Gender Differences, Motor Skills and Physical Fitness Heterogeneity in Adults with Down’s Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Characteristics
2.2. Experimental Protocol
2.3. Measures
- -
- Flexibility. The sit and reach flexibility test is a common measure of flexibility of the lower back and hamstring muscles. The participant was sitting on the floor, legs stretched out, and was asked to bend the trunk and reach forward as far as possible. Any measure beyond the toes line was positive, and any measure below was negative. A higher score (expressed in centimeters) indicated better flexibility.
- -
- Explosive strength. The participant jumps as far as possible during a broad jump test. The horizontal distance achieved is measured in centimeters
- -
- Isometric strength. A handgrip test (Sensel Measurements force sensor, France) recorded the maximum voluntary force of preferential hand squeezing. A higher score (expressed in Newton) indicated better isometric strength.
- -
- Static balance. Time held on one leg is timed in seconds.
- -
- Static balance (both feet aligned on a beam: time (in seconds) held in balance and which determines a score between 0 and 4.
- -
- Dynamic balance (number of steps backward without imbalance on a line drawn on the ground and which determines a score between 0 and 4).
2.4. Statistical Analysis
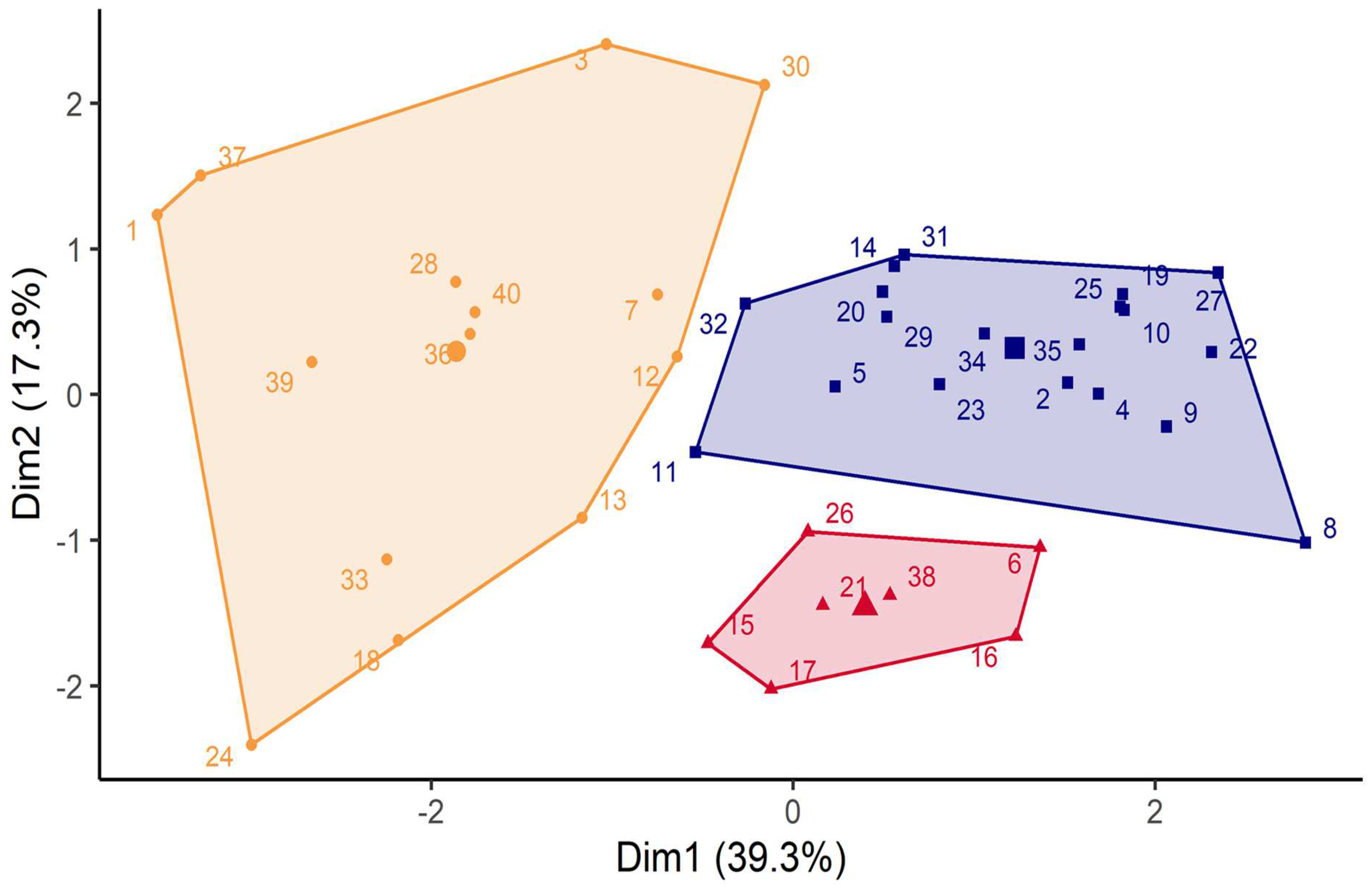
3. Results
4. Discussion
4.1. Gender Differences
4.2. Physical Activity Levels and Their Potential Determinants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Graaf, G.; Buckley, F.; Skotko, B.G. Estimation of the number of people with Down syndrome in the United States. Genet. Med. 2017, 19, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J. Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics 2011, 128, 393–406. [Google Scholar] [CrossRef]
- Finesilver, C. A new age for childhood diseases. Down syndrome. RN 2002, 65, 43–48. [Google Scholar]
- Rodríguez-Grande, E.I.; Vargas-Pinilla, O.; Torres-Narvaez, M.R.; Rodríguez-Malagón, N. Neuromuscular exercise in children with Down Syndrome: A systematic review. Sci. Rep. 2022, 12, 14988. [Google Scholar] [CrossRef] [PubMed]
- FFox, B.; Moffett, G.E.; Kinnison, C.; Brooks, G. LEC Physical Activity Levels of Children With Down Syndrome. Pediatr. Phys. Ther. 2019, 31, 33–41. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee. In Guidelines on Physical Activity, Sedentary Behaviour and Sleep for Children under 5 Years of Age; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Agiovlasitis, S.; Choi, P.; Allred, A.T.; Xu, J.; Motl, R.W. Systematic review of sedentary behaviour in people with Down syndrome across the lifespan: A clarion call. J. Appl. Res. Intellect. Disabil. 2019, 33, 146–159. [Google Scholar] [CrossRef]
