Revisiting the Rotational Field TMS Method for Neurostimulation
Abstract
1. Introduction
2. TMS Principles
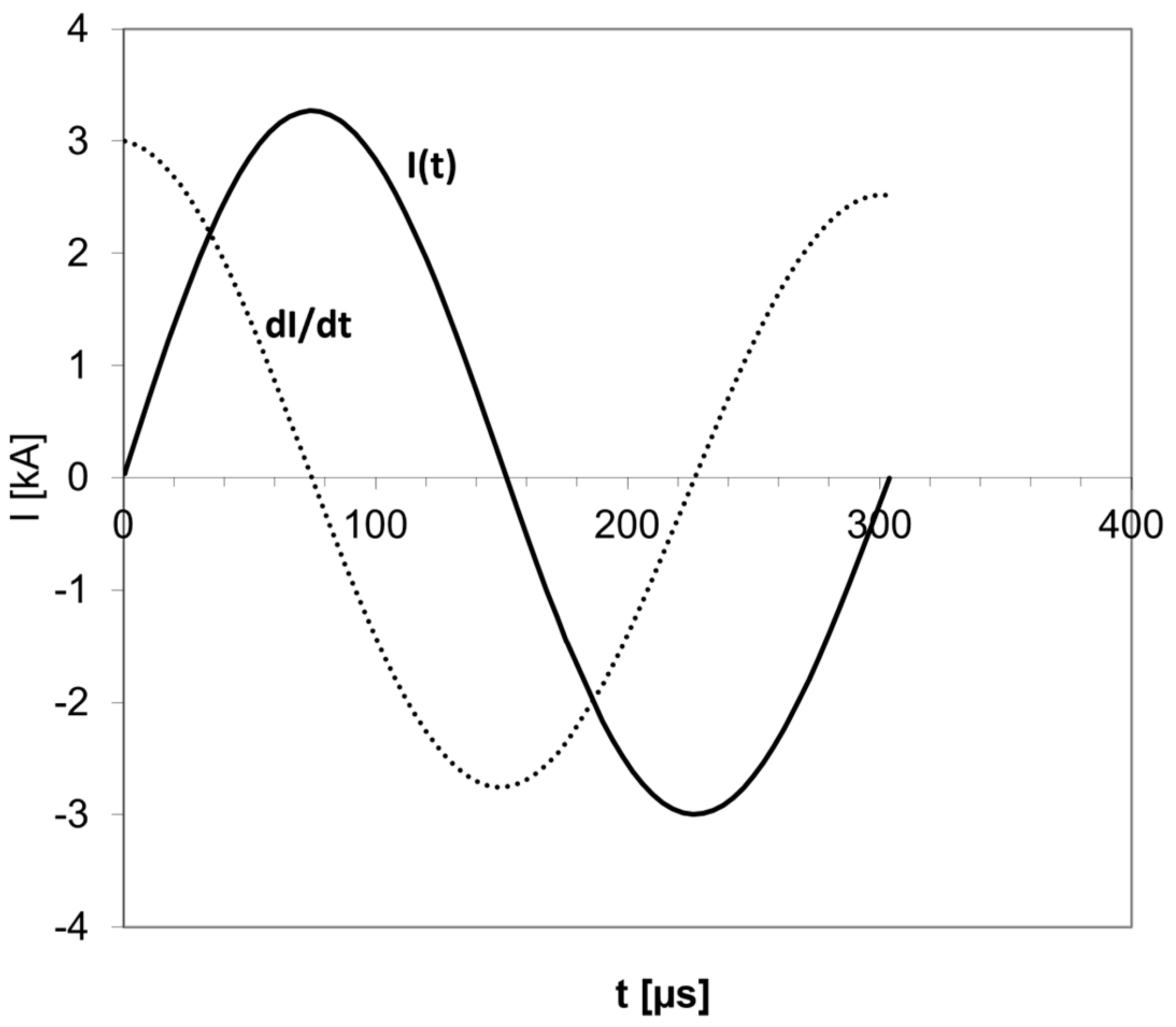
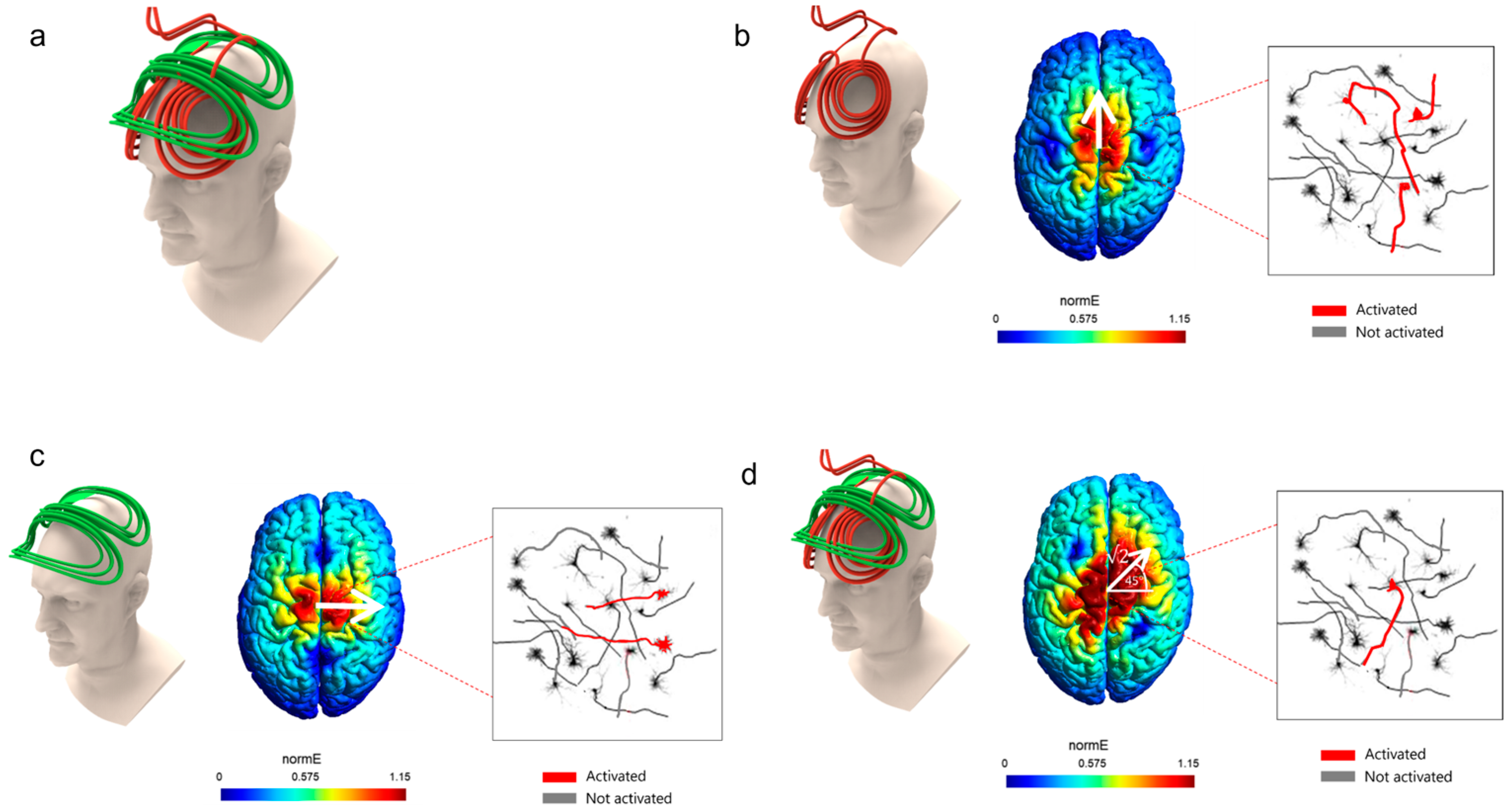
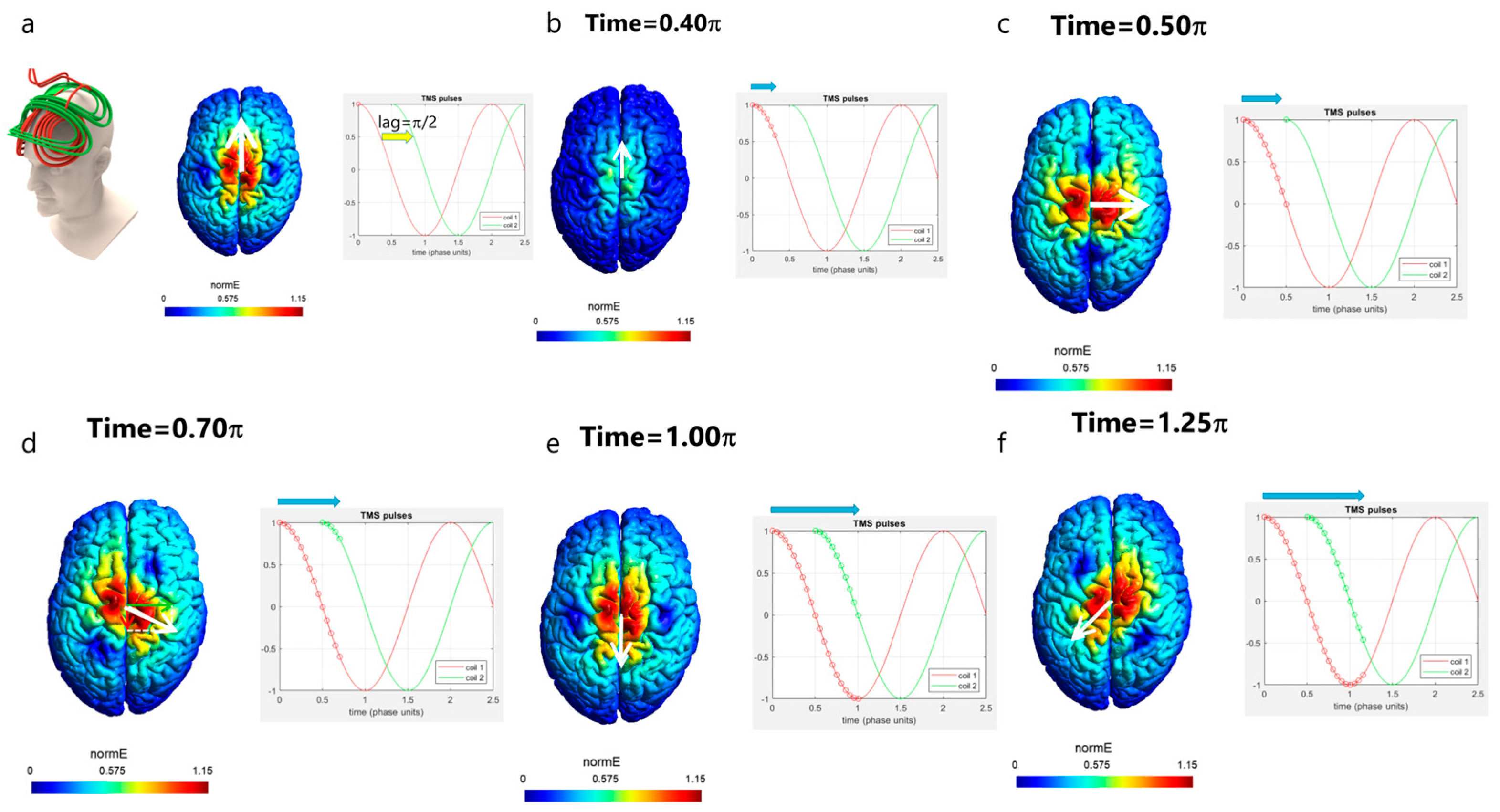
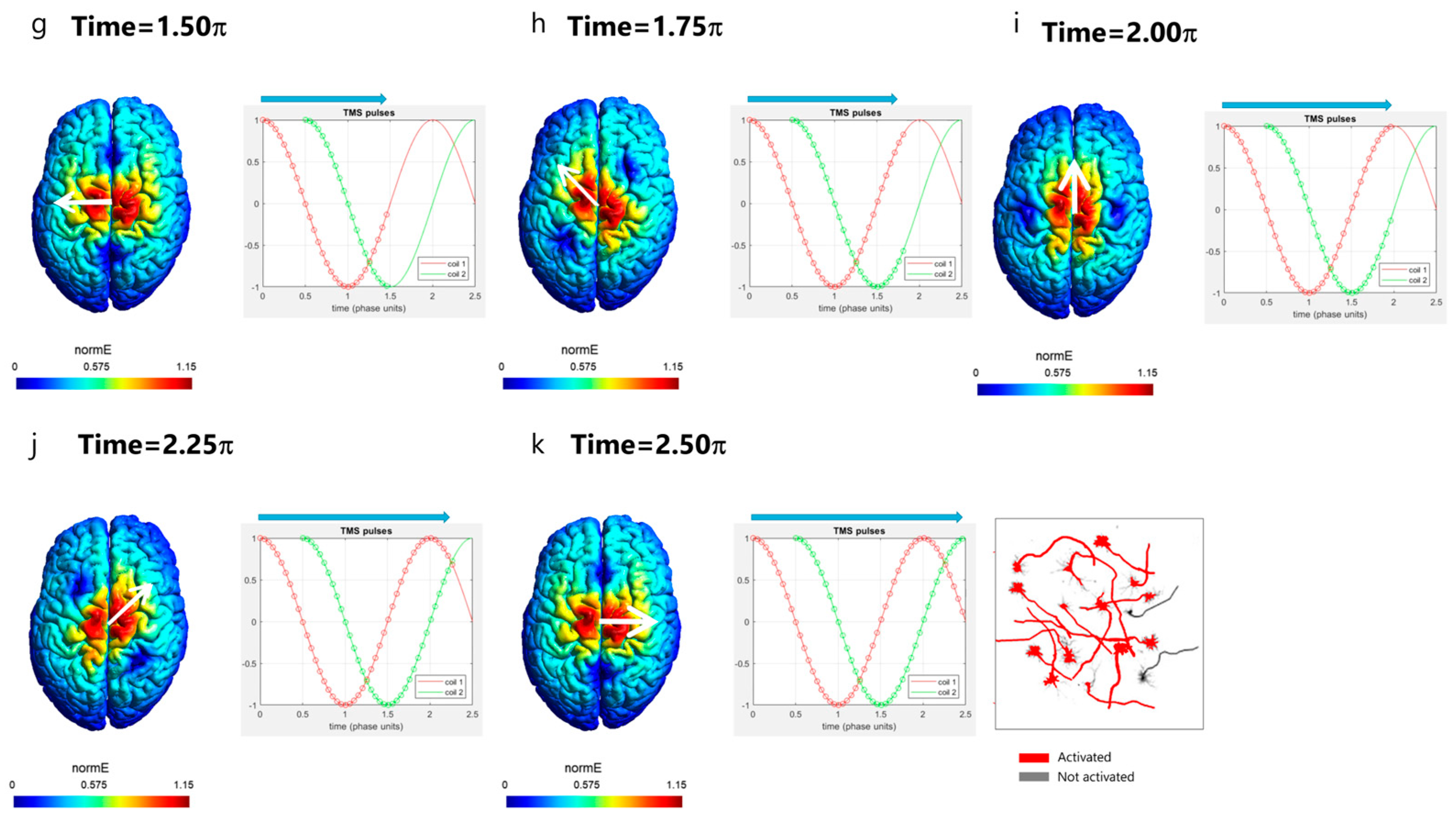
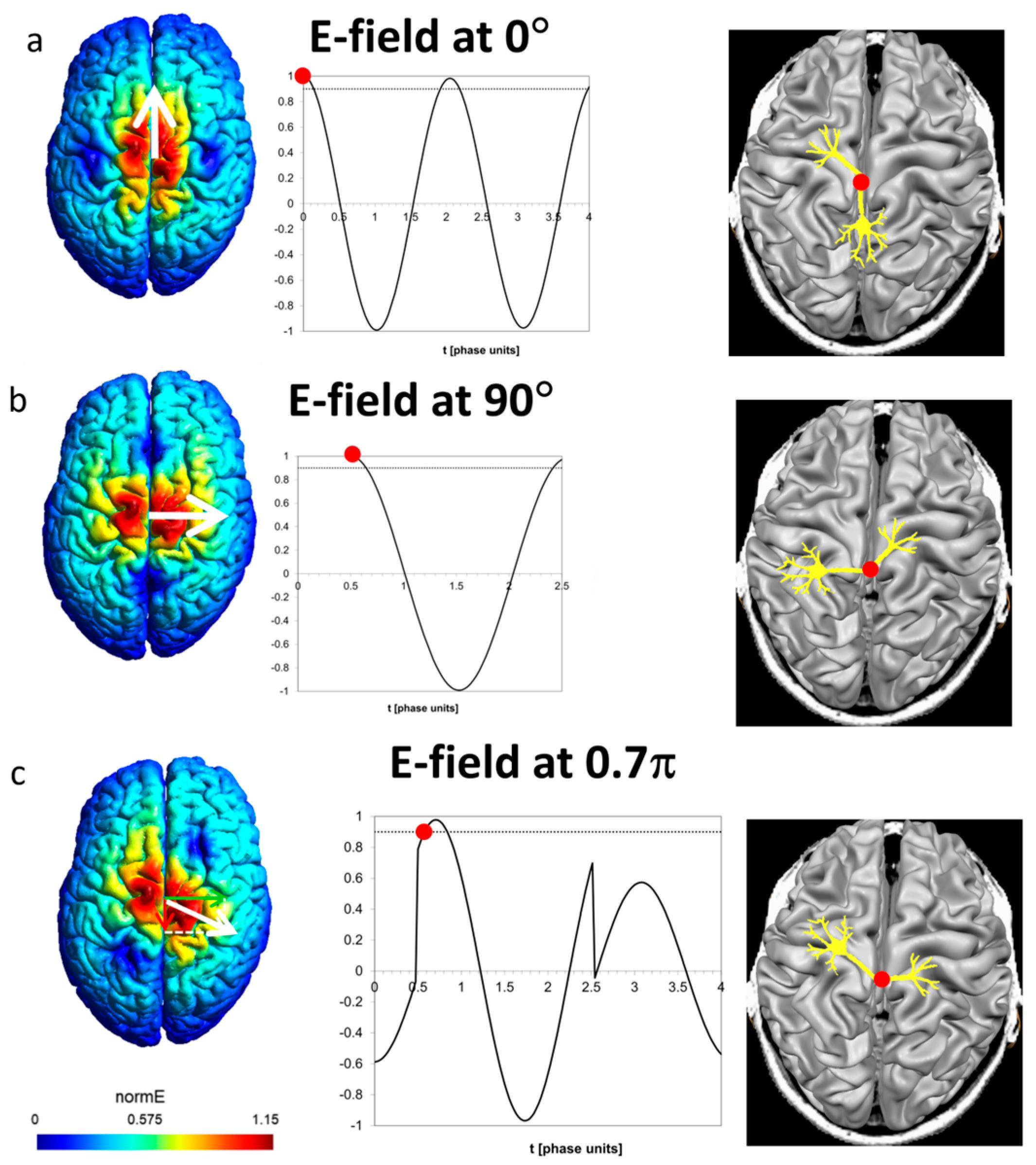
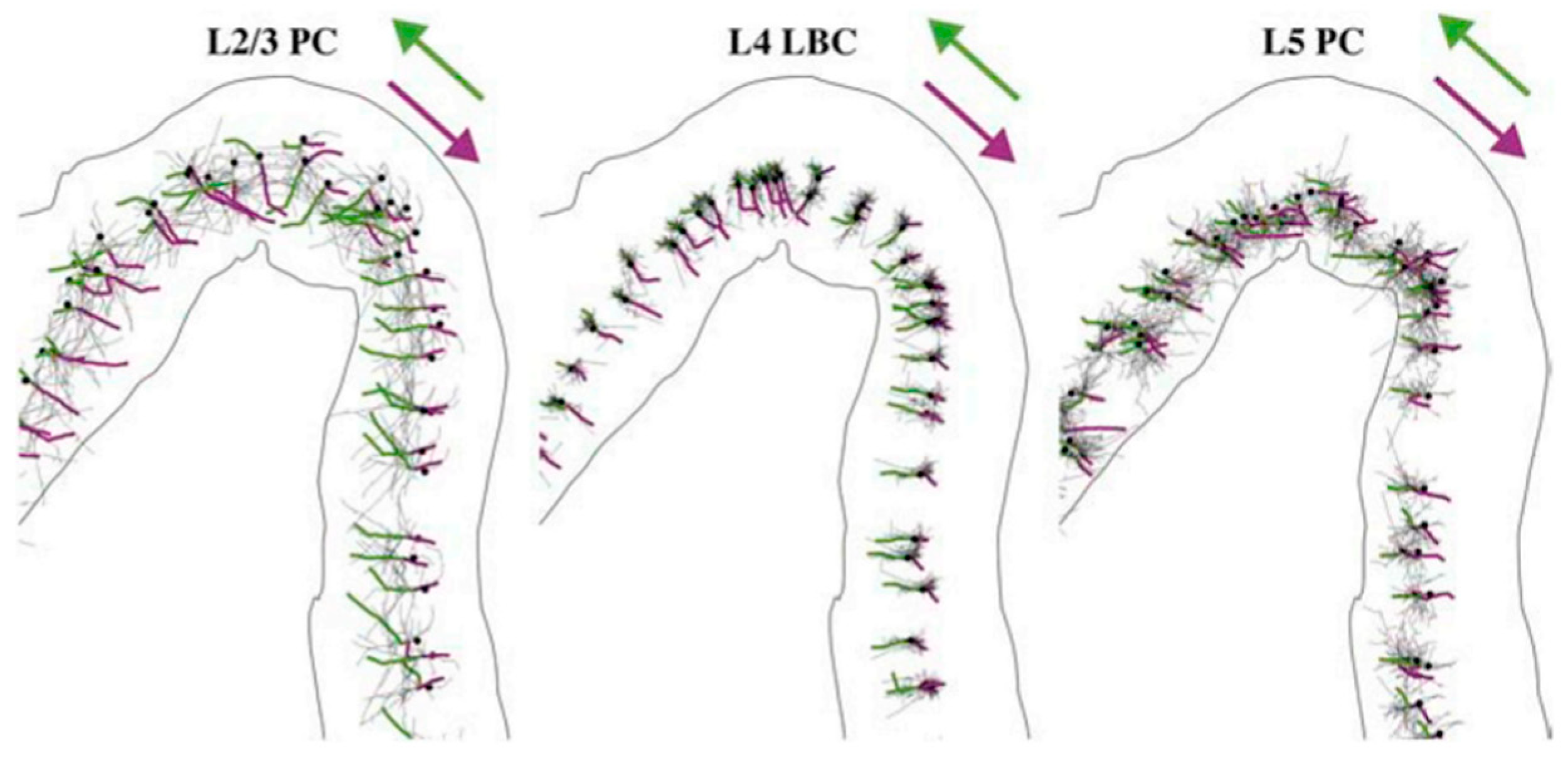
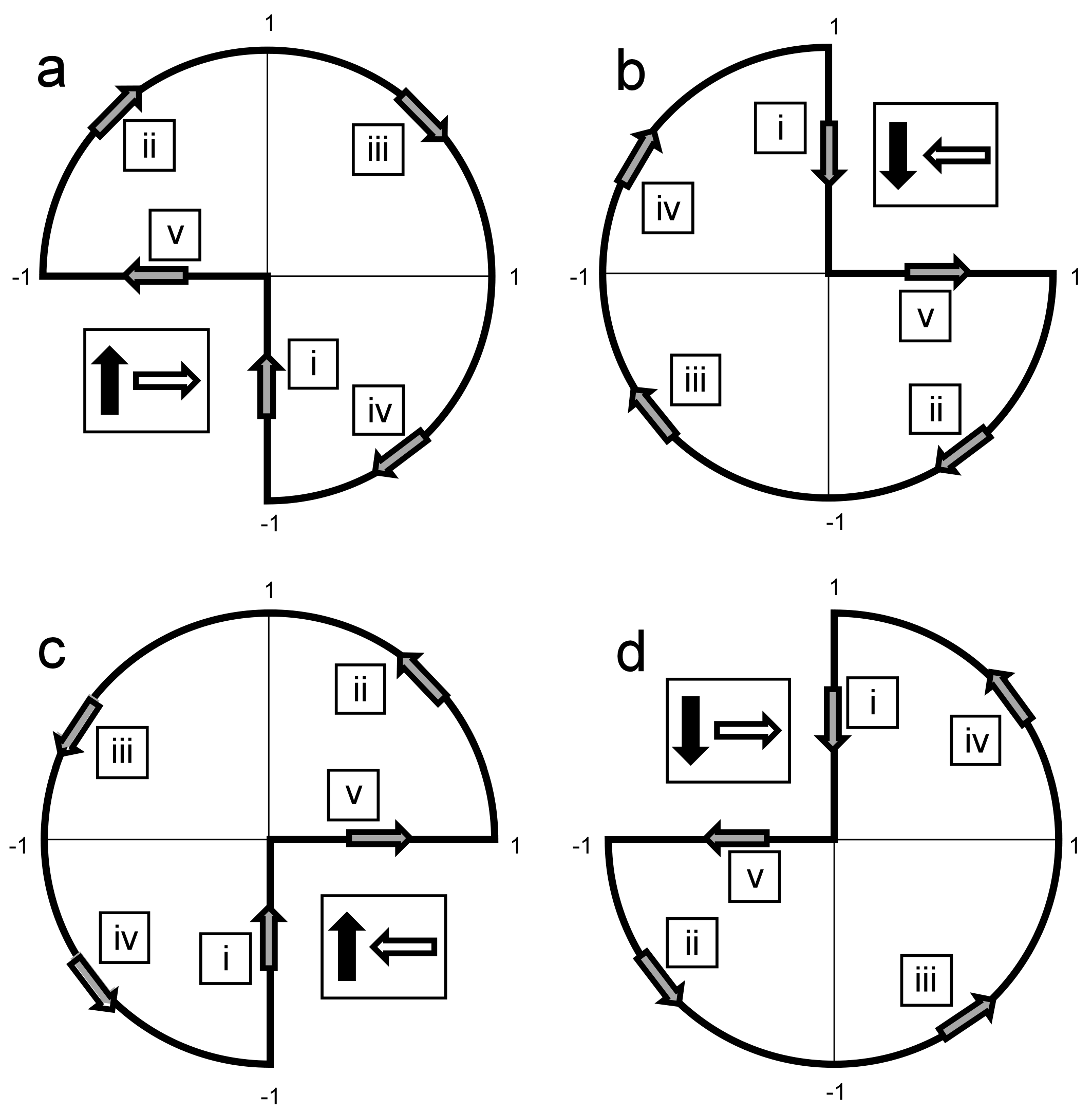
3. Rotational Field TMS (rfTMS) Method
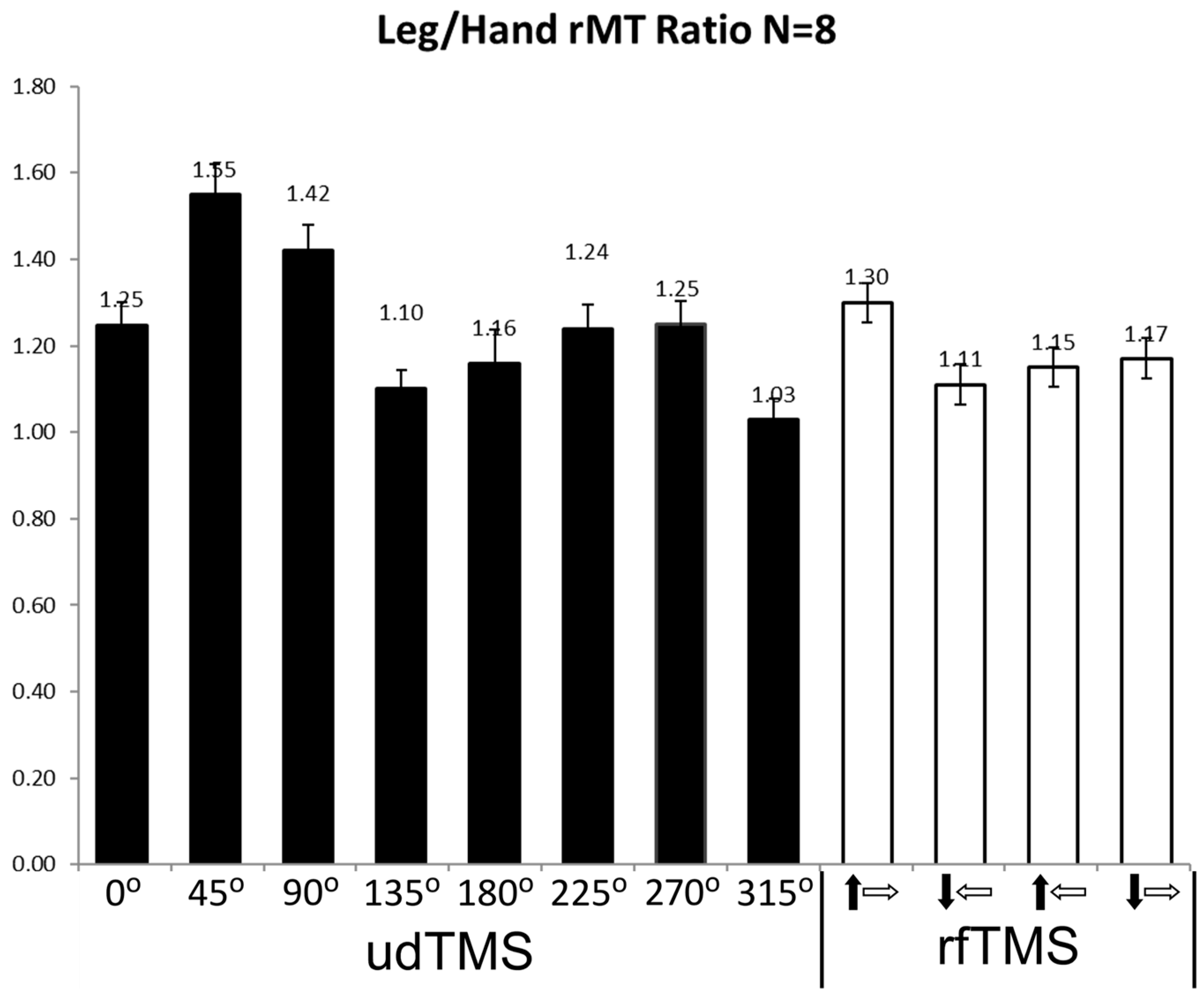
4. Re-Visiting the rfTMS Clinical Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
References
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2020, 132, 269–306. [Google Scholar] [CrossRef]
- Perera, T.; George, M.S.; Grammar, G.; Janicak, P.G.; Pascual-Leone, A.; Wirecki, T.S. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016, 9, 336–346. [Google Scholar] [CrossRef]
- Voigt, J.; Carpenter, L.; Leuchter, A. Cost effectiveness analysis comparing repetitive transcranial magnetic stimulation to antidepressant medications after a first treatment failure for major depressive disorder in newly diagnosed patients—A lifetime analysis. PLoS ONE 2017, 12, e0186950. [Google Scholar] [CrossRef]
- O’Reardon, J.P.; Solvason, H.B.; Janicak, P.G.; Sampson, S.; Isenberg, K.E.; Nahas, Z.; McDonald, W.M.; Avery, D.; Fitzgerald, P.B.; Loo, C.; et al. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol. Psychiatry 2007, 62, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Lisanby, S.H.; Avery, D.; McDonald, W.M.; Durkalski, V.; Pavlicova, M.; Anderson, B.; Nahas, Z.; Bulow, P.; Zarkowski, P.; et al. Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial. Arch. Gen. Psychiatry 2010, 67, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Levkovitz, Y.; Isserles, M.; Padberg, F.; Lisanby, S.H.; Bystritsky, A.; Xia, G.; Tendler, A.; Daskalakis, Z.J.; Winston, J.L.; Dannon, P.; et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: A prospective multicenter randomized controlled trial. World Psychiatry 2015, 14, 64–73. [Google Scholar] [CrossRef]
