier, H.H.W.; Hoeve, H.L.J.; König, A.M.; van der Schroeff, M.P.; Hoekstra, C.E.L.; Veder, L.L.; van der Pol, R.J.; et al. Reliability of the Reflux Finding Score for Infants in Flexible versus Rigid Laryngoscopy. Int. J. Pediatr. Otorhinolaryngol. 2016, 86, 37–42. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, H.S.; Kim, K.M.; Lee, Y.J.; Jung, M.H.; Park, J.J.; Kim, J.P.; Woo, S.H. Extra-Esophageal Pepsin from Stomach Refluxate Promoted Tonsil Hypertrophy. PLoS ONE 2016, 11, e0152336. [Google Scholar] [CrossRef]
- Duncan, D.R.; Amirault, J.; Johnston, N.; Mitchell, P.; Larson, K.; Rosen, R.L. Gastroesophageal Reflux Burden, Even in Children That Aspirate, Does Not Increase Pediatric Hospitalization. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 210–217. [Google Scholar] [CrossRef]
- Dy, F.; Amirault, J.; Mitchell, P.D.; Rosen, R. Salivary Pepsin Lacks Sensitivity as a Diagnostic Tool to Evaluate Extraesophageal Reflux Disease. J. Pediatr. 2016, 177, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Doğru, M.; Kuran, G.; Haytoğlu, S.; Dengiz, R.; Arıkan, O.K. Role of Laryngopharyngeal Reflux in the Pathogenesis of Otitis Media with Effusion. J. Int. Adv. Otol. 2015, 11, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Formánek, M.; Komínek, P.; Matoušek, P.; Tomanova, R.; Urban, O.; Zeleník, K. Comparison of Three Methods Used in the Diagnosis of Extraesophageal Reflux in Children with Chronic Otitis Media with Effusion. Gastroenterol. Res. Pract. 2015, 2015, 547959. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, R.C.; Soundar, S.; Tonb, D.; Bolling, L.; Yoo, E.; Nadal, T.; Grindle, C.; Field, E.; He, Z. The Role of Gastric Pepsin in the Inflammatory Cascade of Pediatric Otitis Media. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Formánek, M.; Zeleník, K.; Komínek, P.; Matoušek, P. Diagnosis of Extraesophageal Reflux in Children with Chronic Otitis Media with Effusion Using Peptest. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 677–679. [Google Scholar] [CrossRef]
- Luo, H.N.; Yang, Q.M.; Sheng, Y.; Wang, Z.H.; Zhang, Q.; Yan, J.; Hou, J.; Zhu, K.; Cheng, Y.; Wang, B.T.; et al. Role of Pepsin and Pepsinogen: Linking Laryngopharyngeal Reflux with Otitis Media with Effusion in Children. Laryngoscope 2014, 124, E294–E300. [Google Scholar] [CrossRef]
- Baudoin, T.; Kosec, A.; Cor, I.S.; Zaja, O. Clinical Features and Diagnostic Reliability in Paediatric Laryngopharyngeal Reflux. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1101–1106. [Google Scholar] [CrossRef]
- Van Der Pol, R.J.; Singendonk, M.M.J.; König, A.M.; Hoeve, H.; Kammeijer, Q.; Pullens, B.; Van Spronsen, E.; Thomas, G.; Vermeeren, L.; Benninga, M.A.; et al. Development of the Reflux Finding Score for Infants and Its Observer Agreement. J. Pediatr. 2014, 165, 479–484. [Google Scholar] [CrossRef]
- Katra, R.; Kabelka, Z.; Jurovcik, M.; Hradsky, O.; Kraus, J.; Pavlik, E.; Nartova, E.; Lukes, P.; Astl, J. Pilot Study: Association between Helicobacter Pylori in Adenoid Hyperplasia and Reflux Episodes Detected by Multiple Intraluminal Impedance in Children. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1243–1249. [Google Scholar] [CrossRef]
- Aydin, E.; Aydoǧan, F.; Taştan, E.; Arslan, N.; Karaca, G. Does Helicobacter Pylori Have a Role in the Etiology of Adenoid Hypertrophy? Indian J. Otolaryngol. Head Neck Surg. 2014, 66 (Suppl. 1), 65–70. [Google Scholar] [CrossRef]
- Rosen, R.; Amirault, J.; Johnston, N.; Haver, K.; Khatwa, U.; Rubinstein, E.; Nurko, S. The Utility of Endoscopy and Multichannel Intraluminal Impedance Testing in Children with Cough and Wheezing. Pediatr. Pulmonol. 2014, 49, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Andrews, T.M.; Orobello, N. Histologic versus PH Probe Results in Pediatric Laryngopharyngeal Reflux. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.A.; Parakininkas, D.E.; Werlin, S.L.; Southern, J.F.; Johnston, N.; Kerschner, J.E. Prevalence of Pediatric Aspiration-Associated Extraesophageal Reflux Disease. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 996–1001. [Google Scholar] [CrossRef]
- Kilic, M.; Ozturk, F.; Kirmemis, O.; Atmaca, S.; Guner, S.N.; Caltepe, G.; Sancak, R.; Kalayci, A.G. Impact of Laryngopharyngeal and Gastroesophageal Reflux on Asthma Control in Children. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Ummarino, D.; Miele, E.; Masi, P.; Tramontano, A.; Staiano, A.; Vandenplas, Y. Impact of Antisecretory Treatment on Respiratory Symptoms of Gastroesophageal Reflux Disease in Children. Dis. Esophagus 2012, 25, 671–677. [Google Scholar] [CrossRef]
- Greifer, M.; Ng, K.; Levine, J. Impedance and Extraesophageal Manifestations of Reflux in Pediatrics. Laryngoscope 2012, 122, 1397–1400. [Google Scholar] [CrossRef]
