Usefulness of Systemic Venous Ultrasound Protocols in the Prognosis of Heart Failure Patients: Results from a Prospective Multicentric Study
Abstract
1. Introduction
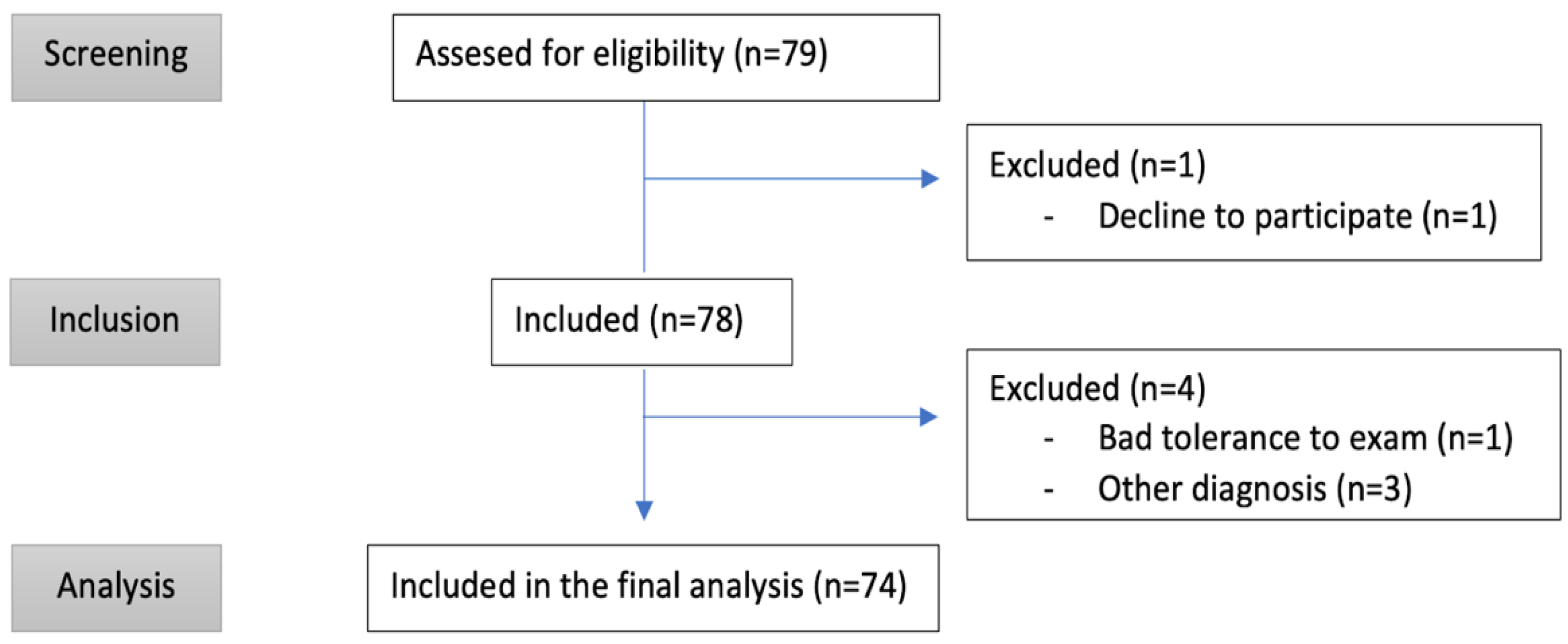
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Initial Assessment
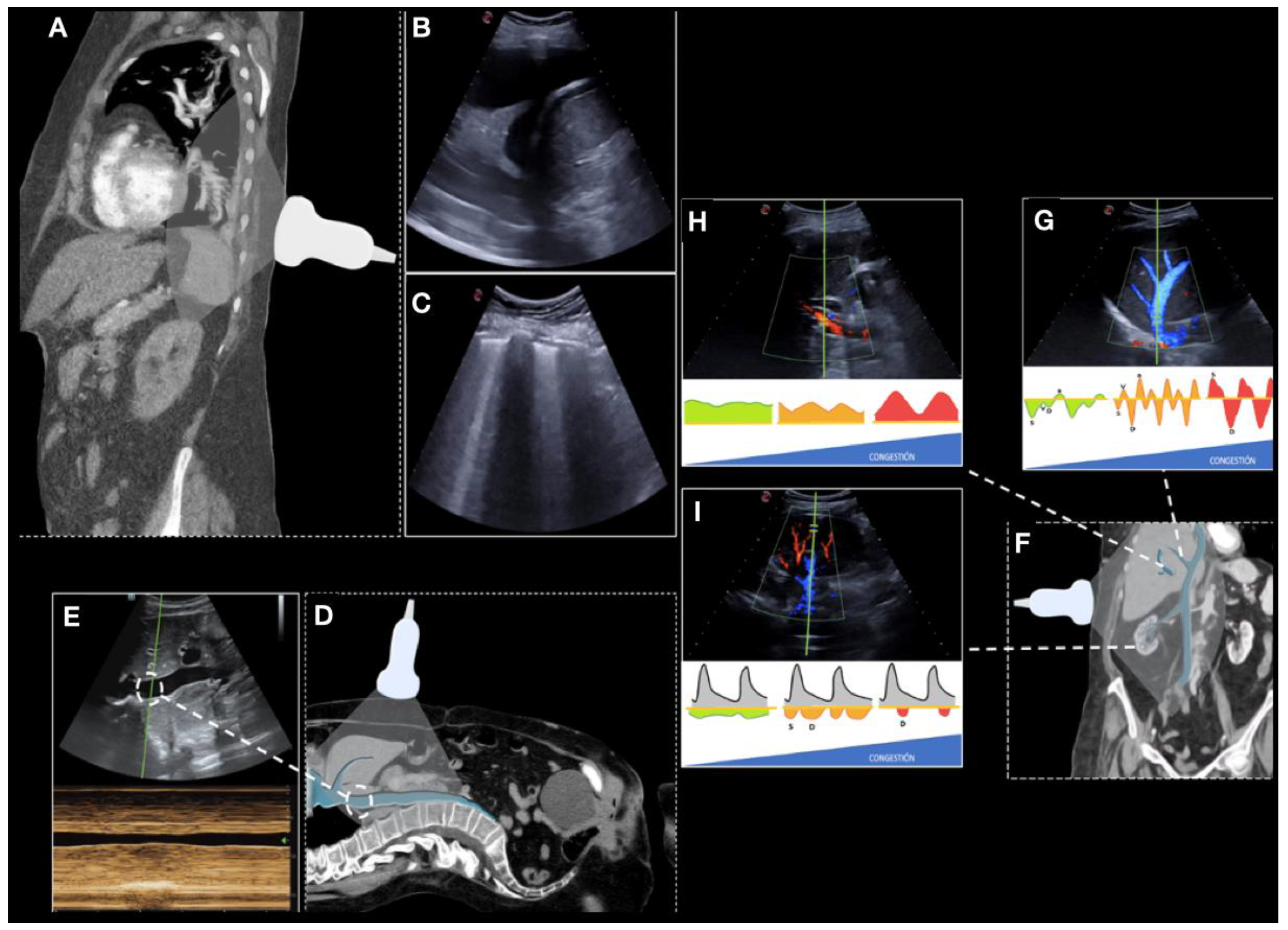
2.3. Collecting Ultrasound Data
2.4. Objective and Definitions
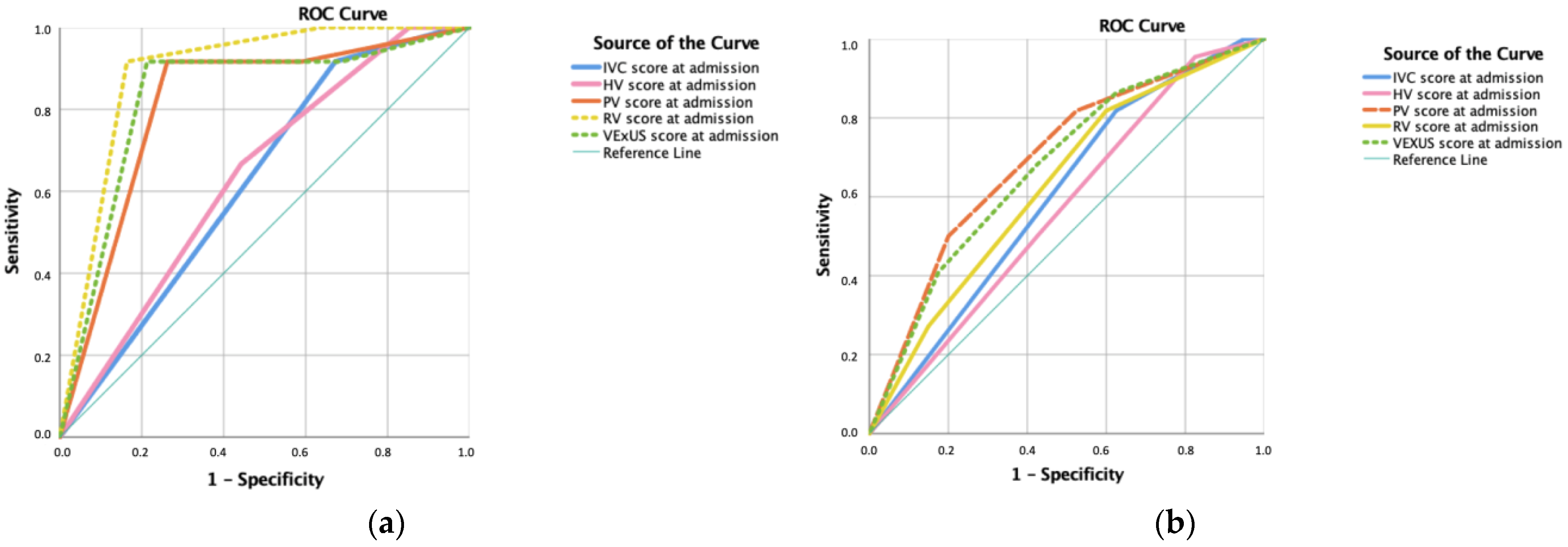
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brouwers, F.P.; De Boer, R.A.; Van Der Harst, P.; Voors, A.A.; Gansevoort, R.T.; Bakker, S.J.; Hillege, H.L.; Van Veldhuisen, D.J.; van Gilst, W. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur. Heart J. 2013, 34, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Motiejūnaitė, J.; Akiyama, E.; Cohen-Solal, A.; Maggioni, A.P.; Mueller, C.; Choi, D.-J.; Kavoliūnienė, A.; Čelutkienė, J.; Parenica, J.; Lassus, J.; et al. The association of long-term outcome and biological sex in patients with acute heart failure from different geographic regions. Eur. Heart J. 2020, 41, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Barasa, A.; Schaufelberger, M.; Lappas, G.; Swedberg, K.; Dellborg, M.; Rosengren, A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur. Heart J. 2014, 35, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, N.H.; Westland, H.; Groenwold, R.H.; Ågren, S.; Anguita, M.; Blue, L.; de la Porte, P.W.B.-A.; DeWalt, D.A.; Hebert, P.L.; Heisler, M.; et al. What Are Effective Program Characteristics of Self-Management Interventions in Patients With Heart Failure? An Individual Patient Data Meta-analysis. J. Card. Fail. 2016, 22, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Van Spall, H.; Rahman, T.; Mytton, O.; Ramasundarahettige, C.; Ibrahim, Q.; Kabali, C.; Coppens, M.; Haynes, B.; Connolly, S. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: A systematic review and network meta-analysis: Comparative effectiveness of transitional care services in patients hospitalized with heart failure. Eur. J Heart Fail. 2017, 19, 1427–1443. [Google Scholar] [PubMed]