- Carmi, L.; Tendler, A.; Bystritsky, A.; Hollander, E.; Blumberger, D.M.; Daskalakis, J.; Ward, H.; Lapidus, K.; Goodman, W.; Casuto, L.; et al. Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. Am. J. Psychiatry 2019, 176, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Zangen, A.; Moshe, H.; Martinez, D.; Barnea-Ygael, N.; Vapnik, T.; Bystritsky, A.; Duffy, W.; Toder, D.; Casuto, L.; Grosz, M.L.; et al. Repetitive transcranial magnetic stimulation for smoking cessation: A pivotal multicenter double-blind randomized controlled trial. World Psychiatry 2021, 20, 397–404. [Google Scholar] [CrossRef]
- Zibman, S.; Pell, G.S.; Barnea-Ygael, N.; Roth, Y.; Zangen, A. Application of transcranial magnetic stimulation for major depression: Coil design and neuroanatomical variability considerations. Eur. Neuropsychopharmacol. 2021, 45, 73–88. [Google Scholar] [CrossRef]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, C.; Wang, J. Updated Review on the Clinical Use of Repetitive Transcranial Magnetic Stimulation in Psychiatric Disorders. Neurosci. Bull. 2017, 33, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U. Thirty years of transcranial magnetic stimulation: Where do we stand? Exp. Brain Res. 2017, 235, 973–984. [Google Scholar] [CrossRef]
- Ridding, M.C.; Ziemann, U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010, 588, 2291–2304. [Google Scholar] [CrossRef]
- Bergmann, T.O. Brain State-Dependent Brain Stimulation. Front. Psychol. 2018, 9, 2108. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, C.; Desideri, D.; Belardinelli, P.; Ziemann, U. Real-time EEG-defined excitability states determine efficacy of TMS-induced plasticity in human motor cortex. Brain Stimul. 2018, 11, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Thielscher, A.; Kammer, T. Linking physics with physiology in TMS: A sphere field model to determine the cortical stimulation site in TMS. Neuroimage 2002, 17, 1117–1130. [Google Scholar] [CrossRef]