- Oreskovic, N.M.; Agiovlasitis, S.; Patsiogiannis, V.; Santoro, S.L.; Nichols, D.; Skotko, B.G. Caregiver perceived physical activity preferences of adults with Down syndrome. J. Appl. Res. Intellect. Disabil. 2022, 35, 910–915. [Google Scholar] [CrossRef]
- Barr, M.A.S.N. Identifying the barriers and facilitators to participation in physical activity for children with Down syndrome. J. Intellect. Disabil. Res. 2011, 55, 1020–1033. [Google Scholar] [CrossRef]
- Shields, N.; Synnot, A.J.; Barr, M. Perceived barriers and facilitators to physical activity for children with disability: A systematic review. Br. J. Sport. Med. 2012, 46, 989–997. [Google Scholar] [CrossRef]
- Fernhall, B. Physical fitness and exercise training of individuals with mental retardation. Med. Sci. Sport. Exerc. 1993, 25, 442–450. [Google Scholar] [CrossRef]
- Fernhall, B.; Pitetti, K.H.; Vukovich, M.D.; Stubbs, N.; Hensen, T.; Winnick, J.P.; Short, F.X. Validation of cardiovascular fitness field tests in children with mental retardation. Am. J. Ment. Retard. 1998, 102, 602–612. [Google Scholar] [CrossRef]
- Pitetti, K.; Baynard, T.; Agiovlasitis, S. Children and adolescents with Down syndrome, physical fitness and physical activity. J. Sport Health Sci. 2013, 2, 47–57. [Google Scholar] [CrossRef]
- Pitetti, K.H.; Boneh, S. Cardiovascular fitness as related to leg strength in adults with mental retardation. Med. Sci. Sport. Exerc. 1995, 27, 423–428. [Google Scholar] [CrossRef]
- Pitetti, K.H.; Campbell, K.D. Mentally retarded individuals-a population at risk? Med. Sci. Sport. Exerc. 1991, 23, 586–593. [Google Scholar] [CrossRef]
- Shields, N.; King, M.; Corbett, M.; Imms, C. Is participation among children with intellectual disabilities in outside school activities similar to their typically developing peers? A systematic review. Dev. Neurorehabilit. 2014, 17, 64–71. [Google Scholar] [CrossRef]
- Wentz, E.E.; Looper, J.; Menear, K.S.; Rohadia, D.; Shields, N. Promoting Participation in Physical Activity in Children and Adolescents With Down Syndrome. Phys. Ther. 2021, 101, pzab032. [Google Scholar] [CrossRef]
- Mendonca, G.V.; Pereira, F.D.; Fernhall, B. Heart rate recovery and variability following combined aerobic and resistance exercise training in adults with and without Down syndrome. Res. Dev. Disabil. 2013, 34, 353–361. [Google Scholar] [CrossRef]
- Beck, V.D.; Baynard, T.; Lefferts, E.C.; Hibner, B.A.; Fernhall, B.; Hilgenkamp, T.I. Anthropometry does not fully explain low fitness among adults with Down syndrome. J. Intellect. Disabil. Res. 2021, 65, 373–379. [Google Scholar] [CrossRef]
