Efficacy of Submicron Dispersible Free Phytosterols on Non-Alcoholic Fatty Liver Disease: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Clinical and Metabolic Assessment
2.5. Liver Steatosis Quantification
2.6. Investigational Product (IP)
2.7. Statistical Analyses
3. Results
3.1. Subjects’ Characteristics
3.2. Liver and Plasma Lipid Level Comparison after Treatment with SDP
3.3. Plasma Concentration of Apoproteins, Lipoproteins and the Size of Lipoproteins after Treatment with SDP
3.4. Biochemical, Anthropometric and Metabolic Parameters after Treatment with SDP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Videla, L.A. Impact of the co-administration of N-3 fatty acids and olive oil components in preclinical nonalcoholic fatty liver disease models: A mechanistic view. Nutrients 2020, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Rajak, S.; Upadhyay, A.; Tewari, A.; Sinha, R.A. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front. Biosci. Landmark Ed. 2021, 26, 206. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegård, L.; Jessup, W.; Jones, P.J.; Lütjohann, D.; Maerz, W.; Masana, L. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef]
- Hassan, A.S.; Rampone, A.J. Effect of β-sitostanol (5α-stigmastan-3β-ol) on cholesterol absorption from micellar solutions in jejunal loops urlbar|situ. Steroids 1980, 36, 731–741. [Google Scholar] [CrossRef]
- Nissinen, M.; Gylling, H.; Vuoristo, M.; Miettinen, T.A. Micellar distribution of cholesterol and phytosterols after duodenal plant stanol ester infusion. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 282, G1009–G1015. [Google Scholar] [CrossRef]
- Brufau, G.; Kuipers, F.; Lin, Y.; Trautwein, E.A.; Groen, A.K. A reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PLoS ONE 2011, 6, e21576. [Google Scholar] [CrossRef]
- Demonty, I.; Ras, R.T.; van der Knaap, H.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: A pooled analysis of 12 randomised controlled trials. Eur. J. Nutr. 2013, 52, 153–160. [Google Scholar] [CrossRef]
- Naumann, E.; Plat, J.; Kester, A.D.; Mensink, R.P. The baseline serum lipoprotein profile is related to plant stanol induced changes in serum lipoprotein cholesterol and triacylglycerol concentrations. J. Am. Coll. Nutr. 2008, 27, 117–126. [Google Scholar] [CrossRef]