- Silva, S.; Basser, P.J.; Miranda, P.C. Elucidating the mechanisms and loci of neuronal excitation by Transcranial Magnetic Stimulation using a finite element model of a cortical sulcus. Clin. Neurophysiol. 2008, 119, 2405–2413. [Google Scholar] [CrossRef]
- Opitz, A.; Windhoff, M.; Heidemann, R.M.; Turner, R.; Thielscher, A. How the brain tissue shapes the electric field induced by transcranial magnetic stimulation. Neuroimage 2011, 58, 849–859. [Google Scholar] [CrossRef]
- Pell, G.S.; Roth, Y.; Zangen, A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: Influence of timing and geometrical parameters and underlying mechanisms. Prog. Neurobiol. 2011, 93, 59–98. [Google Scholar] [CrossRef]
- Salvador, R.; Silva, S.; Basser, P.J.; Miranda, P.C. Determining which mechanisms lead to activation in the motor cortex: A modeling study of transcranial magnetic stimulation using realistic stimulus waveforms and sulcal geometry. Clin. Neurophysiol. 2011, 122, 748–758. [Google Scholar] [CrossRef]
- Thielscher, A.; Opitz, A.; Windhoff, M. Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. Neuroimage 2011, 54, 234–243. [Google Scholar] [CrossRef]
- Aberra, A.S.; Wang, B.; Grill, W.M.; Peterchev, A.V. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul. 2020, 13, 175–189. [Google Scholar] [CrossRef]
- López-Alonso, V.; Cheeran, B.; Río-Rodríguez, D.; Fernández-Del-Olmo, M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.R.; Boniface, S.J.; Schubert, M. Magnetic brain stimulation with a double coil: The importance of coil orientation. Electroencephalogr. Clin. Neurophysiol. 1992, 85, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Balslev, D.; Braet, W.; McAllister, C.; Miall, R.C. Inter-individual variability in optimal current direction for transcranial magnetic stimulation of the motor cortex. J. Neurosci. Methods 2007, 162, 309–313. [Google Scholar] [CrossRef]
- Souza, V.H.; Nieminen, J.O.; Tugin, S.; Koponen, L.M.; Baffa, O.; Ilmoniemi, R.J. TMS with fast and accurate electronic control: Measuring the orientation sensitivity of corticomotor pathways. Brain Stimul. 2022, 15, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Goldman-Rakic, P.S. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb. Cortex 1995, 5, 323–337. [Google Scholar] [CrossRef]