- Ptomey, L.T.; Bodde, A.E.; Hastert, M.; Suire, K.B.; Helsel, B.C.; Gorczyca, A.M.; Washburn, R.A.; Rice, A.M.; Donnelly, J.E. Weight loss in adolescents with down syndrome compared to adolescents with other intellectual disabilities enrolled in an 18-month randomized weight management trial. Front. Pediatr. 2022, 10, 1022738. [Google Scholar] [CrossRef]
- Alesi, M.; Giustino, V.; Gentile, A.; Gómez-López, M.; Battaglia, G. Motor Coordination and Global Development in Subjects with Down Syndrome: The Influence of Physical Activity. J. Clin. Med. 2022, 11, 5031. [Google Scholar] [CrossRef]
- Armstrong, T.; Bull, F. Development of the world health organization global physical activity questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Bricout, V.A.; Pace, M.; Dumortier, L.; Miganeh, S.; Mahistre, Y.; Guinot, M. Motor Capacities in Boys with High Functioning Autism: Which Evaluations to Choose? J. Clin. Med. 2019, 8, 1521. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM's Health-Related Physical Fitness Assessment Manual; Wilkins, L.W., Ed.; American College of Sports Medicine: Baltimore, MD, USA, 2013. [Google Scholar]
- Italian National Olympic Committee. EUROFIT European Test of Physical Fitness; Central Direction for Sport’s Technical Activities Documentation and Information Division: Rome, Italy, 1988. [Google Scholar]
- Henderson, S.E.; Sugden, D.A. Movement Assessment Battery for Children; T.P.C. Ltd.: Moshi, Tanzania, 1992. [Google Scholar]
- Wasserman, K.; Hansen, J.; Sue, D.; Stringer, W.; Whipp, B. Principles of Exercise Testing and Interpretation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; 612p. [Google Scholar]
- Freedson, P.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications Inc. Accelerometer. Med. Sci. Sport. Exerc. 1997, 30, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S. Statistics and Computing; Springer: New York, NY, USA, 2002. [Google Scholar]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. B 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Baynard, T.; Pitetti, K.H.; Guerra, M.; Unnithan, V.B.; Fernhall, B. Age-related changes in aerobic capacity in individuals with mental retardation: A 20-yr review. Med. Sci. Sport. Exerc. 2008, 40, 1984–1989. [Google Scholar] [CrossRef]
- Fernhall, B.; Pitetti, K.H. Limitations to physical work capacity in individuals with mental retardation. Clin. Exerc. Physiol. 2001, 3, 176–185. [Google Scholar]
- Fernhall, B.; Mendonca, G.V.; Baynard, T. Reduced Work Capacity in Individuals with Down Syndrome: A Consequence of Autonomic Dysfunction? Exerc. Sport Sci. Rev. 2013, 41, 138–147. [Google Scholar] [CrossRef]