- Tung-Chen, Y.; Ossaba-Vélez, S.; Velásquez, K.S.A.; Parra-Gordo, M.L.; Díez-Tascón, A.; Villén-Villegas, T.; Montero-Hernández, E.; Gutiérrez-Villanueva, A.; Trueba-Vicente, Á.; Arenas-Berenguer, I.; et al. The Impact of Different Lung Ultrasound Protocols in the Assessment of Lung Lesions in COVID-19 Patients: Is There an Ideal Lung Ultrasound Protocol? J. Ultrasound 2022, 25, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Cubo-Romano, P.; Torres-Macho, J.; Soni, N.J.; Reyes, L.F.; Rodríguez-Almodóvar, A.; Fernández-Alonso, J.M.; González-Davia, R.; Casas-Rojo, J.M.; Restrepo, M.I.; de Casasola, G.G. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J. Hosp. Med. 2016, 11, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Torres-Arrese, M.; de Casasola-Sánchez, G.G.; Méndez-Bailón, M.; Montero-Hernández, E.; Cobo-Marcos, M.; Rivas-Lasarte, M.; Caurcel-Díaz, L.; Rodríguez-Fuertes, P.; Villén-Villegas, T.; Tung-Chen, Y. Usefulness of Serial Multiorgan Point-of-Care Ultrasound in Acute Heart Failure: Results from a Prospective Observational Cohort. Med. Pharmacol. 2021, 58, 124. Available online: https://www.preprints.org/manuscript/202112.0113/v1 (accessed on 14 January 2022). [CrossRef] [PubMed]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar]
- Goonewardena, S.N.; Blair, J.E.; Manuchehry, A.; Brennan, J.M.; Keller, M.; Reeves, R.; Price, A.; Spencer, K.T.; Puthumana, J.; Gheorghiade, M. Use of hand carried ultrasound, B-type natriuretic peptide, and clinical assessment in identifying abnormal left ventricular filling pressures in patients referred for right