Unexpected CD5+ B Cell Lymphocytosis during SARS-CoV-2 Infection: Relevance for the Pathophysiology of Chronic Lymphocytic Leukemia
Abstract
1. Introduction
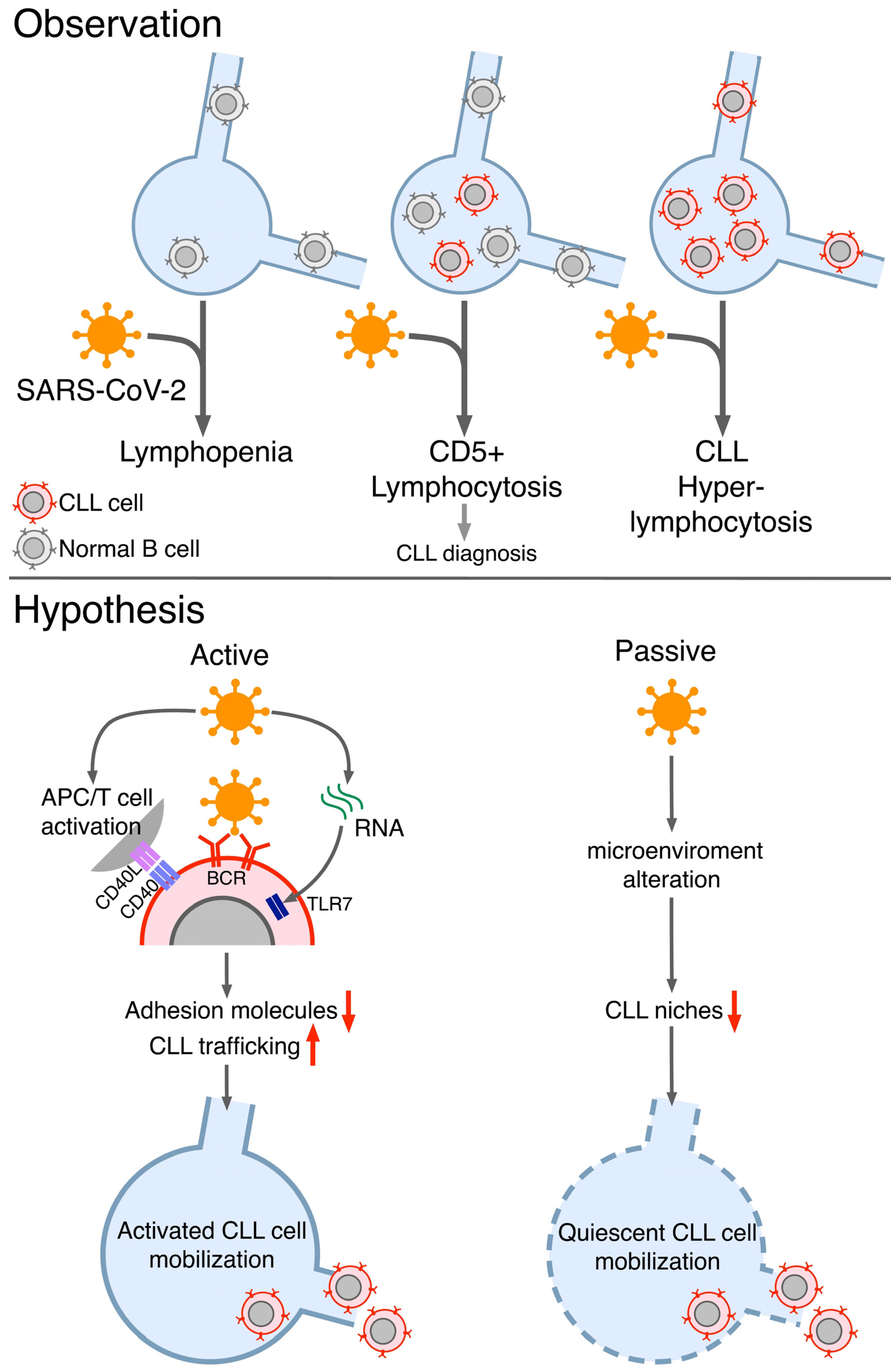
2. Evidencesof Unexpeceted Lymphocytosis of CLL B Cells during COVID-19
3. Plausible Explanations of the Observed CLL B Cellslymhocitosis during COVID-19
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep Immune Profiling of COVID-19 Patients Reveals Distinct Immunotypes with Therapeutic Implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef]
- Haslbauer, J.D.; Matter, M.S.; Stalder, A.K.; Tzankov, A. Histomorphological Patterns of Regional Lymph Nodes in COVID-19 Lungs. Pathologe 2021, 42, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Feng, Z.; Diao, B.; Tu, C.; Qiao, Q.; Yang, H.; Zhang, Y.; Wang, G.; Wang, H.; Wang, C.; et al. SARS-CoV-2 Induces Lymphocytopenia by Promoting Inflammation and Decimates Secondary Lymphoid Organs. Front. Immunol. 2021, 12, 661052. [Google Scholar] [CrossRef] [PubMed]
- Scarfò, L.; Chatzikonstantinou, T.; Rigolin, G.M.; Quaresmini, G.; Motta, M.; Vitale, C.; Garcia-Marco, J.A.; Hernández-Rivas, J.Á.; Mirás, F.; Baile, M.; et al. COVID-19 Severity and Mortality in Patients with Chronic Lymphocytic Leukemia: A Joint Study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia 2020, 34, 2354–2363. [Google Scholar] [CrossRef]
- Yang, L.; Chai, P.; Yu, J.; Fan, X. Effects of Cancer on Patients with COVID-19: A Systematic Review and Meta-Analysis of 63,019 Participants. Cancer Biol. Med. 2021, 18, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Blixt, L.; Bogdanovic, G.; Buggert, M.; Gao, Y.; Hober, S.; Healy, K.; Johansson, H.; Kjellander, C.; Mravinacova, S.; Muschiol, S.; et al. Covid-19 in Patients with Chronic Lymphocytic Leukemia: Clinical Outcome and B- and T-Cell Immunity during 13 Months in Consecutive Patients. Leukemia 2022, 36, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Llamas, A.A.; Vela-Ojeda, J.; Hernandez-Caballero, A. Chronic Lymphocytic Leukemia in the SARS-CoV-2 Pandemic. Curr. Oncol. Rep. 2022, 24, 209–213. [Google Scholar] [CrossRef]
- Fiorcari, S.; Atene, C.G.; Maffei, R.; Debbia, G.; Potenza, L.; Luppi, M.; Marasca, R. Ibrutinib Interferes with Innate Immunity in Chronic Lymphocytic Leukemia Patients during COVID-19 Infection. Haematologica 2021, 106, 2265–2268. [Google Scholar] [CrossRef]
- Lanza, L.; Koroveshi, B.; Barducchi, F.; Lorenzo, A.; Venturino, E.; Cappelli, E.; Lillo, F.; Bain, B.J. A New Diagnosis of Monoclonal B-Cell Lymphocytosis with Cytoplasmic Inclusions in a Patient with COVID-19. Am. J. Hematol. 2022, 97, 1372–1373. [Google Scholar] [CrossRef]
- Saluja, P.; Gautam, N.; Amisha, F.; Safar, M.; Bartter, T. Emergence of Chronic Lymphocytic Leukemia During Admission for COVID-19: Cause or Coincidence? Cureus 2022, 14, e23470. [Google Scholar] [CrossRef]