- Islam, N.N.; Sumit, A.F.; Chowdhury, M.M.; Ullah, M.A.; Araf, Y.; Sarkar, B.; Gozal, D. Age and gender-related differences in quality of life of Bangladeshi patients with Down Syndrome: A cross-sectional study. Heliyon 2022, 8, e08777. [Google Scholar] [CrossRef]
- Jensen, K.M.; Davis, M.M. Health care in adults with Down syndrome: A longitudinal cohort study. J. Intellect. Disabil. Res. 2013, 57, 947–958. [Google Scholar] [CrossRef]
- Mendonca, G.V.; Pereira, F.D.; Fernhall, B. Reduced exercise capacity in persons with Down syndrome: Cause, effect, and management. Ther. Clin. Risk Manag. 2010, 6, 601–610. [Google Scholar] [CrossRef]
- Suarez-Villadat, B.; Villagra, A.; Veiga, O.L.; Cabanas-Sanchez, V.; Izquierdo-Gomez, R. Prospective Associations of Physical Activity and Health-Related Physical Fitness in Adolescents with Down Syndrome: The UP&DOWN Longitudinal Study. Int. J. Environ. Res. Public Health 2021, 18, 5521. [Google Scholar]
- Höög, S.; Andersson, E.P. Sex and age-group differences in strength, jump, speed, flexibility, and endurance performances of Swedish elite gymnasts competing in TeamGym. Front. Sport. Act. Living 2021, 3, 653503. [Google Scholar] [CrossRef]
- Winders, P.; Wolter-Warmerdam, K.; Hickey, F. A schedule of gross motor development for children with Down syndrome. J. Intellect. Disabil. Res. 2019, 63, 346–356. [Google Scholar] [CrossRef]
- Oppewal, A.; Hilgenkamp, T.I.M. Physical fitness is predictive for 5-year survival in older adults with intellectual disabilities. J. Appl. Res. Intellect. Disabil. 2019, 32, 958–966. [Google Scholar] [CrossRef]
- Guerra, M.; Gine-Garriga, M.; Fernhall, B. Reliability of Wingate testing in adolescents with Down syndrome. Pediatr. Exerc. Sci. 2009, 21, 47–54. [Google Scholar] [CrossRef]
- Izquierdo-Gomez, R.; Veiga, Ó.L.; Sanz, A.; Díaz-Cueto, M.; Villagra, A. Correlates of objectively measured physical activity in adolescents with Down syndrome: The UP & DOWN study. Nutr. Hosp. 2015, 31, 2606–2617. [Google Scholar]
- Guerra, M.; Llorens, N.; Fernhall, B. Chronotropic incompetence in persons with down syndrome. Arch. Phys. Med. Rehabil. 2003, 84, 1604–1608. [Google Scholar] [CrossRef]
- Mendonca, G.V.; Pereira, F.D. Heart rate recovery after exercise in adults with the Down syndrome. Am. J. Cardiol. 2010, 105, 1470–1473. [Google Scholar] [CrossRef]
- Pastore, A.; Tozzi, G.; Gaeta, L.M.; Giannotti, A.; Bertini, E.; Federici, G.; Digilio, M.C.; Piemonte, F. Glutathione metabolism and antioxidant enzymes in children with Down syndrome. J. Pediatr. 2003, 142, 583–585. [Google Scholar] [CrossRef]
- Bull, M.J. Down Syndrome. N. Engl. J. Med. 2020, 382, 2344–2352. [Google Scholar] [CrossRef]