- Banaszkiewicz, A.; Dembinski, L.; Zawadzka-Krajewska, A.; Dziekiewicz, M.; Albrecht, P.; Kulus, M.; Radzikowski, A. Evaluation of Laryngopharyngeal Reflux in Pediatric Patients with Asthma Using a New Technique of Pharyngeal PH-Monitoring. Adv. Exp. Med. Biol. 2013, 755, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Baran, M.; Özgenç, F.; Arikan, Ç.; Çakir, M.; Ecevit, Ç.Ö.; Aydoǧdu, S.; Yaǧci, R.V. Gastroesophageal Reflux in Children with Functional Constipation. Turkish J. Gastroenterol. 2012, 23, 634–638. [Google Scholar] [CrossRef]
- Ozmen, S.; Demirceken, F.; Barut, Y.; Dibek Misirlioglu, E. Role of Laryngoscopy in Children with Respiratory Complaints and Suspected Reflux. Allergol. Immunopathol. 2012, 40, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Koufman, J.A. The Otolaryngologic Manifestations of Gastroesophageal Reflux Disease (Gerd): A Clinical Investigation of 225 Patients Using Ambulatory 24-Hour Ph Monitoring and an Experimental Investigation of the Role of Acid and Pepsin in the Development of Laryngeal. Laryngoscope 1991, 101, 1–78. [Google Scholar] [CrossRef]
- Babaei, A.; Venu, M.; Naini, S.R.; Gonzaga, J.; Lang, I.M.; Massey, B.T.; Jadcherla, S.; Shaker, R. Impaired Upper Esophageal Sphincter Reflexes in Patients with Supraesophageal Reflux Disease. Gastroenterology 2015, 149, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, E.C.; Blumin, J.H.; Bock, J.M. Dual PH with Multichannel Intraluminal Impedance Testing in the Evaluation of Subjective Laryngopharyngeal Reflux Symptoms. Otolaryngol. Head Neck. Surg. 2016, 155, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, D.C.; Shuck, T.J.; Bordeaux, R.A.; Winship, D.H. Human Upper Esophageal Sphincter. Response to Volume, Osmotic, and Acid Stimuli. Gastroenterology 1978, 75, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Vincent, D.A.; Garrett, J.D.; Radionoff, S.L.; Reussner, L.A.; Stasney, C.R. The Proximal Probe in Esophageal PH Monitoring: Development of a Normative Database. J. Voice 2000, 14, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Koufman, J.A. Laryngopharyngeal Reflux Is Different from Classic Gastroesophageal Reflux Disease. Ear Nose Throat J. 2002, 81 (Suppl. 2), 7–9. [Google Scholar]
- Czinn, S.J.; Blanchard, S. Gastroesophageal Reflux Disease in Neonates and Infants: When and How to Treat. Pediatr. Drugs 2013, 15, 19–27. [Google Scholar] [CrossRef]
- Nelson, S.P.; Chen, E.H.; Syniar, G.M.; Christoffel, K.K. Prevalence of Symptoms of Gastroesophageal Reflux during Infancy: A Pediatric Practice-Based Survey. Arch. Pediatr. Adolesc. Med. 1997, 151, 569–572. [Google Scholar] [CrossRef]
- Karkos, P.D.; Leong, S.C.; Apostolidou, M.T.; Apostolidis, T. Laryngeal Manifestations and Pediatric Laryngopharyngeal Reflux. Am. J. Otolaryngol. Head Neck Med. Surg. 2006, 27, 200–203. [Google Scholar] [CrossRef]
- Rybak, A.; Pesce, M.; Thapar, N.; Borrelli, O. Gastro-Esophageal Reflux in Children. Int. J. Mol. Sci. 2017, 18, 1671. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Rudolph, C.D.; Di Lorenzo, C.; Hassall, E.; Liptak, G.; Mazur, L.; Sondheimer, J.; Staiano, A.; Thomson, M.; Veereman-Wauters, G.; et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J. Pediatr. Gastroenterol. Nutr. 2009, 49, 498–547. [Google Scholar] [CrossRef]
- Koebnick, C.; Getahun, D.; Smith, N.; Porter, A.H.; Der-Sarkissian, J.K.; Jacobsen, S.J. Extreme Childhood Obesity Is Associated with Increased Risk for Gastroesophageal Reflux Disease in a Large Population-Based Study. Int. J. Pediatr. Obes. 2011, 6, e257–e263. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, A.M.; Priston, J.; Thoen, R.H.; Medeiros, T.; Assunção, A.R. Laryngopharyngeal Reflux: Diagnosis, Treatment, and Latest Research. Int. Arch. Otorhinolaryngol. 2014, 18, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.; Machado, R.; Orsi, M.; Chao, C.S.; Alhajj, T.; Alhajj, M.; Port, C.; Skaggs, B.; Woodley, F.W. Combined Multichannel Intraluminal Impedance-PH (MII-PH): Multicenter Report of Normal Values from 117 Children. Curr. Gastroenterol. Rep. 2014, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Pilic, D.; Fröhlich, T.; Nöh, F.; Pappas, A.; Schmidt-Choudhury, A.; Köhler, H.; Skopnik, H.; Wenzl, T.G. Detection of Gastroesophageal Reflux in Children Using Combined Multichannel Intraluminal Impedance and PH Measurement: Data from the German Pediatric Impedance Group. J. Pediatr. 2011, 158, 650–654.e1. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Arrigo, S.; Luini, C.; Vandenplas, Y. Esophageal Impedance in Children: Symptom-Based Results. J. Pediatr. 2010, 157, 949–954.e2. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, K.; Mertens, V.; Dupont, L.; Pauwels, A.; Farré, R.; Malfroot, A.; De Wachter, E.; De Schutter, I.; Hauser, B.; Vandenplas, Y.; et al. The Relationship between Gastroesophageal Reflux and Cough in Children with Chronic Unexplained Cough Using Combined Impedance-PH-Manometry Recordings. Pediatr. Pulmonol. 2011, 46, 286–294. [Google Scholar] [CrossRef]