- Gylling, H.; Simonen, P. Phytosterols, phytostanols, and lipoprotein metabolism. Nutrients 2015, 7, 7965–7977. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Wang, T.; Zheng, F.; Wang, H.; Wang, C. Dietary wood pulp-derived sterols modulation of cholesterol metabolism and gut microbiota in high-fat-diet-fed hamsters. Food Funct. 2019, 10, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Qu, D.; Zhang, Q.; Zhou, H.; Jiang, R.; Li, Y.; Zhang, Y.; Yan, H. Phytosterol esters attenuate hepatic steatosis in rats with non-alcoholic fatty liver disease rats fed a high-fat diet. Sci. Rep. 2017, 7, 41604. [Google Scholar] [CrossRef]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Plat, J.; Hendrikx, T.; Bieghs, V.; Jeurissen, M.L.; Walenbergh, S.M.; van Gorp, P.J.; De Smet, E.; Konings, M.; Vreugdenhil, A.C.; Guichot, Y.D. Protective role of plant sterol and stanol esters in liver inflammation: Insights from mice and humans. PLoS ONE 2014, 9, e110758. [Google Scholar] [CrossRef]
- Chen, D.-L.; Huang, P.-H.; Chiang, C.-H.; Leu, H.-B.; Chen, J.-W.; Lin, S.-J. Phytosterols increase circulating endothelial progenitor cells and insulin-like growth factor-1 levels in patients with nonalcoholic fatty liver disease: A randomized crossover study. J. Funct. Foods 2015, 13, 148–157. [Google Scholar] [CrossRef]
- Song, L.; Zhao, X.G.; Ouyang, P.L.; Guan, Q.; Yang, L.; Peng, F.; Du, H.; Yin, F.; Yan, W.; Yu, W.J. Combined effect of n-3 fatty acids and phytosterol esters on alleviating hepatic steatosis in non-alcoholic fatty liver disease subjects: A double-blind placebo-controlled clinical trial. Br. J. Nutr. 2020, 123, 1148–1158. [Google Scholar] [CrossRef]
- Cicero, A.F.; Fogacci, F.; Bove, M.; Veronesi, M.; Rizzo, M.; Giovannini, M.; Borghi, C. Short-term effects of a combined nutraceutical on lipid level, fatty liver biomarkers, hemodynamic parameters, and estimated cardiovascular disease risk: A double-blind, placebo-controlled randomized clinical trial. Adv. Ther. 2017, 34, 1966–1975. [Google Scholar] [CrossRef]
- Mohammad Shahi, M.; Javanmardi, M.A.; Seyedian, S.S.; Haghighizadeh, M.H. Effects of phytosterol supplementation on serum levels of lipid profiles, liver enzymes, inflammatory markers, adiponectin, and leptin in patients affected by nonalcoholic fatty liver disease: A double-blind, placebo-controlled, randomized clinical trial. J. Am. Coll. Nutr. 2018, 37, 651–658. [Google Scholar] [CrossRef]
- Kurano, M.; Hasegawa, K.; Kunimi, M.; Hara, M.; Yatomi, Y.; Teramoto, T.; Tsukamoto, K. Sitosterol prevents obesity-related chronic inflammation. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2018, 1863, 191–198. [Google Scholar] [CrossRef]