- Rotem, A.; Neef, A.; Neef, N.E.; Agudelo-Toro, A.; Rakhmilevitch, D.; Paulus, W.; Moses, E. Solving the Orientation Specific Constraints in Transcranial Magnetic Stimulation by Rotating Fields. PLoS ONE 2014, 9, e86794. [Google Scholar] [CrossRef]
- Roth, Y.; Pell, G.S.; Ankry, M.; Hadad, Y.; Eisen, A.; Burnishev, Y.; Tendler, A.; Moses, E.; Zangen, A. Comparing rotational-field-dTMS to unidirectional-dTMS in healthy volunteers. Brain Stimul. 2019, 12, 390. [Google Scholar] [CrossRef]
- Roth, Y.; Pell, G.S.; Barnea-Ygael, N.; Ankry, M.; Hadad, Y.; Eisen, A.; Burnishev, Y.; Tendler, A.; Moses, E.; Zangen, A. Rotational field TMS: Comparison with conventional TMS based on motor evoked potentials and thresholds in the hand and leg motor cortices. Brain Stimul. 2020, 13, 900–907. [Google Scholar] [CrossRef]
- Peterchev, A.V.; D’Ostilio, K.; Rothwell, J.C.; Murphy, D.L. Controllable pulse parameter transcranial magnetic stimulator with enhanced circuit topology and pulse shaping. J. Neural Eng. 2014, 11, 056023. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Pilato, F.; Saturno, E.; Dileone, M.; Mazzone, P.; Insola, A.; Tonali, P.A.; Rothwell, J.C. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin. Neurophysiol. 2004, 115, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, A.; Cohen, L.G.; Mall, V.; Kaelin-Lang, A.; Mima, T.; Rossi, S.; Thickbroom, G.W.; et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef]
- Epstein, C.M.; Schwartzberg, D.G.; Davey, K.R.; Sudderth, D.B. Localizing the site of magnetic brain stimulation in humans. Neurology 1990, 49, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Rudiak, D.; Marg, E. Finding the depth of magnetic brain stimulation: A re-evaluation. Electroencephalogr. Clin. Neurophys. 1994, 93, 358–371. [Google Scholar] [CrossRef]