- Borrelli, O.; Marabotto, C.; Mancini, V.; Aloi, M.; MacRì, F.; Falconieri, P.; Lindley, K.J.; Cucchiara, S. Role of Gastroesophageal Reflux in Children with Unexplained Chronic Cough. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 287–292. [Google Scholar] [CrossRef]
- Ghezzi, M.; Silvestri, M.; Guida, E.; Pistorio, A.; Sacco, O.; Mattioli, G.; Jasonni, V.; Rossi, G.A. Acid and Weakly Acid Gastroesophageal Refluxes and Type of Respiratory Symptoms in Children. Respir. Med. 2011, 105, 972–978. [Google Scholar] [CrossRef]
- Sereg-Bahar, M.; Jerin, A.; Jansa, R.; Stabuc, B.; Hocevar-Boltezar, I. Pepsin and Bile Acids in Saliva in Patients with Laryngopharyngeal Reflux—A Prospective Comparative Study. Clin. Otolaryngol. 2015, 40, 234–239. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Zhou, B.; Tang, Y.; Liu, X.; Wu, Y.; Wang, Y.; Kong, J.; Xu, T.; He, C.; et al. Effects of Acids, Pepsin, Bile Acids, and Trypsin on Laryngopharyngeal Reflux Diseases: Physiopathology and Therapeutic Targets. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 2743–2752. [Google Scholar] [CrossRef]
- Johnston, N.; Dettmar, P.W.; Bishwokarma, B.; Lively, M.O.; Koufman, J.A. Activity/Stability of Human Pepsin: Implications for Reflux Attributed Laryngeal Disease. Laryngoscope 2007, 117, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Samuels, T.L.; Johnston, N. Pepsin as a Causal Agent of Inflammation during Nonacidic Reflux. Otolaryngol. Head Neck Surg. 2009, 141, 559–563. [Google Scholar] [CrossRef]
- Bulmer, D.; Ross, P.E.; Axford, S.E.; Johnston, N.; Gill, G.A.; Pearson, J.P.; Dettmar, P.W.; Panetti, M.; Pignatelli, M.; Koufman, J.A. Cell Biology of Laryngeal Epithelial Defenses in Health and Disease: Further Studies. Ann. Otol. Rhinol. Laryngol. 2003, 112, 481–491. [Google Scholar] [CrossRef]
- Johnston, N.; Knight, J.; Dettmar, P.W.; Lively, M.O.; Koufman, J. Pepsin and Carbonic Anhydrase Isoenzyme III as Diagnostic Markers for Laryngopharyngeal Reflux Disease. Laryngoscope 2004, 114, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, D.L.; Pathmeswaran, A.; de Silva, H.J.; Ranasinha, C.D. Response of the Airways and Autonomic Nervous System to Acid Perfusion of the Esophagus in Patients with Asthma: A Laboratory Study. BMC Pulm. Med. 2013, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.; Kilty, S.J.; Hutton, B.; Bonaparte, J.P. The Role of Helicobacter Pylori in Laryngopharyngeal Reflux. Otolaryngol. Head Neck Surg. 2017, 156, 255–262. [Google Scholar] [CrossRef]
- Stavroulaki, P. Diagnostic and Management Problems of Laryngopharyngeal Reflux Disease in Children. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 579–590. [Google Scholar] [CrossRef]
- Brodsky, L.; Carr, M.M. Extraesophageal Reflux in Children. Curr. Opin. Otolaryngol. Head Neck Surg. 2006, 14, 387–392. [Google Scholar] [CrossRef]
- Galluzzi, F.; Schindler, A.; Gaini, R.M.; Garavello, W. The Assessment of Children with Suspected Laryngopharyngeal Reflux: An Otorhinolaringological Persepective. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1613–1619. [Google Scholar] [CrossRef]
- Saniasiaya, J.; Kulasegarah, J. Dysphonia and Reflux in Children: A Systematic Review. Int. J. Pediatr. Otorhinolaryngol. 2020, 139, 110473. [Google Scholar] [CrossRef]
- Carr, M.M.; Nagy, M.L.; Pizzuto, M.P.; Poje, C.P.; Brodsky, L.S. Correlation of Findings at Direct Laryngoscopy and Bronchoscopy with Gastroesophageal Reflux Disease in Children: A Prospective Study. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. Validity and Reliability of the Reflux Symptom Index (RSI). J. Voice 2002, 16, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. The Validity and Reliability of the Reflux Finding Score (RFS). Laryngoscope 2001, 111, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Mitchell, P.D.; Amirault, J.; Amin, M.; Watters, K.; Rahbar, R. The Edematous and Erythematous Airway Does Not Denote Pathologic Gastroesophageal Reflux. J. Pediatr. 2017, 183, 127–131. [Google Scholar] [CrossRef]
- Weitzendorfer, M.; Antoniou, S.A.; Schredl, P.; Witzel, K.; Weitzendorfer, I.C.; Majerus, A.; Emmanuel, K.; Koch, O.O. Pepsin and Oropharyngeal PH Monitoring to Diagnose Patients with Laryngopharyngeal Reflux. Laryngoscope 2020, 130, 1780–1786. [Google Scholar] [CrossRef]
- Klimara, M.J.; Samuels, T.L.; Johnston, N.; Chun, R.H.; McCormick, M.E. Detection of Pepsin in Oral Secretions of Infants with and without Laryngomalacia. Ann. Otol. Rhinol. Laryngol. 2020, 129, 224–229. [Google Scholar] [CrossRef]