- Shaghaghi, M.A.; Harding, S.V.; Jones, P.J. Water dispersible plant sterol formulation shows improved effect on lipid profile compared to plant sterol esters. J. Funct. Foods 2014, 6, 280–289. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Patel, J.; Bettencourt, R.; Cui, J.; Salotti, J.; Hooker, J.; Bhatt, A.; Hernandez, C.; Nguyen, P.; Aryafar, H.; Valasek, M. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Ther. Adv. Gastroenterol. 2016, 9, 692–701. [Google Scholar] [CrossRef]
- Mallol, R.; Amigó, N.; Rodríguez, M.A.; Heras, M.; Vinaixa, M.; Plana, N.; Rock, E.; Ribalta, J.; Yanes, O.; Masana, L. Liposcale: A novel advanced lipoprotein test based on 2D diffusion-ordered 1H NMR spectroscopy [S]. J. Lipid Res. 2015, 56, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Ahmida, H.M.; Bertucci, P.; Franzò, L.; Massoud, R.; Cortese, C.; Lala, A.; Federici, G. Simultaneous determination of plasmatic phytosterols and cholesterol precursors using gas chromatography–mass spectrometry (GC–MS) with selective ion monitoring (SIM). J. Chromatogr. B 2006, 842, 43–47. [Google Scholar] [CrossRef]
- Herrera, R.; Peñaloza, F.; Arrieta, C.; Zacconi, F.; Saavedra, V.; Saavedra, C.; Brañes, C.; Hack, T.; Uribe, S. Quantification of liver fat infiltration by magnetic resonance. Rev. Med. Chile 2019, 147, 821–827. [Google Scholar] [CrossRef]
- Tang, A.; Desai, A.; Hamilton, G.; Wolfson, T.; Gamst, A.; Lam, J.; Clark, L.; Hooker, J.; Chavez, T.; Ang, B.D. Accuracy of MR imaging–estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 2015, 274, 416. [Google Scholar] [CrossRef]
- Campos-Murguía, A.; Ruiz-Margáin, A.; González-Regueiro, J.A.; Macías-Rodríguez, R.U. Clinical assessment and management of liver fibrosis in non-alcoholic fatty liver disease. World J. Gastroenterol. 2020, 26, 5919. [Google Scholar] [CrossRef]
- German, J.B.; Smilowitz, J.T.; Zivkovic, A.M. Lipoproteins: When size really matters. Curr. Opin. Colloid Interface Sci. 2006, 11, 171–183. [Google Scholar] [CrossRef]
- Frasinariu, O.; Serban, R.; Trandafir, L.M.; Miron, I.; Starcea, M.; Vasiliu, I.; Alisi, A.; Temneanu, O.R. The Role of Phytosterols in Nonalcoholic Fatty Liver Disease. Nutrients 2022, 14, 2187. [Google Scholar] [CrossRef]
- Othman, R.A.; Moghadasian, M.H. Beyond cholesterol-lowering effects of plant sterols: Clinical and experimental evidence of anti-inflammatory properties. Nutr. Rev. 2011, 69, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sala, A.; Blanco-Morales, V.; Cilla, A.; Silvestre, R.Á.; Hernández-Álvarez, E.; Granado-Lorencio, F.; Barberá, R.; Garcia-Llatas, G. A positive impact on the serum lipid profile and cytokines after the consumption of a plant sterol-enriched beverage with a milk fat globule membrane: A clinical study. Food Funct. 2018, 9, 5209–5219. [Google Scholar] [CrossRef]