- Terao, Y.; Ugawa, Y.; Sakai, K.; Miyauchi, S.; Fukuda, H.; Sasaki, Y.; Takino, R.; Hanajima, R.; Furubayashi, T.; Pütz, B.; et al. Localizing the site of magnetic brain stimulation by functional MRI. Exp. Brain Res. 1998, 121, 145–152. [Google Scholar] [CrossRef]
- Roth, Y.; Pell, G.S.; Chistyakov, A.V.; Sinai, A.; Zangen, A.; Zaaroor, M. Motor cortex activation by H-coil and figure-8 coil at different depths. Combined motor threshold and electric field distribution study. Clin. Neurophysiol. 2014, 125, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Terao, Y.; Ugawa, Y.; Hanajima, R.; Machii, K.; Furubayashi, T.; Mochizuki, H.; Enomoto, H.; Shiio, Y.; Uesugi, H.; Iwata, N.K.; et al. Predominant activation of I1-waves from the leg motor area by transcranial magnetic stimulation. Brain Res. 2000, 859, 137–146. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Profice, P.; Meglio, M.; Cioni, B.; Tonali, P.; Rothwell, J.C. Descending spinal cord volleys evoked by transcranial magnetic and electrical stimulation of the motor cortex leg area in conscious humans. J. Physiol. 2001, 537, 1047–1058. [Google Scholar] [CrossRef]
- Ho, K.; Cirillo, J.; Ren, A.; Byblow, W.D. Intracortical facilitation and inhibition in human primary motor cortex during motor skill acquisition. Exp. Brain Res. 2022, 240, 3289–3304. [Google Scholar] [CrossRef]
- Filipčić, I.; Filipčić, I.Š.; Milovac, Ž.; Sučić, S.; Gajšak, T.; Ivezić Bašić, S.; Bajić, Ž.; Heilig, M. Efficacy of repetitive transcranial magnetic stimulation using a figure-8-coil or an H1-coil in treatment of major depressive disorder; a randomized clinical trial. J. Psychiatr. Res. 2019, 114, 113–119. [Google Scholar] [CrossRef]
- Petrides, M. Lateral prefrontal cortex: Architectonic and functional organization. Philos. Trans. R Soc. Lond. B Biol. Sci. 2005, 360, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Tames, J.; Hamasaka, A.; Laakso, I.; Hirata, A.; Ugawa, Y. Atlas of Optimal Coil Orientation and Position for TMS: A Computational Study. Brain Stimul. 