heart catheterization. J. Card. Fail. 2010, 16, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Khandwalla, R.M.; Birkeland, K.T.; Zimmer, R.; Henry, T.D.; Nazarian, R.; Sudan, M.; Mirocha, J.; Cha, J.; Kedan, I. Usefulness of Serial Measurements of Inferior Vena Cava Diameter by Vscan TM to Identify Patients With Heart Failure at High Risk of Hospitalization. Am. J. Cardiol. 2017, 119, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Bouabdallaoui, N.; Beaubien-Souligny, W.; Oussaïd, E.; Henri, C.; Racine, N.; Denault, A.; Rouleau, J. Assessing Splanchnic Compartment Using Portal Venous Doppler and Impact of Adding It to the EVEREST Score for Risk Assessment in Heart Failure. CJC Open 2020, 2, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Husain-Syed, F.; Birk, H.; Ronco, C.; Schörmann, T.; Tello, K.; Richter, M.J.; Wilhelm, J.; Sommer, N.; Steyerberg, E.; Bauer, P.; et al. Doppler-Derived Renal Venous Stasis Index in the Prognosis of Right Heart Failure. J. Am. Heart Assoc. 2019, 8, e013584. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, A.; Watanabe, K.; Sato, Y.; Ishibashi, S.; Matsuda, M.; Yamadera, Y.; Ichijo, Y.; Yokokawa, T.; Misaka, T.; Oikawa, M.; et al. Intrarenal Doppler ultrasonography reflects hemodynamics and predicts prognosis in patients with heart failure. Sci. Rep. 2020, 10, 22257. [Google Scholar] [CrossRef] [PubMed]
- Brisco, M.A.; Zile, M.; Hanberg, J.S.; Wilson, F.; Parikh, C.; Coca, S.; Tang, W.W.; Testani, J.M. Relevance of Changes in Serum Creatinine During a Heart Failure Trial of Decongestive Strategies: Insights From the DOSE Trial. J. Card. Fail. 2016, 22, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Jackson, K.; Rao, V.S.; Tang, W.W.; Brisco-Bacik, M.A.; Chen, H.H.; Felker, G.M.; Hernandez, A.F.; O’Connor, C.M.; Sabbisetti, V.S.; et al. Worsening Renal Function in Patients With Acute Heart Failure Undergoing Aggressive Diuresis Is Not Associated With Tubular Injury. Circulation 2018, 137, 2016–2028. [Google Scholar] [CrossRef] [PubMed]