- Palmeiro-Silva, Y.K.; Aravena, R.I.; Ossio, L.; Parro Fluxa, J. Effects of daily consumption of an aqueous dispersion of free-phytosterols nanoparticles on individuals with metabolic syndrome: A randomised, double-blind, placebo-controlled clinical trial. Nutrients 2020, 12, 2392. [Google Scholar] [CrossRef] [PubMed]
- Fransen, H.P.; de Jong, N.; Wolfs, M.; Verhagen, H.; Verschuren, W.M.; Lütjohann, D.; von Bergmann, K.; Plat, J.; Mensink, R.P. Customary use of plant sterol and plant stanol enriched margarine is associated with changes in serum plant sterol and stanol concentrations in humans. J. Nutr. 2007, 137, 1301–1306. [Google Scholar] [CrossRef]
- Gylling, H.; Hallikainen, M.; Raitakari, O.T.; Laakso, M.; Vartiainen, E.; Salo, P.; Korpelainen, V.; Sundvall, J.; Miettinen, T.A. Long-term consumption of plant stanol and sterol esters, vascular function and genetic regulation. Br. J. Nutr. 2008, 101, 1688–1695. [Google Scholar] [CrossRef]
- Kelly, E.R.; Plat, J.; Mensink, R.P.; Berendschot, T.T. Effects of long term plant sterol and-stanol consumption on the retinal vasculature: A randomized controlled trial in statin users. Atherosclerosis 2011, 214, 225–230. [Google Scholar] [CrossRef]
- de Jong, A.; Plat, J.; Lütjohann, D.; Mensink, R.P. Effects of long-term plant sterol or stanol ester consumption on lipid and lipoprotein metabolism in subjects on statin treatment. Br. J. Nutr. 2008, 100, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology 2018, 68, 763–772. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver fat imaging—A clinical overview of ultrasound, CT, and MR imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef]
- Jones, P.J. Inter-individual variability in response to plant sterol and stanol consumption. J. AOAC Int. 2015, 98, 724–728. [Google Scholar] [CrossRef]
- Patel, K.; Harrison, S.A.; Elkhashab, M.; Trotter, J.F.; Herring, R.; Rojter, S.E.; Kayali, Z.; Wong, V.W.S.; Greenbloom, S.; Jayakumar, S. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: A phase 2 randomized controlled trial. Hepatology 2020, 72, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Farooqui, K.J.; Singh, M.K.; Wasir, J.S.; Bansal, B.; Kaur, P.; Jevalikar, G.; Gill, H.K. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: A randomized controlled trial (E-LIFT Trial). Diabetes Care 2018, 41, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.M.; Guzman, G.; Correa De Mello, L.L.; Trein, B.; Spina, L.; Bussade, I.; Marques Prata, J.; Sajoux, I.; Countinho, W. Efficacy of a 2-month very low-calorie ketogenic diet (VLCKD) compared to a standard low-calorie diet in reducing visceral and liver fat accumulation in patients with obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a low-fat vegan diet on body weight, insulin sensitivity, postprandial metabolism, and intramyocellular and hepatocellular lipid levels in overweight adults: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2025454. [Google Scholar] [CrossRef]