2018, 11, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Liu, H.; Pascual-Leone, A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. NeuroImage 2013, 66, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.; Horn, A.; Caballero, R.; Cooke, D.; Stern, A.P.; Taylor, S.F.; Press, D.; Pascual-Leone, A.; Fox, M.D. Prospective Validation That Subgenual Connectivity Predicts Antidepressant Efficacy of Transcranial Magnetic Stimulation Sites. Biol. Psychiatry 2018, 84, 28–37. [Google Scholar] [CrossRef]
- Cash, R.F.H.; Cocchi, L.; Lv, J.; Fitzgerald, P.B.; Zalesky, A. Functional Magnetic Resonance Imaging–Guided Personalization of Transcranial Magnetic Stimulation Treatment for Depression. JAMA Psychiatry 2021, 78, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Balderston, N.L.; Beer, J.C.; Seok, D.; Makhoul, W.; Deng, Z.D.; Girelli, T.; Teferi, M.; Smyk, N.; Jaskir, M.; Oathes, D.J.; et al. Proof of concept study to develop a novel connectivity-based electric-field modelling approach for individualized targeting of transcranial magnetic stimulation treatment. Neuropsychopharmacology 2022, 47, 588–598. [Google Scholar] [CrossRef]
- Ziemann, U.; Paulus, W.; Nitsche, M.A.; Pascual-Leone, A.; Byblow, W.D.; Berardelli, A.; Siebner, H.R.; Classen, J.; Cohen, L.G.; Rothwell, J.C. Consensus: Motor cortex plasticity protocols. Brain Stimul. 2008, 1, 164–182. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta burst stimulation of the human motor cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef]
- Hamada, M.; Murase, N.; Hasan, A.; Balaratnam, M.; Rothwell, J.C. The Role of Interneuron Networks in Driving Human Motor Cortical Plasticity. Cereb. Cortex 2013, 23, 1593–1605. [Google Scholar] [CrossRef]
- Nettekoven, C.; Volz, L.J.; Leimbach, M.; Pool, E.M.; Rehme, A.K.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Inter-individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. Neuroimage 2015, 118, 209–218. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef]