| Demographic | N (%) | Outcomes | N (%) | Echocardiographic Findings | N (%) |
|---|---|---|---|---|---|
| Days of admission—mean (SD) | 9.8 (4.72) | Death | 19 (25.7) | TR | 71 (95.9) |
| Age—mean (SD) | 79.55 (12.5) | Death during hospitalization | 10 (13.5) | Mild TR | 18 (24.3) |
| Female (N, %) | 38 (51.4) | HF-related death | 12 (16.2) | Moderate TR | 29 (39.2) |
| Male (N, %) | 36 (48.6) | Death in the first month after discharge | 5 (6.8) | Severe TR | 24 (32.4) |
| Heart disease (N, %) | 58 (78.4) | Death from day 30 to 90 | 4 (5.4) | Dilated RA | 55 (74.3) |
| Atrial Fibrillation (N, %) | 44 (59.5) | Unexpected visit | 16 (21.6) | RV greater than LV | 16 (21.6) |
| PH (N, %) | 39 (52.7) | Admission in the first month | 15 (20.3) | Anomalous movement of the sept | 17 (23) |
| HFpEF (N, %) | 56 (75.7) | Admission in the first three months | 26 (35.1) | Outflow RV tract acceleration time < 105 ms | 61 (82.4) |
| HFmrEF (N, %) | 10 (13.5) | Admission related to HF | 22 (29.7) | Pulmonary artery diameter > 25 mm | 17 (23) |
| HFrEF (N, %) | 8 (13.5) | Impaired kidney function | 16 (21.8) | Low PH probability | 14 (18.9) |
| Lung disease (N,%) | 32 (43.2) | Admission hypertonic use | 10 (13.5) | Intermediate PH probability | 11 (14.9) |
| COPD (N, %) | 14 (18.9) | Hypertonic use at follow-up | 9 (12.2) | High PH probability | 49 (66.2) |
| SAHS (N, %) | 7 (9.5) | Vasoactive drugs | 4 (5.4) | HFpEF | 41 (55.4) |
| Interstitial disease (N, %) | 0 (0) | Increase in diuretic treatment at follow-up | 29 (39.2) | HFmrEF | 8 (10.8) |
| Asthma (N, %) | 9 (12.2) | HFrEF | 25 (33.8) | ||
| Arterial hypertension (N, %) | 65 (87.8) | TAPSE less than 17mm | 31 (41.9) | ||