- Wainwright, P.; Byrne, C.D. Bidirectional relationships and disconnects between NAFLD and features of the metabolic syndrome. Int. J. Mol. Sci. 2016, 17, 367. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wong, G.H.; Chan, H.Y.; Chan, H.Y.; Yeung, D.W.; Chan, R.M.; Chim, A.L.; Chan, A.H.; Choi, P.L.; Woo, J. PNPLA 3 gene polymorphism accounts for fatty liver in community subjects without metabolic syndrome. Aliment. Pharmacol. Ther. 2014, 39, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Kantartzis, K.; Peter, A.; Machicao, F.; Machann, J.; Wagner, S.; Königsrainer, I.; Königsrainer, A.; Schick, F.; Fritsche, A.; Häring, H.-U. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes 2009, 58, 2616–2623. [Google Scholar] [CrossRef]
- Plat, J.; Brufau, G.; Dallinga-Thie, G.M.; Dasselaar, M.; Mensink, R.P. A plant stanol yogurt drink alone or combined with a low-dose statin lowers serum triacylglycerol and non-HDL cholesterol in metabolic syndrome patients. J. Nutr. 2009, 139, 1143–1149. [Google Scholar] [CrossRef]
- Plat, J.; Mensink, R.P. Plant stanol esters lower serum triacylglycerol concentrations via a reduced hepatic VLDL-1 production. Lipids 2009, 44, 1149–1153. [Google Scholar] [CrossRef]
- Holmes, M.V.; Millwood, I.Y.; Kartsonaki, C.; Hill, M.R.; Bennett, D.A.; Boxall, R.; Guo, Y.; Xu, X.; Bian, Z.; Hu, R. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J. Am. Coll. Cardiol. 2018, 71, 620–632. [Google Scholar] [CrossRef]
- Garoufi, A.; Vorre, S.; Soldatou, A.; Tsentidis, C.; Kossiva, L.; Drakatos, A.; Marmarinos, A.; Gourgiotis, D. Plant sterols–enriched diet decreases small, dense LDL-cholesterol levels in children with hypercholesterolemia: A prospective study. Ital. J. Pediatr. 2014, 40, 42. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Miettinen, T.A. Effects of inhibiting cholesterol absorption and synthesis on cholesterol and lipoprotein metabolism in hypercholesterolemic non-insulin-dependent diabetic men. J. Lipid Res. 1996, 37, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Volek, J.S.; Udani, J.; Wood, R.J.; Greene, C.M.; Aggarwal, D.; Contois, J.H.; Kavoussi, B.; Fernandez, M.L. A combination therapy including psyllium and plant sterols lowers LDL cholesterol by modifying lipoprotein metabolism in hypercholesterolemic individuals. J. Nutr. 2006, 136, 2492–2497. [Google Scholar] [CrossRef] [PubMed]