- Farhath, S.; He, Z.; Nakhla, T.; Saslow, J.; Soundar, S.; Camacho, J.; Stahl, G.; Shaffer, S.; Mehta, D.I.; Aghai, Z.H. Pepsin, a Marker of Gastric Contents, Is Increased in Tracheal Aspirates from Preterm Infants Who Develop Bronchopulmonary Dysplasia. Pediatrics 2008, 121, e253–e259. [Google Scholar] [CrossRef]
- Hayat, J.O.; Gabieta-Somnez, S.; Yazaki, E.; Kang, J.Y.; Woodcock, A.; Dettmar, P.; Mabary, J.; Knowles, C.H.; Sifrim, D. Pepsin in Saliva for the Diagnosis of Gastro-Oesophageal Reflux Disease. Gut 2015, 64, 373–380. [Google Scholar] [CrossRef]
- Na, S.Y.; Kwon, O.E.; Lee, Y.C.; Eun, Y.G. Optimal Timing of Saliva Collection to Detect Pepsin in Patients with Laryngopharyngeal Reflux. Laryngoscope 2016, 126, 2770–2773. [Google Scholar] [CrossRef]
- Calvo-Henríquez, C.; Ruano-Ravina, A.; Vaamonde, P.; Martínez-Capoccioni, G.; Martín-Martín, C. Is Pepsin a Reliable Marker of Laryngopharyngeal Reflux? A Systematic Review. Otolaryngol Head Neck Surg. 2017, 157, 385–391. [Google Scholar] [CrossRef]
- Klimara, M.J.; Randall, D.R.; Allen, J.; Figueredo, E.; Johnston, N. Proximal Reflux: Biochemical Mediators, Markers, Therapeutic Targets, and Clinical Correlations. Ann. N. Y. Acad. Sci. 2020, 1481, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, N.N.; Pine, H.S.; Underbrink, M. Laryngopharyngeal Reflux Disease in Children. Pediatr. Clin. N. Am. 2013, 60, 865–878. [Google Scholar] [CrossRef]
- Horvath, A.; Dziechciarz, P.; Szajewska, H. The Effect of Thickened-Feed Interventions on Gastroesophageal Reflux in Infants: Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Pediatrics 2008, 122, e1268–e1277. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.R.; Hanlon-Dearman, A.; Sinclair, C.; Taback, S.P.; Moffatt, M. Metoclopramide, Thickened Feedings, and Positioning for Gastro-Oesophageal Reflux in Children under Two Years. Cochrane Database Syst. Rev. 2004, 18, CD003502. [Google Scholar] [CrossRef]
- Heacock, H.J.; Jeffery, H.E.; Baker, J.L.; Page, M. Influence of Breast versus Formula Milk on Physiological Gastroesophageal Reflux in Healthy, Newborn Infants. J. Pediatr. Gastroenterol. Nutr. 1992, 14, 41–46. [Google Scholar] [CrossRef]
- Campanozzi, A.; Boccia, G.; Pensabene, L.; Panetta, F.; Marseglia, A.; Strisciuglio, P.; Barbera, C.; Magazzu, G.; Pettoello-Mantovani, M.; Staiano, A. Prevalence and Natural History of Gastroesophageal Reflux: Pediatric Prospective Survey. Pediatrics 2009, 123, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Omari, T.I.; Barnett, C.P.; Benninga, M.A.; Lontis, R.; Goodchild, L.; Haslam, R.R.; Dent, J.; Davidson, G.P. Mechanisms of Gastro-Oesophageal Reflux in Preterm and Term Infants with Reflux Disease. Gut 2002, 51, 475–479. [Google Scholar] [CrossRef]
- Djeddi, D.; Stephan-Blanchard, E.; Léké, A.; Ammari, M.; Delanaud, S.; Lemaire-Hurtel, A.S.; Bach, V.; Telliez, F. Effects of Smoking Exposure in Infants on Gastroesophageal Reflux as a Function of the Sleep-Wakefulness State. J. Pediatr. 2018, 201, 147–153. [Google Scholar] [CrossRef]
- Moon, R.Y.; Darnall, R.A.; Feldman-Winter, L.; Goodstein, M.H.; Hauck, F.R. SIDS and Other Sleep-Related Infant Deaths: Evidence Base for 2016 Updated Recommendations for a Safe Infant Sleeping Environment. Pediatrics 2016, 138, e20162940. [Google Scholar] [CrossRef]
- Jeffery, H.E.; Ius, D.; Page, M. The Role of Swallowing during Active Sleep in the Clearance of Reflux in Term and Preterm Infants. J. Pediatr. 2000, 137, 545–548. [Google Scholar] [CrossRef]
- Loots, C.; Kritas, S.; Van Wijk, M.; McCall, L.; Peeters, L.; Lewindon, P.; Bijlmer, R.; Haslam, R.; Tobin, J.; Benninga, M.; et al. Body Positioning and Medical Therapy for Infantile Gastroesophageal Reflux Symptoms. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, H.E.; Megevand, A.; Page, M. Why the Prone Position Is a Risk Factor for Sudden Infant Death Syndrome. Pediatrics 1999, 104, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.O. Optimizing Medical Therapy for Gastroesophageal Reflux Disease: State of the Art. Rev. Gastroenterol. Disord. 2003, 3, 59–69. Available online: https://europepmc.org/article/med/12776003 (accessed on 3 October 2022). [PubMed]
- Meyer, T.K.; Olsen, E.; Merati, A. Contemporary Diagnostic and Management Techniques for Extraesophageal Reflux Disease. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 519–524. [Google Scholar] [CrossRef]
- Hatlebakk, J.G.; Katz, P.O.; Camacho-Lobato, L.; Castell, D.O. Proton Pump Inhibitors: Better Acid Suppression When Taken before a Meal than without a Meal. Aliment. Pharmacol. Ther. 2000, 14, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Drugs for GERD in Children—UpToDate. Available online: https://www.uptodate.com/contents/image?imageKey=PEDS%2F55435 (accessed on 3 October 2022).