| Diabetes (N, %) | 35 (45.9) | ||||
| Dyslipidemia (N, %) | 33 (44.6) | ||||
| Advanced CKD (N, %) | 27 (36.5) | ||||
| Obesity (N, %) | 29 (39) | ||||
| Admission in the previous three months (N, %) | 17 (23) |
| On Admission | At Discharge | At Follow-Up | |
|---|---|---|---|
| Systolic blood pressure—mean (SD) | 136.31 (21.14) | 121.27(17.80) | 129.25 (25.08) |
| Diastolic blood pressure—mean (SD) | 77.26 (17.79) | 69.53 (12.18) | 65.53 (11.25) |
| Oxygen saturation—mean (SD) | 90.07 (7.46) | 95.26 (2.31) | 94.72 (4.21) |
| Weight (kg)—mean (SD) | 72 (17.05) | 69.45 (17.26) | 70.86 (17.29 |
| NYHA I (N, %) | 0 (0) | 37 (50) | 28 (37.8) |
| NYHA II (N, %) | 5 (6.8) | 28 (37.8) | 22 (29.7) |
| NYHA III (N, %) | 46 (62.2) | 1 (1.4) | 8 (10.8) |
| NYHA IV (N, %) | 22 (29.7) | 0 (0) | 2 (2.7) |
| EVEREST score—mean (DE) | 7.76, 8 (3.1) | 1.42 (1.17) | 2.27 (2.43) |
| Urea—mean (SD) | 74.99 (44.04) | 86.98 (47.68) | 72.76 (36.98) |
| Creatinine—mean (SD) | 1.58 (1.03) | 1.47 (0.94) | 1.52 (0.83) |
| Creatine deteriorated—N (%) | 55 (74.3) | 59 (79.7) | 59 (79.7) |
| Sodium—mean (SD) | 138 (5.6) | 139 (3.4) | 138 (3.9) |
| NT-proBNP—mean (SD) | 10278.5 (12740.7) | 6156.14(7889.63) | 5438.93 (5712.1) |
| GPT—mean (SD) | 49.68 (48.17) | 35.78 (25.18) | 36.98 (14.2) |
| GOT—mean (SD) | 41.90 (27.4) | 28.27 (13.73) | 31.90 (14.29) |
| Leukocytes—mean (SD) | 8812.45 (3325.4) | 7610.4 (2155.44) | 7221.30 (2705.7) |
| Haemoglobin—mean (SD) | 12.6 (2.28) | 12.77 (2.14) | 13.15 (2.14) |
| On Admission (N = 74) | At Discharge (N = 64) | p-Value | At Follow-Up (N = 55) | p-Value | |
|---|---|---|---|---|---|
| IVC (cm)–mean (SD) | 2.25 (0.53) | 1.81 (0.42) | <0.001 | 1.85 (0.43) | <0.001 |
| Absence of collapsibility–N (%) | 71 (95.9) | 41 (55.4) | 0.023 | 38 (51.4) | 0.321 |