- Schulze, L.; Feffer, K.; Lozano, C.; Giacobbe, P.; Daskalakis, Z.J.; Blumberger, D.M.; Downar, J. Number of pulses or number of sessions? An open-label study of trajectories of improvement for once-vs. twice-daily dorsomedial prefrontal rTMS in major depression. Brain Stimul. 2018, 11, 327–336. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Hoy, K.E.; Reynolds, J.; Singh, A.; Gunewardene, R.; Slack, C.; Ibrahim, S.; Daskalakis, Z.J. A pragmatic randomized controlled trial exploring the relationship between pulse number and response to repetitive transcranial magnetic stimulation treatment in depression. Brain Stimul. 2020, 13, 145–152. [Google Scholar] [CrossRef]
- McCalley, D.M.; Lench, D.H.; Doolittle, J.D.; Imperatore, J.P.; Hoffman, M.; Hanlon, C.A. Determining the optimal pulse number for theta burst induced change in cortical excitability. Sci. Rep. 2021, 11, 8726. [Google Scholar] [CrossRef]
- Day, B.L.; Dressler, D.; Maertens de Noordhout, A.; Marsden, C.D.; Nakashima, K.; Rothwell, J.C.; Thompson, P.D. Electric and magnetic stimulation of human motor cortex: Surface EMG and single motor unit responses. J. Physiol. 1989, 412, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Ugawa, Y.; Terao, Y.; Hanajima, R.; Furubayashi, T.; Kanazawa, I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp. Brain Res. 1997, 113, 24–32. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Profice, P.; Saturno, E.; Pilato, F.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr. Clin. Neurophysiol. 1998, 109, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Volz, L.J.; Hamada, M.; Michely, J.; Pool, E.M.; Nettekoven, C.; Rothwell, J.C.; Grefkes Hermann, C. Modulation of I-wave generating pathways by theta-burst stimulation: A model of plasticity induction. J. Physiol. 2019, 597, 5963–5971. [Google Scholar] [CrossRef]
- Tang, A.; Thickbroom, G.; Rodger, J. Repetitive Transcranial Magnetic Stimulation of the Brain: Mechanisms from Animal and Experimental Models. Neuroscientist 2017, 23, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Müller-Dahlhaus, F.; Ziemann, U. Metaplasticity in human cortex. Neuroscientist 2015, 21, 185–202. [Google Scholar] [CrossRef]