- Tjon, J.A.; Pe, M.; Soscia, J.; Mahant, S. Efficacy and Safety of Proton Pump Inhibitors in the Management of Pediatric Gastroesophageal Reflux Disease. Pharmacotherapy 2013, 33, 956–971. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pol, R.J.; Smits, M.J.; Van Wijk, M.P.; Omari, T.I.; Tabbers, M.M.; Benning, M.A. Efficacy of Proton-Pump Inhibitors in Children with Gastroesophageal Reflux Disease: A Systematic Review. Pediatrics 2011, 127, 925–935. [Google Scholar] [CrossRef]
- Park, W.; Hicks, D.M.; Khandwala, F.; Richter, J.E.; Abelson, T.I.; Milstein, C.; Vaezi, M.F. Laryngopharyngeal Reflux: Prospective Cohort Study Evaluating Optimal Dose of Proton-Pump Inhibitor Therapy and Pretherapy Predictors of Response. Laryngoscope 2005, 115, 1230–1238. [Google Scholar] [CrossRef]
- Eusebi, L.H.; Rabitti, S.; Artesiani, M.L.; Gelli, D.; Montagnani, M.; Zagari, R.M.; Bazzoli, F. Proton Pump Inhibitors: Risks of Long-Term Use. J. Gastroenterol. Hepatol. 2017, 32, 1295–1302. [Google Scholar] [CrossRef]
- D’Agostino, J.A.; Passarella, M.; Martin, A.E.; Lorch, S.A. Use of Gastroesophageal Reflux Medications in Premature Infants after NICU Discharge. Pediatrics 2016, 138, 20161977. [Google Scholar] [CrossRef]
- Zentilin, P.; Dulbecco, P.; Savarino, E.; Parodi, A.; Iiritano, E.; Bilardi, C.; Reglioni, S.; Vigneri, S.; Savarino, V. An Evaluation of the Anti-reflux Properties of Sodium Alginate by Means of Combined Multichannel Intraluminal Impedance and PH-Metry. Aliment. Pharmacol. Ther. 2005, 21, 29–34. [Google Scholar] [CrossRef] [PubMed]
- McGlashan, J.A.; Johnstone, L.M.; Sykes, J.; Strugala, V.; Dettmar, P.W. The Value of a Liquid Alginate Suspension (Gaviscon Advance) in the Management of Laryngopharyngeal Reflux. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, M.D.; Fraser, H.M.; Raja, H. Gaviscon® Advance Alone versus Co-Prescription of Gaviscon® Advance and Proton Pump Inhibitors in the Treatment of Laryngopharyngeal Reflux. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2515–2521. [Google Scholar] [CrossRef]
- Ummarino, D.; Miele, E.; Martinelli, M.; Scarpato, E.; Crocetto, F.; Sciorio, E.; Staiano, A. Effect of Magnesium Alginate plus Simethicone on Gastroesophageal Reflux in Infants. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 230–235. [Google Scholar] [CrossRef]
- Baldassarre, M.E.; Di Mauro, A.; Pignatelli, M.C.; Fanelli, M.; Salvatore, S.; Di Nardo, G.; Chiaro, A.; Pensabene, L.; Laforgia, N. Magnesium Alginate in Gastro-Esophageal Reflux: A Randomized Multicenter Cross-over Study in Infants. Int. J. Environ. Res. Public Health 2020, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Ripepi, A.; Huysentruyt, K.; van de Maele, K.; Nosetti, L.; Agosti, M.; Salvatoni, A.; Vandenplas, Y. The Effect of Alginate in Gastroesophageal Reflux in Infants. Pediatr. Drugs 2018, 20, 575–583. [Google Scholar] [CrossRef]
- Iwanaka, T.; Kanamori, Y.; Sugiyama, M.; Komura, M.; Tanaka, Y.; Kodaka, T.; Ishimaru, T. Laparoscopic Fundoplication for Gastroesophageal Refl Ux Disease in Infants and Children. Surg. Today 2010, 40, 393–397. [Google Scholar] [CrossRef]
- Rothenberg, S.S. Two Decades of Experience with Laparoscopic Nissen Fundoplication in Infants and Children: A Critical Evaluation of Indications, Technique, and Results. J. Laparoendosc. Adv. Surg. Tech. 2013, 23, 791–794. [Google Scholar] [CrossRef]
- Steyaert, H.; Al Mohaidly, M.; Lembo, M.A.; Carfagna, L.; Tursini, S.; Valla, J.S. Long-Term Outcome of Laparoscopic Nissen and Toupet Fundoplication in Normal and Neurologically Impaired Children. Surg. Endosc. Other Interv. Tech. 2003, 17, 543–546. [Google Scholar] [CrossRef]
- Capito, C.; Leclair, M.D.; Piloquet, H.; Plattner, V.; Heloury, Y.; Podevin, G. Long-Term Outcome of Laparoscopic Nissen-Rossetti Fundoplication for Neurologically Impaired and Normal Children. Surg. Endosc. Other Interv. Tech. 2008, 22, 875–880. [Google Scholar] [CrossRef]

| Study | Design | Population (Age Range) | Diagnostic Modality | Scope/Subject | Conclusions/Clinical Relevance |
|---|---|---|---|---|---|
| Mahoney et al. (2021) [20] | Prospective | 43 children with chronic respiratory symptoms (1–18 y.o.) | MII-pH | Evaluate the metabolite profile in BAL fluid from children with suspected LPR and assess the impact of reflux treatment on these metabolites | The study explored the impact of reflux and PPIs on the lungs and provided a foundation for future studies for novel potential biomarkers of LPR |
| Samuels et al. (2021) [21] | Prospective | 30 pediatric subjects undergoing tympanostomy tube placement for COME (0–12 y.o.) | Patients were not diagnosed with LPR, but pepsin in middle ear was thought to arise from LPR | Investigate the association of pepsin with middle ear inflammatory signaling | Pepsin was observed in middle ear fluid in 77% of patients, and this correlated with increased IL-6 and -8, neutrophil elastase, and mucin 5B; therefore, pepsin may contribute to inflammatory signaling |
| Ugras et al. (2021) [22] | Retrospective | 52 children that underwent gastroesophagoscopy and had LPR symptoms (5–17 y.o.) | RSI and RFS | Explore associations between RFS, RSI, and the severity of esophagitis on histopathology | A correlation between RFS and histopathological findings was found |
| Tumgor et al. (2021) [16] | Prospective | 44 children requiring adenotonsillectomy and 22 children without reflux symptoms as a control group (>3 y.o.) | GERD diagnosed by esophageal pH monitoring and histopathological evaluation for esophagitis. | Determination of GERD incidence in children requiring adenotonsillectomy due to adenotonsillar hypertrophy | Reflux was detected in 72.7% of children requiring adenotonsillectomy vs. none in the control group |
| Lei et al. (2021) [23] | Prospective | 65 children diagnosed with COME (2–14 y.o.) | No LPR-diagnosed subjects were included, but pepsin in the middle ear was thought to derive from LPR | Correlation between pepsin A and DNA from bacteria with IL-8 and TNF-a in the effusion of children with COME | The study showed that bacterial infection and LPR might act in synergy in the pathogenesis of COME |