| Lung score–mean (SD) | 17.74 (7.23) | 6.9 (5.62) | 0.015 | 8.4 (7.8) | <0.001 |
| Hepatic vein (SD) | 1.34 (0.69) | 0.98 (0.839) | <0.001 | 0.85 (0.81) | <0.001 |
| S > D at hepatic vein flow–N (%) | 9 (12.2) | 20 (27) | 23 (31.1) | ||
| S < D at hepatic vein flow–N (%) | 29 (39.2) | 26 (35.1) | 18 (24.3) | ||
| S Reversal at hepatic vein–N (%) | 35 (47.3) | 19 (25.7) | 14 (18.9) | ||
| Portal vein (SD) | 0.94 (0.839) | 0.32 (0.612) | <0.001 | 0.47 (0.56) | 0.19 |
| Pulsatility < 30%–N (%) | 27 (36.5) | 50 (67.6) | 33 (44.6) | ||
| Pulsatility 30–50%–N (%) | 20 (27) | 11 (14.9) | 23 (31.1) | ||
| Pulsatility > 50%–N (%) | 27 (36.5) | 5 (6.8) | 2 (2.7) | ||
| Intra-renal vein (SD) | 0.88 (0.734) | 0.58 (0.74) | <0.001 | 0.64 (0.76) | <0.001 |
| Continuous–N (%) | 23 (31.1) | 38 (51.4) | 31 (41.9) | ||
| Discontinuous Biphasic N (%) | 30 (40.5) | 18 (24.3) | 17 (23) | ||
| Discontinuous monophasic–N (%) | 21 (28.4) | 10 (13.5) | 10 (13.5) | ||
| VExUS score (SD) | 1.50 (1.18) | 0.65 (1.015) | <0.001 | 0.95 (1.09) | 0.052 |
| VExUS 0–N (%) | 21 (28.4) | 42 (56.8) | 29 (39.2) | ||
| VExUS 1–N (%) | 13 (17.6) | 12 (16.2) | 8 (10.8) | ||
| VExUS 2–N (%) | 16 (21.6) | 5 (6.8) | 14 (18.9) | ||
| VExUS 3 -N (%) | 24 (32.4) | 7 (9.5) | 6 (8.1) |
| Death | Death during Admission | HF-Related Death | Re-Admission | Re-Admission (First Month) | HF-Related Re-Admission |
|---|---|---|---|---|---|
| IVCa (r = 0.432) | IVCa (r = 0.516) | ||||
| SPSa (r = 0.320) | SPSa (r = 0.357) | SPSa (r = 0.504) | SPSa (r = 0.363) | SPSa (r = 0.317) | |
| SI-rSa (r = 0.440) | SI-rSa (r = 0.540) | SI-rSa (r = 0.618) | SI-rSa (r = 0.393) | ||
| VExUS 3a (r = 0.377) | VExUS 3a (r = 0.402) | VExUS 3a (r = 0.557) | VExUS 3a (r = 0.444) | ||
| SI-rSd (r = 0.358) | SI-rSd (r = 0.346) | ||||
| IVCf (r = 0.438) | IVCf (r = 0.442) | ||||
| SSSf (r = 0.426) | SSSf (r = 0.399) | SSSf (r = 0.356) | |||