- Thomson, A.C.; Sack, A.T. How to Design Optimal Accelerated rTMS Protocols Capable of Promoting Therapeutically Beneficial Metaplasticity. Front Neurol. 2020, 11, 599918. [Google Scholar] [CrossRef]
- Cantone, M.; Lanza, G.; Ranieri, F.; Opie, G.M.; Terranova, C. Editorial: Non-invasive Brain Stimulation in the Study and Modulation of Metaplasticity in Neurological Disorders. Front Neurol. 2021, 12, 721906. [Google Scholar] [CrossRef]
- Green, J.B. Brain reorganization after stroke. Top. Stroke Rehabil. 2003, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tofts, P.S. The distribution of induced currents in magnetic stimulation of the nervous system. Phys. Med. Biol. 1990, 35, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Tofts, P.S.; Branston, N.M. The measurement of electric field, and the influence of surface charge, in magnetic stimulation. Electroencephal. Clin. Neurophysiol. 1991, 81, 238–239. [Google Scholar] [CrossRef]
- Eaton, H. Electric field induced in a spherical volume conductor from arbitrary coils: Application to magnetic stimulation and MEG. Med. Biol. Eng. Comput. 1992, 30, 433–440. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, Y.; Zibman, S.; Pell, G.S.; Zangen, A.; Tendler, A. Revisiting the Rotational Field TMS Method for Neurostimulation. J. Clin. Med. 2023, 12, 983. https://doi.org/10.3390/jcm12030983
Roth Y, Zibman S, Pell GS, Zangen A, Tendler A. Revisiting the Rotational Field TMS Method for Neurostimulation. Journal of Clinical Medicine. 2023; 12(3):983. https://doi.org/10.3390/jcm12030983
Chicago/Turabian StyleRoth, Yiftach, Samuel Zibman, Gaby S. Pell, Abraham Zangen, and Aron Tendler. 2023. "Revisiting the Rotational Field TMS Method for Neurostimulation" Journal of Clinical Medicine 12, no. 3: 983. https://doi.org/10.3390/jcm12030983
APA StyleRoth, Y., Zibman, S., Pell, G. S., Zangen, A., & Tendler, A. (2023). Revisiting the Rotational Field TMS Method for Neurostimulation. Journal of Clinical Medicine, 12(3), 983. https://doi.org/10.3390/jcm12030983