| Singh et al. (2020) [24] | Prospective | 50 children with recurrent tonsillitis (6–18 y.o.) | RSI and RFS | Detection of H. pylori and LPR in patients with recurrent tonsillitis | LPR was not found to be a significant factor in the colonization of tonsils by H. pylori |
| Dziekiewicz et al. (2020) [15] | Prospective | 38 subjects with adenoid hypertrophy (3–15 y.o.) | MII-pH | Estimate the frequency of GERD among children with adenoid hypertrophy | First study to use MII-pH to assess GERD in children with adenoid hypertrophy. Only 13.2% of patients had GERD |
| Mantegazza et al. (2020) [25] | Prospective | 197 children with suspected LPR (0–17 y.o.) | MII-pH | Investigation of the reliability of RFS and RSI in assessing pediatric GERD/LPR | RSI and RFS were not found accurate in predicting pediatric GERD/LPR |
| Upendran et al. (2020) [26] | Prospective | 18 children with repaired esophageal atresia (6–16 y.o.) | Pepsin A with Peptest™ and ELISA | Investigate pepsin in exhaled breath condensate and saliva as a potential non-invasive marker of reflux aspiration in children with esophageal atresia | Salivary pepsin was detected in a large proportion of subjects, and this was associated with GERD symptoms or wheezing, substantiating a role for pepsin as a probable non-invasive marker of reflux aspiration |
| Wertz et al. (2020) [27] | Retrospective | 163 children with LPR (2.5–17 y.o.) | Clinical diagnosis based on symptoms and endoscopic findings | Laryngologic/dysphonia assessment (pVHI, acoustic analysis, laryngeal endoscopy) in LPR-diagnosed children | Pediatric patients with clinically diagnosed LPR have pVHI, jitter, and shimmer scores that are comparable to those of pediatric patients with dysphonia due to other etiologies |
| Košec et al. (2020) [28] | Retrospective | 89 children who presented with extra-esophageal GERD symptoms (NA) | 24-h double-probe pH monitoring for both GER and LPR diagnosis | Evaluate the prevalence of extraesophageal symptoms and the reliability of a novel screening score | 52 patients were diagnosed with GERD, and out of them 50 had LPR. Obesity was frequent in patients placed in higher risk groups for a positive GERD diagnosis |
| Maholarnkij et al. (2020) [29] | Prospective | 15 children with esophageal atresia (1.4–12.9 y.o.) | MII-pH | To explore associations of symptoms (esophageal and extraesophageal) and MII-pH findings in children with esophageal atresia | Prevalence of GERD in children with atresia was high and MII-pH showed a high diagnostic value in the specific population |
| Lee et al. (2020) [18] | Retrospective | 5747 children diagnosed with GERD within the first year of life and 14,877 children without GERD serving as controls (0–1 y.o.) | NA | Evaluate otologic outcomes in children with a diagnosis of GERD using a large pediatric hearing database | GERD diagnosed within the first year of life is associated with otologic issues including hearing loss, otitis media, eustachian tube dysfunction, and need for tympanostomy tubes |
| Plocek et al. (2019) [30] | Prospective | 23 children with suspected LPR (3–16 y.o.) | 24-h pharyngeal pH monitoring and MII-pH | Assess the correlation between acid reflux episodes recorded by pharyngeal pH monitoring and GER detected via MII-pH | Findings show that the efficacy of the exclusive application of pharyngeal pH monitoring for LPR diagnosis is uncertain |
| Formánek et al. (2019) [14] | Prospective | 11 children with JORRP (4–14 y.o.) | Presence of pepsin in biopsy specimens | Explore associations between LPR and JORRP | Pepsin was detected in 45.5% of specimens, implying LPR as a probable risk factor for JORRP |
| Galli et al. (2019) [31] | Prospective | 35 children (0–16 y.o.) | RSI and RSF | Evaluate the value of NBI in identifying LPR signs in the pediatric population | NBI could help improve the identification of endoscopic signs of LPR |
| Wlodarczyk et al. (2019) [32] | Prospective | 68 children (3–18 y.o.) | 24-h pharyngeal pH monitoring | Correlate laryngoscopic findings with RFS and 24-h pharyngeal pH monitoring in children with voice disorders | Vocal fold edema, laryngeal edema, and posterior commissure mucosal hypertrophy were found to be important determinants of LPR |
| Klimara et al. (2019) [33] | Prospective | 16 laryngomalacia children and 16 controls (0–2 y.o.) | Salivary pepsin A | Explore associations between salivary pepsin and the presence and severity of laryngomalacia | Pepsin was detected in 13 patients with laryngomalacia vs. 2 controls, implying a probable role for LPR in the pathogenesis of laryngomalacia |
| Baran et al. (2017) [34] | Prospective | 55 children with functional constipation and suspected GERD (5–18 y.o.) | GERD diagnosed with esophageal 24-h pH monitoring | Explore the impact of constipation treatment on GERD symptoms and 24-h pH monitoring | Treatment of constipation can improve the reflux symptoms and abnormal acid reflux |
| Siupsinskiene et al. (2017) [35] | Prospective | 97 patients undergoing tonsillectomy, of which 36 were children (<18 y.o.) | RSI and RFS | Identify H. pylori infection in tonsillar tissue from patients undergoing tonsillectomy and explore its relationship with LPR symptoms and laryngoscopic signs | 56.5% of patients with chronic tonsillitis had H. pylori, and this was associated with signs of vocal fold edema, diffuse laryngeal edema, and hypertrophy of the posterior commissure |
| Luebke et al. (2017) [13] | Prospective | 15 children undergoing airway surgery; 10 with laryngomalacia and 5 controls (0–3 y.o.) | Pepsin A in supraglottic lavages and arytenoid biopsies | Explore correlations between the presence of pepsin in the airways of laryngomalacia patients vs. controls | Pepsin in supraglottic specimens demonstrated an association with laryngomalacia, supporting a probable pathogenetic role for LPR |
| Górecka-Tuteja et al. (2016) [6] | Prospective | 28 children with COME (7–10 y.o.) | MII-pH | Detect and characterize reflux events in COME patients | LPR was noted in 68% of children with COME implying a role for LPR in the pathogenesis of the disease |