- Shields, N.; Plant, S.; Warren, C.; Wollersheim, D.; Peiris, C. Do adults with Down syndrome do the same amount of physical activity as adults without disability? A proof of principle study. J. Appl. Res. Intellect. Disabil. 2018, 31, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Pitetti, K.; Miller, R.A.; Loovis, E. Balance and Coordination Proficiency of Age-Matched Male and Female Children and Adolescents With Intellectual Disabilities. Adapt. Phys. Act. Q. 2018, 35, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Bertapelli, F.; Pitetti, K.; Agiovlasitis, S.; Guerra-Junior, G. Overweight and obesity in children and adolescents with Down syndrome- prevalence, determinants, consequences, and interventions: A literature review. Res. Dev. Disabil. 2016, 57, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Bas, B.; Er, S.; Keseroglu, K.; Korkmaz, H. Cervical Vestibular-Evoked Myogenic Potentials and Balance Testing in Children with Down Syndrome. Int. Arch. Otorhinolaryngol. 2021, 25, e580–e584. [Google Scholar] [CrossRef] [PubMed]
- Tsimaras, V.K.; Fotiadou, E.G. Effect of training on the muscle strength and dynamic balance ability of adults with down syndrome. J. Strength Cond. Res. 2004, 18, 343–347. [Google Scholar]
- Villamonte, R.; Vehrs, P.R.; Feland, J.B.; Johnson, A.W.; Seeley, M.K.; Eggett, D. Reliability of 16 balance tests in individuals with Down syndrome. Percept. Mot. Skills 2010, 111, 530–542. [Google Scholar] [CrossRef]
- Cai, W.; Baek, S.S. Effect of 24-week basketball programme on body composition and functional fitness on adults with Down syndrome. J. Intellect. Disabil. Res. 2022, 66, 939–951. [Google Scholar] [CrossRef]
- Gimunová, M.; Bozděch, M.; Skotáková, A.; Grünn, V.; Válková, H. Comparison of forward and backward gait in males with and without intellectual disabilities. J. Intellect. Disabil. Res. 2021, 65, 922–929. [Google Scholar] [CrossRef]
- Carmeli, E.; Barchad, S.; Masharawi, Y.; Coleman, R. Impact of a walking program in people with down syndrome. J. Strength Cond. Res. 2004, 18, 180–184. [Google Scholar]
- Boer, P.H. The effect of 8 weeks of freestyle swim training on the functional fitness of adults with Down syndrome. J. Intellect. Disabil. Res. 2020, 64, 770–781. [Google Scholar] [CrossRef]
- Pieter, H.B.; De Beer, Z. The effects of aquatic exercises on the physical and functional fitness of adults with Down Syndrome: A non-randomised controlled trial. J. Intellect. Disabil. Res. 2019, 63, 1453–1463. [Google Scholar]
- Boer, P.H.; Moss, S.J. Effect of continuous aerobic vs. interval training on selected anthropometrical, physiological and functional parameters of adults with Down syndrome. J. Intellect. Disabil. Res. 2016, 60, 322–334. [Google Scholar] [CrossRef]