- Ali, E.; Badawi, M.; Abdelmahmuod, E.; Kohla, S.; Yassin, M.A. Chronic Lymphocytic Leukemia Concomitant with COVID 19: A Case Report. Am. J. Case Rep. 2020, 21, e926062-1–e926062-4. [Google Scholar] [CrossRef] [PubMed]
- Largeaud, L.; Ribes, A.; Dubois-Galopin, F.; Mémier, V.; Rolland, Y.; Gaudin, C.; Rousset, D.; Geeraerts, T.; Noel-Savina, E.; Rieu, J.B.; et al. Major Rise of a Chronic Lymphoid Leukemia Clone during the Course of COVID-19. Int. J. Lab. Hematol. 2021, 43, e82–e83. [Google Scholar] [CrossRef] [PubMed]
- Paneesha, S.; Pratt, G.; Parry, H.; Moss, P. Covid-19 Infection in Therapy-Naive Patients with B-Cell Chronic Lymphocytic Leukemia. Leuk. Res. 2020, 93, 106366. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.H.; Zheng, K.I.; Pan, K.H.; Xie, Y.P.; Zheng, M.H. COVID-19 in a Patient with Chronic Lymphocytic Leukaemia. Lancet Haematol. 2020, 7, e351–e352. [Google Scholar] [CrossRef]
- Safarpour, D.; Srinivasan, K.; Farooqui, M.; Roth, C.; Ghouse, M. A Case of COVID-19–Induced Lymphocytosis in a Patient With Treatment-Naive CLL: Should It Be Treated? Clin. Lymphoma Myeloma Leuk. 2021, 21, 69–72. [Google Scholar] [CrossRef]
- Balraj, S.; Sarah, A.; Parminder, K.; Ro-Jay, R.; Sachin, G.; Michael, M. COVID-19-Induced Hyperleucocytosis in Chronic Lymphocytic Leukaemia. Eur. J. Case Rep. Intern. Med. 2021, 8, 002348. [Google Scholar] [CrossRef]
- Myhrinder, A.L.; Hellqvist, E.; Sidorova, E.; Söderberg, A.; Baxendale, H.; Dahle, C.; Willander, K.; Tobin, G.; Bäckman, E.; Söderberg, O.; et al. A New Perspective: Molecular Motifs on Oxidized LDL, Apoptotic Cells, and Bacteria Are Targets for Chronic Lymphocytic Leukemia Antibodies. Blood 2008, 111, 3838–3848. [Google Scholar] [CrossRef]
- Hoogeboom, R.; van Kesse, K.P.M.; Hochstenbach, F.; Wormhoudt, T.A.; Reinten, R.J.A.; Wagner, K.; Kater, A.P.; Guikema, J.E.J.; Bende, R.J.; van Noesel, C.J.M. A Mutated B Cell Chronic Lymphocytic Leukemia Subset That Recognizes and Responds to Fungi. J. Exp. Med. 2013, 210, 59–70. [Google Scholar] [CrossRef]
- Vlachonikola, E.; Stamatopoulos, K.; Chatzidimitriou, A. T Cells in Chronic Lymphocytic Leukemia: A Two-Edged Sword. Front. Immunol. 2021, 11, 612244. [Google Scholar] [CrossRef]
- Ferrer, G.; Jung, B.; Chiu, P.Y.; Aslam, R.; Palacios, F.; Mazzarello, A.N.; Vergani, S.; Bagnara, D.; Chen, S.-S.; Yancopoulos, S.; et al. Myeloid-Derived Suppressor Cell Subtypes Differentially Influence T-Cell Function, T-Helper Subset Differentiation, and Clinical Course in CLL. Leukemia 2021, 35, 3163–3175. [Google Scholar] [CrossRef]