| Pavic et al. (2016) [36] | Prospective | 104 children with suspected LPR (0–18 y.o.) | MII-pH | Determine MII-pH characteristics and assess correlation with RFS and RSI | Both acid and non-acid reflux appeared to have a significant role in the pathogenesis of pediatric LPR |
| Mesallam et al. (2016) [37] | Prospective | 26 children with airway-related problems (0–16 y.o.) | 24-h pharyngeal pH monitoring | Study the feasibility of 24-h pharyngeal pH monitoring in the outpatient setup and explore correlations with airway-related problems | 85% of the patients tolerated the pH probe insertion and completed the exam. Laryngomalacia and subglottic stenosis were frequently reported in LPR patients (77%) |
| Singendonk et al. (2016) [38] | Retrospective | 30 infants (0–1 y.o) | Subjects were not LPR patients | Determine the reliability of the RFS-I (RFS for infants) for flexible versus rigid laryngoscopy in infants | The reliability of the RFS-I was moderate with flexible and rigid laryngoscopy. The RFS-I is not suitable for detecting signs or guiding treatment of LPR in infants |
| Kim et al. (2016) [39] | Prospective | 84 patients with tonsillar hypertrophy of which 54 children (4–16 y.o.) | Presence of pepsin in tonsillar tissue was considered to derive from LPR | Explore the role of pepsin in the pathogenesis of tonsillar hypertrophy | Pepsin may play a role as it was detected in the hypertrophic tonsils and its presence correlated with damaged epithelium and expression of various pro-inflammatory mediators |
| Duncan et al. (2016) [40] | Prospective | 116 children undergoing MII-pH (NA) | MII-pH | Determine if rates of hospitalization are affected by reflux burden, even after adjusting for aspiration risk | Even in aspirating children, reflux burden was not found to increase the risk of hospitalization |
| Dy et al. (2016) [41] | Prospective | 50 children (1–19 y.o.) | MII-pH and salivary pepsin using the PepTest™ | Compare salivary pepsin with pH-MII and endoscopy and determine its sensitivity | Salivary pepsin was found to show a low sensitivity for predicting GERD in children |
| Doğru et al. (2015) [42] | Prospective | 31 children with OME and 19 with no effusion (2–15 y.o.) | Pepsinogen levels and H. pylori in middle ear aspirates were considered indicative of LPR | Explore associations between otitis media with effusion and LPR in children | In children with OME, pepsinogen levels in the middle ear were significantly higher than in the serum. 19% of patients were positive for H. pylori in middle ear aspirates |
| Önal et al. (2015) [12] | Prospective | 51 children with recurrent episodes of URTI, recurrent OME, or sinusitis (3–15 y.o.) | Symptoms questioning and endoscopic findings for LPR diagnosis and histology for esophagitis/GERD diagnosis | Explore associations between LPR and GERD with specific symptoms or clinical findings | The likelihood of the occurrence of esophagitis was found to be increased in the presence of recurrent OME and postglottic edema, irrespective of the presence of reflux symptoms |
| Salturk et al. (2015) [17] | Prospective | 60 children undergoing tonsillectomy, of which 18 with LPR and 42 controls (age range: NA) | RSI and RFS | Calculate rates of complications post-tonsillectomy in LPR patients vs. controls | LPR is a probable risk factor for complications following tonsillectomy |
| Iannella et al. (2015) [11] | Prospective | 20 LPR-diagnosed children and 20 controls (1–15 y.o.) | MII-pH | Identify the presence or absence of pepsin in tears from children with LPR | 20% of LPR subjects showed pepsin in tears, implying a role in the pathogenesis of sinusitis or dacryostenosis. |
| Formánek et al. (2015) [43] | Prospective | 24 children with COME (1–7 y.o.) | 24-h pharyngeal pH monitoring and pepsin in middle ear fluid | Compare 3 modalities of detecting LPR in COME patients (pharyngeal pHmetry, Peptest® to detect pepsin in ear fluid, and pepsin in adenoids by immunochemistry) | pH monitoring was pathological in 13/21 (61.9%) children. Pepsin in the middle ear fluid was present in 5/21 (23.8%) children; these 5 patients had more severe LPR in pH monitoring |
| O’Reilly et al. (2015) [44] | Prospective | 129 children with COME, altogether 199 ear samples (0–16 y.o.) | No LPR-diagnosed subjects were included, but pepsin in the middle ear was thought to derive from LPR | Investigate the correlation of pepsin (measured by immunoassay) with cytokines, bacterial infection, and clinical outcomes | Levels of pepsin were correlated with IL-8 levels and the need for further tube placements; thus, LPR may play a role in the pathogenesis of COME |
| Formánek et al. (2015) [45] | Prospective | 44 children with COME, altogether 59 ears (1–7 y.o.) | No LPR-diagnosed subjects were included, but pepsin in the middle ear was thought to derive from LPR | Investigate whether Peptest™ could be used to identify pepsin in the middle ear fluid children with COME | Pepsin was detected in 19/59 (32.2%) of middle ear specimens, implying a role for LPR in the pathogenesis of COME |
| Nation et al. (2014) [10] | Retrospective | 63 children with rhinorrhea, nasal congestion, and chronic cough (0–10 y.o.) | Histology showing esophagitis was considered diagnostic for GERD | Explore associations between pediatric CRS and GERD in children with symptoms of rhinorrhea, nasal congestion, and chronic cough | Over 40% of all patients had positive gastroesophageal biopsies |
| Luo et al. (2014) [46] | Prospective | 48 children with COME, 50 with adenoid hypertrophy, and 30 controls (2–8 y.o.) | Pepsin and pepsinogen concentrations in ear fluid and plasma | Explore the relationship between LPR and COME | Pepsin and pepsinogen were increased in middle ear effusion; thus, LPR may be involved in the pathogenesis of COME |
| Baudoin et al. (2014) [47] | Retrospective | 89 children (1–18 y.o.) with suspected LPR | 24 h double probe pH monitoring | Development of new probability score based on symptoms, local findings, and comorbidities | Presented an attempt to classify diagnosis likelihood by defining groups at higher risk |