| SI-rSf (r = 0.524) | |||||
| VExUS 3f (r = 0.453) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Arrese, M.; Mata-Martínez, A.; Luordo-Tedesco, D.; García-Casasola, G.; Alonso-González, R.; Montero-Hernández, E.; Cobo-Marcos, M.; Sánchez-Sauce, B.; Cuervas-Mons, V.; Tung-Chen, Y. Usefulness of Systemic Venous Ultrasound Protocols in the Prognosis of Heart Failure Patients: Results from a Prospective Multicentric Study. J. Clin. Med. 2023, 12, 1281. https://doi.org/10.3390/jcm12041281
Torres-Arrese M, Mata-Martínez A, Luordo-Tedesco D, García-Casasola G, Alonso-González R, Montero-Hernández E, Cobo-Marcos M, Sánchez-Sauce B, Cuervas-Mons V, Tung-Chen Y. Usefulness of Systemic Venous Ultrasound Protocols in the Prognosis of Heart Failure Patients: Results from a Prospective Multicentric Study. Journal of Clinical Medicine. 2023; 12(4):1281. https://doi.org/10.3390/jcm12041281
Chicago/Turabian StyleTorres-Arrese, Marta, Arantzazu Mata-Martínez, Davide Luordo-Tedesco, Gonzalo García-Casasola, Rodrigo Alonso-González, Esther Montero-Hernández, Marta Cobo-Marcos, Beatriz Sánchez-Sauce, Valentín Cuervas-Mons, and Yale Tung-Chen. 2023. "Usefulness of Systemic Venous Ultrasound Protocols in the Prognosis of Heart Failure Patients: Results from a Prospective Multicentric Study" Journal of Clinical Medicine 12, no. 4: 1281. https://doi.org/10.3390/jcm12041281
APA StyleTorres-Arrese, M., Mata-Martínez, A., Luordo-Tedesco, D., García-Casasola, G., Alonso-González, R., Montero-Hernández, E., Cobo-Marcos, M., Sánchez-Sauce, B., Cuervas-Mons, V., & Tung-Chen, Y. (2023). Usefulness of Systemic Venous Ultrasound Protocols in the Prognosis of Heart Failure Patients: Results from a Prospective Multicentric Study. Journal of Clinical Medicine, 12(4), 1281. https://doi.org/10.3390/jcm12041281