- Calissano, C.; Damle, R.N.; Marsilio, S.; Yan, X.J.; Yancopoulos, S.; Hayes, G.; Emson, C.; Murphy, E.J.; Hellerstein, M.K.; Sison, C.; et al. Intraclonal Complexity in Chronic Lymphocytic Leukemia: Fractions Enriched in Recently Born/Divided and Older/Quiescent Cells. Mol. Med. 2011, 17, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Coelho, V.; Krysov, S.; Steele, A.; Hidalgo, M.S.; Johnson, P.W.; Chana, P.S.; Packham, G.; Stevenson, F.K.; Forconi, F. Identification in CLL of Circulating Intraclonal Subgroups with Varying B-Cell Receptor Expression and Function. Blood 2013, 12, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Herndon, T.M.; Chen, S.S.; Saba, N.S.; Valdez, J.; Emson, C.; Gatmaitan, M.; Tian, X.; Hughes, T.E.; Sun, C.; Arthur, D.C.; et al. Direct in Vivo Evidence for Increased Proliferation of CLL Cells in Lymph Nodes Compared to Bone Marrow and Peripheral Blood. Leukemia 2017, 31, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Vlad, A.; Deglesne, P.A.; Letestu, R.; Saint-Georges, S.; Chevallier, N.; Baran-Marszak, F.; Varin-Blank, N.; Ajchenbaum-Cymbalista, F.; Ledoux, D. Down-Regulation of CXCR4 and CD62L in Chronic Lymphocytic Leukemia Cells Is Triggered by B-Cell Receptor Ligation and Associated with Progressive Disease. Cancer Res. 2009, 69, 6387–6395. [Google Scholar] [CrossRef]
- Bagnara, D.; Mazzarello, A.N.; Ghiotto, F.; Colombo, M.; Cutrona, G.; Fais, F.; Ferrarini, M. Old and New Facts and Speculations on the Role of the B Cell Receptor in the Origin of Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2022, 23, 14249. [Google Scholar] [CrossRef]
- Mazzarello, A.N.; Fitch, M.; Hellerstein, M.K.; Chiorazzi, N. Measurement of Leukemic B-Cell Growth Kinetics in Patients with Chronic Lymphocytic Leukemia. Methods Mol. Biol. 2019, 1881, 129–151. [Google Scholar]
- Mazzarello, A.N.; Gentner-Göbel, E.; Dühren-vonMinden, M.; Tarasenko, T.N.; Nicolò, A.; Ferrer, G.; Vergani, S.; Liu, Y.; Bagnara, D.; Rai, K.R.; et al. B Cell Receptor Isotypes Differentially Associate with Cell Signaling, Kinetics, and Outcome in Chronic Lymphocytic Leukemia. J. Clin. Investig. 2022, 132, e149308. [Google Scholar] [CrossRef]
- Bagnara, D.; Tang, C.; Brown, J.R.; Kasar, S.; Fernandes, S.; Colombo, M.; Vergani, S.; Mazzarello, A.N.; Ghiotto, F.; Bruno, S.; et al. Post-Transformation IGHV-IGHD-IGHJ Mutations in Chronic Lymphocytic Leukemia B Cells: Implications for Mutational Mechanisms and Impact on Clinical Course. Front. Oncol. 2021, 11, 640731. [Google Scholar] [CrossRef]
- Catera, R.; Silverman, G.J.; Hatzi, K.; Seiler, T.; Didier, S.; Zhang, L.; Hervé, M.; Meffre, E.; Oscier, D.G.; Vlassara, H.; et al. Chronic Lymphocytic Leukemia Cells Recognize Conserved Epitopes Associated with Apoptosis and Oxidation. Mol. Med. 2008, 14, 665–674. [Google Scholar] [CrossRef]
- Chu, C.C.; Catera, R.; Hatzi, K.; Yan, X.J.; Zhang, L.; Wang, X.B.; Fales, H.M.; Allen, S.L.; Kolitz, J.E.; Rai, K.R.; et al. Chronic Lymphocytic Leukemia Antibodies with a Common Stereotypic Rearrangement Recognize Nonmuscle Myosin Heavy Chain IIA. Blood 2008, 112, 5122–5129. [Google Scholar] [CrossRef]