| Van der pol et al. (2014) [48] | Retrospective | 52 infants (0–1 y.o.) | Clinical diagnosis based on flexible laryngoscopy | Development and validation of a modified edition of RFS for infants | The new tool reached only moderate interobserver agreement with a highly variable intraobserver agreement |
| Katra et al. (2014) [49] | Prospective | 30 children with adenoid hyperplasia (2–8 y.o.) | MII-pH | Investigate associations between LPR and H. pylori in adenoid hyperplasia | Patients with H. pylori had significantly more reflux episodes; thus, LPR may play a role in the transmission of H. pylori to adenoids and contribute to hyperplasia |
| Aydin et al. (2014) [50] | Prospective | 32 patients with adenoid hypertrophy (4–13 y.o.) | 24-h dual probe pH monitoring | Investigate the role of LPR and H. pylori colonization in adenoid hypertrophy | Only 5 patients had LPR, none of which had a H. pylori positive adenoidectomy sample |
| Rosen et al. (2014) [51] | Prospective | 112 children with chronic cough and wheezing (1–16 y.o.) | MII-pH and gastrointestinal endoscopy | Evaluate the role of reflux testing (endoscopy and pH-MII) in the specific group of patients | There is a high yield to reflux testing in children with chronic cough and wheezing, as 58% had either an abnormal MII-pH, or endoscopy |
| Andrews et al. (2013) [52] | Retrospective | 63 children with suspected LPR (0–17 y.o.) | 24-h pharyngeal pH monitoring | Comparison of histologic findings from the post-cricoid region versus pharyngeal pH probe results | 24 h pharyngeal pH monitoring was well tolerated and with no complications, representing a useful tool in confirming clinical suspicion |
| Abdel-aziz et al. (2013) [9] | Prospective | 50 children with COME (1–10 y.o.) | 24 h dual-probe pH monitoring and pepsin A in middle ear effusions | Evaluate the clinical role of pepsin and LPR in children with COME | There was a significant correlation between the level of pepsin in the effusions and the number of reflux episodes implying a role for LPR in the pathogenesis of COME |
| Kelly et al. (2013) [53] | Prospective | 76 patients with chronic pulmonary disease, of which 65 study patients and 11 controls (0–24 y.o./only two were >18) | Pepsin A detection by Western blot in BAL | Determine the prevalence of aspiration-associated extra-esophageal reflux in patients with chronic respiratory symptoms by detecting the presence of pepsin in BAL specimens | 72% of study group had (+) BAL vs. 0% of controls. Detection of pepsin in BAL can serve as a biomarker for aspiration-associated extra-esophageal reflux disease |
| Kilic et al. (2013) [54] | Prospective | 50 children with persistent asthma (7–17 y.o.) | 24-h double probe pH monitoring | Explore correlations of LPR and GERD diagnosis with RSI and RFS, and status of asthma (controlled vs. uncontrolled) | RSI and RFS did not seem reliable in diagnosing LPR and GER in children. There was no association between asthma control status and LPR or GERD |
| Ummarino et al. (2012) [55] | Prospective | 35 children with extraesophageal symptoms and positive MII-pH (0–15 y.o.) | MII-pH | Compare treatment outcomes of 3 months ± another 3 months if not symptom-free with omeprazole versus ranitidine | The efficacy of omeprazole was superior to that of ranitidine in these children |
| Greifer et al. (2012) [56] | Retrospective | 63 children with extraesophageal symptoms (0–17 y.o.) | MII-pH | Explore associations of extraesophageal symptoms with pathological acid or nonacid reflux | No association was demonstrated between the extraesophageal signs and symptoms and pathological reflux in MII-pH |
| Banaszkiewicz et al. (2012) [57] | Prospective | 21 children with difficult-to-treat asthma (7–17 y.o.) | 24-h pharyngeal pH monitoring | Assess the prevalence of LPR in children with difficult-to-treat asthma | LPR was diagnosed in 61.9% of children. There was a correlation between LPR and the degree of asthma control |
| Baran et al. (2012) [58] | Prospective | 38 children with functional constipation and 40 children with suspected GERD (4–16 y.o.) | 24-h esophageal pH monitoring | Investigate the frequency of GERD in children with functional constipation | GERD should be considered in the treatment and monitoring of patients with functional constipation. |
| Ozmen et al. (2012) [59] | Retrospective | 49 children with suspected LPR (1–16 y.o.) | 24-h double probe pH monitoring and/or scintigraphy | Assess the laryngoscopic findings in children diagnosed with LPR or GERD | 12 of 30 patients diagnosed with LPR or GERD had a positive laryngeal finding. Laryngoscopy showed a 40% sensitivity and 50% specificity in the diagnosis of LPR/GERD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sofokleous, V.; Papadopoulou, A.-M.; Giotakis, E.; Delides, A.; Kyrodimos, E.; Maragoudakis, P.; Psarommatis, I. Pediatric Laryngopharyngeal Reflux in the Last Decade: What Is New and Where to Next? J. Clin. Med. 2023, 12, 1436. https://doi.org/10.3390/jcm12041436
Sofokleous V, Papadopoulou A-M, Giotakis E, Delides A, Kyrodimos E, Maragoudakis P, Psarommatis I. Pediatric Laryngopharyngeal Reflux in the Last Decade: What Is New and Where to Next? Journal of Clinical Medicine. 2023; 12(4):1436. https://doi.org/10.3390/jcm12041436
Chicago/Turabian StyleSofokleous, Valentinos, Anna-Maria Papadopoulou, Evangelos Giotakis, Alexander Delides, Efthymios Kyrodimos, Pavlos Maragoudakis, and Ioannis Psarommatis. 2023. "Pediatric Laryngopharyngeal Reflux in the Last Decade: What Is New and Where to Next?" Journal of Clinical Medicine 12, no. 4: 1436. https://doi.org/10.3390/jcm12041436
APA StyleSofokleous, V., Papadopoulou, A.-M., Giotakis, E., Delides, A., Kyrodimos, E., Maragoudakis, P., & Psarommatis, I. (2023). Pediatric Laryngopharyngeal Reflux in the Last Decade: What Is New and Where to Next? Journal of Clinical Medicine, 12(4), 1436. https://doi.org/10.3390/jcm12041436