- Sialvera, T.; Pounis, G.; Koutelidakis, A.; Richter, D.; Yfanti, G.; Kapsokefalou, M.; Goumas, G.; Chiotinis, N.; Diamantopoulos, E.; Zampelas, A. Phytosterols supplementation decreases plasma small and dense LDL levels in metabolic syndrome patients on a westernized type diet. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. Int. J. Mol. Sci. 2022, 23, 3967. [Google Scholar] [CrossRef]
- Khera, A.V.; Demler, O.V.; Adelman, S.J.; Collins, H.L.; Glynn, R.J.; Ridker, P.M.; Rader, D.J.; Mora, S. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: An analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 2017, 135, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Chapman, M.J. Antiatherogenic small, dense HDL—Guardian angel of the arterial wall? Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 144–153. [Google Scholar] [CrossRef]
- Baumgartner, S.; Ras, R.T.; Trautwein, E.A.; Mensink, R.P.; Plat, J. Plasma fat-soluble vitamin and carotenoid concentrations after plant sterol and plant stanol consumption: A meta-analysis of randomized controlled trials. Eur. J. Nutr. 2017, 56, 909–923. [Google Scholar] [CrossRef]
- Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Vitamin D and metabolic dysfunction-associated fatty liver disease (MAFLD): An update. Nutrients 2020, 12, 3302. [Google Scholar] [CrossRef]
- Roth, C.L.; Elfers, C.T.; Figlewicz, D.P.; Melhorn, S.J.; Morton, G.J.; Hoofnagle, A.; Yeh, M.M.; Nelson, J.E.; Kowdley, K.V. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology 2012, 55, 1103–1111. [Google Scholar] [CrossRef]
- Beilfuss, A.; Sowa, J.-P.; Sydor, S.; Beste, M.; Bechmann, L.P.; Schlattjan, M.; Syn, W.-K.; Wedemeyer, I.; Mathé, Z.; Jochum, C.; et al. Vitamin D counteracts fibrogenic TGF-β signalling in human hepatic stellate cells both receptor-dependently and independently. Gut 2015, 64, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J. Vitamin D metabolism and its clinical application. J. Bone Jt. Surg. Br. Vol. 1982, 64, 542–560. [Google Scholar] [CrossRef]
- Yue, S.Y.P.; Rideout, T.C.; Harding, S.V. Effects of plant sterol and stanol consumption on blood pressure and endothelial function. J. AOAC Int. 2015, 98, 729–734. [Google Scholar] [CrossRef]
- Turpeinen, A.M.; Ikonen, M.; Kivimäki, A.S.; Kautiainen, H.; Vapaatalo, H.; Korpela, R. A spread containing bioactive milk peptides Ile–Pro–Pro and Val–Pro–Pro, and plant sterols has antihypertensive and cholesterol-lowering effects. Food Funct. 2012, 3, 621–627. [Google Scholar] [CrossRef]