- Kocic, M.; Bojic, I.; Aleksandrovic, M.; Ignjatovic, A. Physical activity in adolescent with mental retardation: Is basketball training is adequate stimulus to improve cardiorespiratoy fitness and sports skills performance? Acta Falcutatis Med. Naissensis 2017, 34, 159–168. [Google Scholar] [CrossRef]
- Bartlo, P.; Klein, P.J. Physical activity benefits and needs in adults with intellectual disabilities: Systematic review of the literature. Am. J. Intellect. Dev. Disabil. 2011, 116, 220–232. [Google Scholar] [CrossRef]
- Baumer, N.; Davidson, E.J. Supporting a happy, healthy adolescence for young people with Down syndrome and other intellectual disabilities: Recommendations for clinicians. Curr. Opin. Pediatr. 2014, 26, 428–434. [Google Scholar] [CrossRef]
- Bricout, V.-A.; Guinot, M.; Favre-Juvin, A.; Amblard, F.; Devillard, F. A Successful Harmonious Development by Sport of a Child with Down Syndrome: Fifteen Years of Sport Medical Follow-Up. J. Psychiatry Psychiatr. Disord. 2021, 5, 76–88. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Meng How, Y.; Zhang, A.L. Benefits of physical exercise intervention on fitness of individuals with Down syndrome: A systematic review of randomized-controlled trials. Int. J. Rehabil. Res. 2013, 36, 187–195. [Google Scholar] [CrossRef]
- Tsou, A.Y.; Bulova, P.; Capone, G.; Chicoine, B.; Gelaro, B.; Harville, T.O.; Martin, B.A.; McGuire, D.E.; McKelvey, K.D.; Peterson, M.; et al. Medical Care of Adults With Down Syndrome: A Clinical Guideline. Global Down Syndrome Foundation Medical Care Guidelines for Adults with Down Syndrome Workgroup. JAMA 2020, 324, 1543–1556. [Google Scholar] [CrossRef]
- Shields, N.; Van Den Bos, R.; Buhlert-Smith, K.; Prendergast, L.; Taylor, N. A community-based exercise program to increase participation in physical activities among youth with disability: A feasibility study. Disabil. Rehabil. 2019, 41, 1152–1159. [Google Scholar] [CrossRef]

| Men (n = 24) | Women (n = 16) | ||
|---|---|---|---|
| Demographic | Age (years) | 29.8 ± 6.2 | 28.8 ± 9.2 |
| Height (cm) | 161 ± 5 | 146 ± 5 *** | |
| Weight (kg) | 63.98 ± 9.1 | 53.9 ± 9.6 ** | |
| BMI (kg/m2) | 24.7 ± 3.6 | 25.0 ± 4.4 | |
| Physical fitness | VO2peak (mLO2·kg−1·min−1) | 36.1 ± 7.7 | 29.5 ± 7.9 ** |
| VO2peak (L·min−1) | 2.27 ± 0.45 | 1.52 ± 0.38 *** | |
| VO2predicted (mLO2·kg−1·min−1) | 39.45 ± 2.32 | 32.60 ± 4.64 *** | |
| %VO2predicted | 91.4 ± 18.3 | 90.8 ± 21.0 | |
| Flexibility (cm) | 26.3 ± 10.2 | 32.0 ± 9.2 * | |
| Explosive strength (cm) | 100.9 ± 36.5 | 89.3 ± 32.6 | |
| Isometric strength (N) | 280.4 ± 98.6 | 182.8 ± 72.7 ** | |
| Static balance (s) | 11″96 ± 6″49 | 13″58 ± 5″93 | |
| Dynamic balance (score) | 10.50 ± 5.63 | 11.38 ± 4.76 | |
| Physical activity | GPAQ | 2940 ± 2173 | 2019 ± 1311 |
| Sedentary behavior (min) | 469.5 ± 98.1 | 483.8 ± 77.5 | |