- Wolska, A.; Cebula-Obrzut, B.; Smolewski, P.; Robak, T. Effects of Toll-like Receptor 7 and Toll-like Receptor 9 Signaling Stimulators and Inhibitors on Chronic Lymphocytic Leukemia Cells Ex Vivo and Their Interactions with Cladribine. Leuk. Lymphoma 2013, 54, 1268–1278. [Google Scholar] [CrossRef]
- de Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; lo Tartaro, D.; Mattioli, M.; et al. Marked T Cell Activation, Senescence, Exhaustion and Skewing towards TH17 in Patients with COVID-19 Pneumonia. Nat. Commun. 2020, 11, 3434. [Google Scholar] [CrossRef] [PubMed]
- Hoferkova, E.; Kadakova, S.; Mraz, M. In Vitro and In Vivo Models of CLL–T Cell Interactions: Implications for Drug Testing. Cancers 2022, 14, 3087. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Ghiotto, F.; Tenca, C.; Gugiatti, E.; Santamaria, S.; Ledda, B.; Ibatici, A.; Cutrona, G.; Mazzarello, A.N.; Bagnara, D.; et al. Berberine Affects Mitochondrial Activity and Cell Growth of Leukemic Cells from Chronic Lymphocytic Leukemia Patients. Sci. Rep. 2020, 10, 16519. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.; Avots, A.; Kreth, H.W.; Serfling, E.; Schuster, V. Bruton’s Tyrosine Kinase Is Activated upon CD40 Stimulation in Human B Lymphocytes. Immunobiology 2002, 206, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Lee, K.G.; Ou, X.; Lam, K.P. Bruton’s Tyrosine Kinase and Protein Kinase C μ Are Required for TLR7/9-Induced IKKα and IRF-1 Activation and Interferon-β Production in Conventional Dendritic Cells. PLoS ONE 2014, 9, e105420. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzarello, A.N.; Koroveshi, B.; Guardo, D.; Lanza, L.; Ghiotto, F.; Bruno, S.; Cappelli, E. Unexpected CD5+ B Cell Lymphocytosis during SARS-CoV-2 Infection: Relevance for the Pathophysiology of Chronic Lymphocytic Leukemia. J. Clin. Med. 2023, 12, 998. https://doi.org/10.3390/jcm12030998
Mazzarello AN, Koroveshi B, Guardo D, Lanza L, Ghiotto F, Bruno S, Cappelli E. Unexpected CD5+ B Cell Lymphocytosis during SARS-CoV-2 Infection: Relevance for the Pathophysiology of Chronic Lymphocytic Leukemia. Journal of Clinical Medicine. 2023; 12(3):998. https://doi.org/10.3390/jcm12030998
Chicago/Turabian StyleMazzarello, Andrea Nicola, Brisejda Koroveshi, Daniela Guardo, Lorella Lanza, Fabio Ghiotto, Silvia Bruno, and Enrico Cappelli. 2023. "Unexpected CD5+ B Cell Lymphocytosis during SARS-CoV-2 Infection: Relevance for the Pathophysiology of Chronic Lymphocytic Leukemia" Journal of Clinical Medicine 12, no. 3: 998. https://doi.org/10.3390/jcm12030998
APA StyleMazzarello, A. N., Koroveshi, B., Guardo, D., Lanza, L., Ghiotto, F., Bruno, S., & Cappelli, E. (2023). Unexpected CD5+ B Cell Lymphocytosis during SARS-CoV-2 Infection: Relevance for the Pathophysiology of Chronic Lymphocytic Leukemia. Journal of Clinical Medicine, 12(3), 998. https://doi.org/10.3390/jcm12030998