| MVPA (min) | 29.37 ± 23.02 | 21.92 ± 10.23 | |
| Energy expenditure | 269.4 ± 99.1 | 225.6 ± 109.6 | |
| Number of steps | 6301 ± 1716 | 6125 ± 1841 |
| Cluster 1 (n = 14) | Cluster 2 (n = 19) | Cluster 3 (n = 7) | ||
|---|---|---|---|---|
| Demographic | Age (years) | 34.4 ± 7.5 | 27.1 ± 6.2 * | 25.7 ± 5.6 * |
| Height (cm) | 1.53 ± 0.08 | 1.56 ± 0.08 | 1.56 ± 0.08 | |
| Weight (kg) | 65.9 ± 11.4 | 56.9 ± 9.1 | 56.3 ± 7.2 | |
| BMI (kg/m2) | 28.3 ± 4.3 | 22.9 ± 2.0 *** | 22.9 ± 1.9 ** | |
| Physical fitness | VO2peak (mLO2·kg−1min−1) | 25.41 ± 4.37 | 37.69 ± 6.90 ** | 38.19 ± 5.89 *** |
| VO2theo (L·min−1) | 1.85 ± 0.58 | 1.95 ± 0.54 | 2.03 ± 0.48 | |
| VO2predicted (mLO2·kg−1·min−1) | 33.44 ± 5.31 | 38.32 ± 3.73 * | 38.88 ± 2.84 | |
| %VO2predicted | 77.0 ± 13.8 | 98.9 ± 18.6 ** | 98.4 ± 14.8 * | |
| Flexibility (cm) | 25.01 ± 12.55 | 30.41 ± 8.77 | 30.71 ± 7.05 | |
| Explosive strength (cm) | 68.85 ± 31.26 | 108.92 ± 29.66 ** | 116.86 ± 21.63 ** | |
| Isometric strength (N) | 186.26 ± 92.55 | 269.35 ± 101.84 | 275.57 ± 71.62 | |
| Static balance (s) | 7″41 ± 4″38 | 17″50 ± 3″08 *** | 9″71 ± 5″92 $ | |
| Dynamic balance (score) | 9.57 ± 6.05 | 14.00 ± 2.16 * | 4.86 ± 2.97 $$$ | |
| Physical activity | GPAQ | 1473 ± 1134 | 3060 ± 1836 * | 3443 ± 2529 **$ |
| Sedentary behavior (min) | 478 ± 101 | 485 ± 75 | 442 ± 108 | |
| MVPA (min) | 20.27 ± 13.68 | 29.32 ± 22.43 | 30.67 ± 18.23 | |
| Energy expenditure | 252.7 ± 98.8 | 237.1 ± 112.3 | 290.0 ± 96.7 | |
| Number of steps | 5716 ± 1596 | 6161 ± 1553 | 7450 ± 2166 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covain, S.; Baillieul, S.; Nguyen, T.D.; Guinot, M.; Doutreleau, S.; Bricout, V.-A. Gender Differences, Motor Skills and Physical Fitness Heterogeneity in Adults with Down’s Syndrome. J. Clin. Med. 2023, 12, 1367. https://doi.org/10.3390/jcm12041367
Covain S, Baillieul S, Nguyen TD, Guinot M, Doutreleau S, Bricout V-A. Gender Differences, Motor Skills and Physical Fitness Heterogeneity in Adults with Down’s Syndrome. Journal of Clinical Medicine. 2023; 12(4):1367. https://doi.org/10.3390/jcm12041367
Chicago/Turabian StyleCovain, Sandro, Sébastien Baillieul, Thai Duy Nguyen, Michel Guinot, Stéphane Doutreleau, and Véronique-Aurélie Bricout. 2023. "Gender Differences, Motor Skills and Physical Fitness Heterogeneity in Adults with Down’s Syndrome" Journal of Clinical Medicine 12, no. 4: 1367. https://doi.org/10.3390/jcm12041367
APA StyleCovain, S., Baillieul, S., Nguyen, T. D., Guinot, M., Doutreleau, S., & Bricout, V.-A. (2023). Gender Differences, Motor Skills and Physical Fitness Heterogeneity in Adults with Down’s Syndrome. Journal of Clinical Medicine, 12(4), 1367. https://doi.org/10.3390/jcm12041367