| Variable | Median | p25 | p75 |
|---|---|---|---|
| Sociodemographic variables | |||
| Age | 54 | 48 | 59 |
| Male | 9 | - | - |
| Female | 17 | - | - |
| General health-related information | |||
| HBP | 8 | - | - |
| T2D | 1 | - | - |
| Alcohol consumption (yes) | 17 | - | - |
| Audit points | 1.5 | 0.0 | 3.8 |
| Variable | Baseline | End | Relative Change | p-Value |
|---|---|---|---|---|
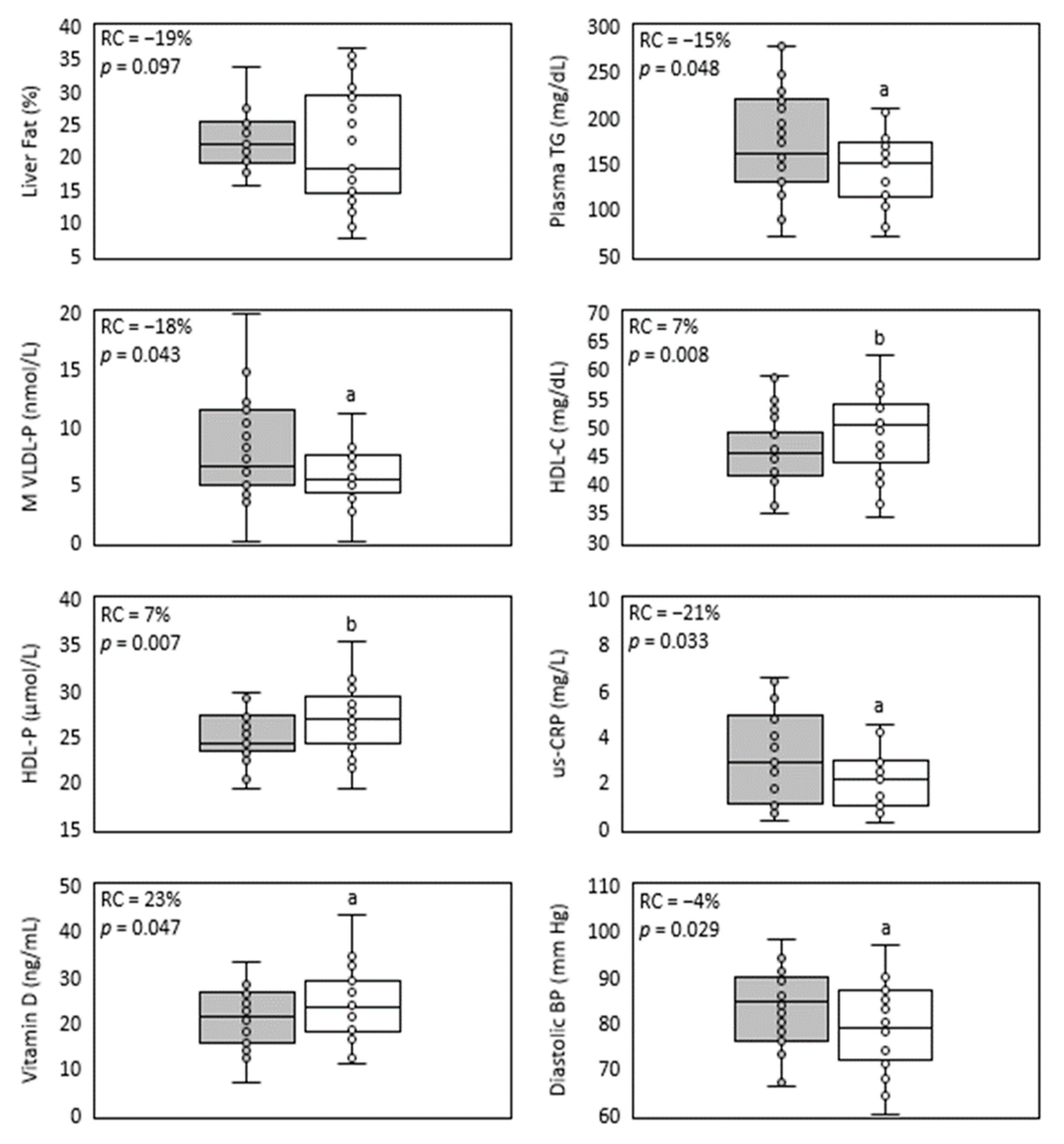
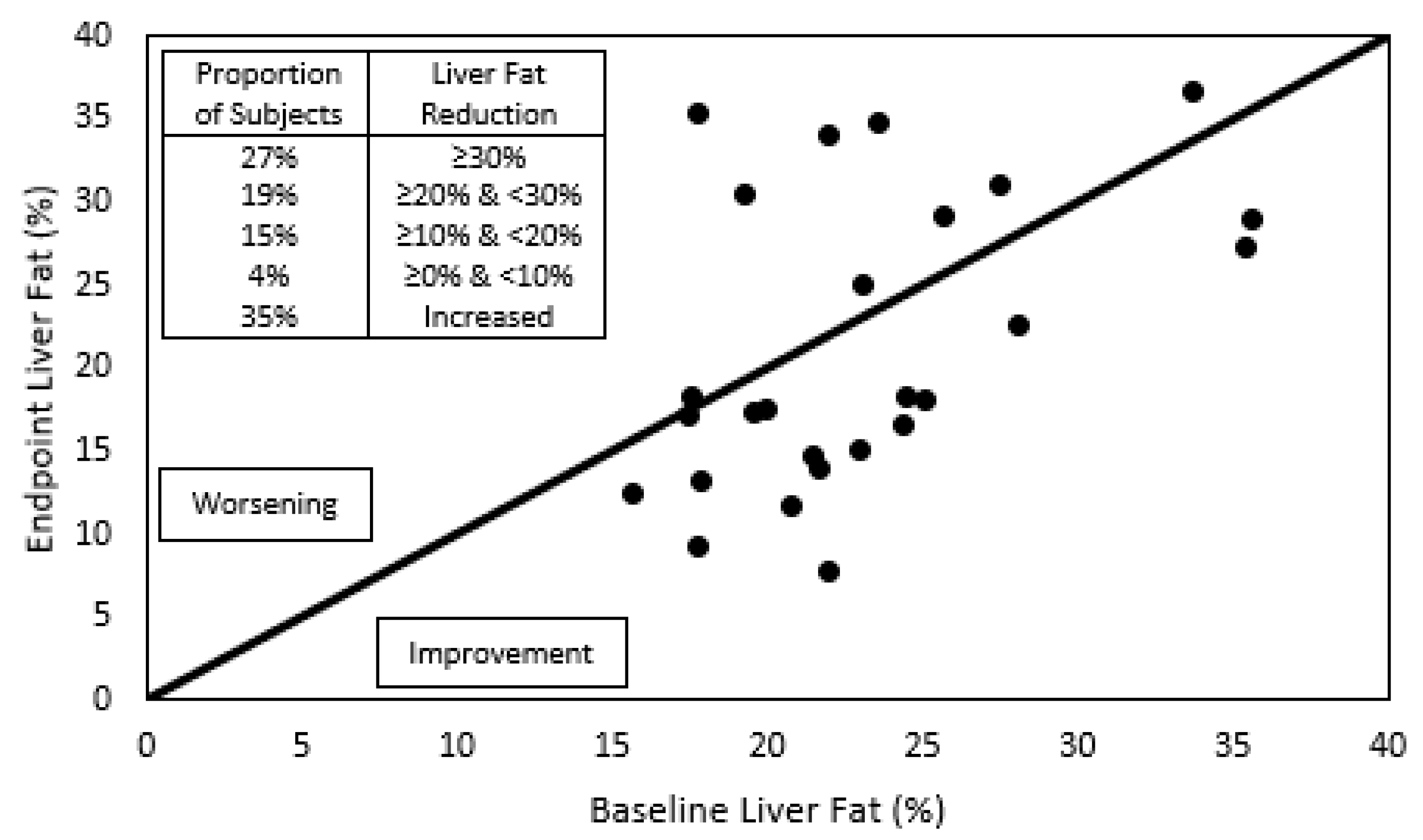
| Liver fat (%) | 22.0 (19.4; 25.0) | 18.2 (14.8; 29.1) | −19% | 0.0972 |
| TG (mg/dL) | 160.0 (130.3; 215.9) | 144.5 a (110.9; 172.8) | −15% | 0.0478 |
| VLDL-TG (mg/dL) | 105.2 (85.2; 156.7) | 93.8 a (67.9; 114.4) | −19% | 0.0454 |
| IDL-TG (mg/dL) | 14.0 (11.8; 15.4) | 12.7 (11.1; 14.9) | −4% | 0.0853 |
| LDL-TG (mg/dL) | 21.5 (18.8; 23.5) | 20.1 (17.6; 22.9) | −1% | 0.2254 |
| HDL-TG (mg/dL) | 18.6 (14.7; 23.0) | 18.5 (15.3; 22.2) | 0% | 0.2581 |
| Tot-C (mg/dL) | 232.5 (215.3; 248.5) | 239.0 (216.6; 251.6) | 1% | 0.4051 |
| VLDL-C (mg/dL) | 27.0 (19.9; 36.7) | 23.4 (17.9; 29.8) | −17% | 0.0815 |
| IDL-C (mg/dL) | 14.2 (12.1; 16.1) | 13.1 (10.2; 15.3) | −7% | 0.0977 |
| LDL-C (mg/dL) | 147.5 (129.3; 153.9) | 148.6 (131.0; 168.7) | 0% | 0.4002 |
| HDL-C (mg/dL) | 45.0 (41.4; 48.2) | 49.8 b (43.3; 53.5) | 7% | 0.0079 |
| Variable | Baseline | End | Relative Change | p-Value |
|---|---|---|---|---|
| Apo-B (mg/dL) | 127.0 (113.0; 147.0) | 117.0 b (107.2; 130.2) | −7% | 0.005 |
| VLDL-P (nmol/L) | 83.3 (61.7; 108.7) | 68.3 (50.8; 84.9) | −15% | 0.050 |
| L VLDL-P (nmol/L) | 2.0 (1.6; 2.5) | 1.7 a (1.4; 2.0) | −14% | 0.037 |
| M VLDL-P (nmol/L) | 6.6 (5.0; 11.2) | 5.4 a (4.5; 7.4) | −18% | 0.043 |
| S VLDL-P (nmol/L) | 72.4 (56.1; 94.7) | 60.1 a (44.2; 76.4) | −14% | 0.045 |
| LDL-P (nmol/L) | 1517.1 (1409.6; 1749.8) | 1601.5 (1348.6; 1702.9) | −1% | 0.452 |
| L LDL-P (nmol/L) | 202.0 (175.6; 225.9) | 198.6 (170.4; 218.1) | 0% | 0.340 |
| M LDL-P (nmol/L) | 432.7 (387.7; 502.5) | 433.5 (362.1; 499.3) | −5% | 0.293 |
| S LDL-P (nmol/L) | 882.2 (822.8; 1009.8) | 892.7 (787.0; 993.7) | −1% | 0.431 |
| Apo-A (mg/dL) | 128.0 (115.0; 147.0) | 133.0 (122.1; 140.0) | 4% | 0.063 |
| HDL-P (nmol/L) | 24.3 (23.3; 27.0) | 26.9 b (24.3; 28.3) | 7% | 0.007 |
| L HDL-P (nmol/L) | 0.3 (0.3; 0.3) | 0.3 (0.3; 0.3) | 0% | 0.428 |
| M HDL-P (nmol/L) | 9.2 (8.7; 9.9) | 9.9 (8.7; 10.7) | 3% | 0.089 |
| S HDL-P (nmol/L) | 15.0 (13.7; 16.5) | 16.5 a (13.9; 18.3) | 5% | 0.043 |
| VLDL-Z (nm) | 42.0 (41.6; 42.1) | 42.0 (41.9; 42.2) | 0% | 0.072 |
| LDL-Z (nm) | 20.9 (20.7; 21.0) | 21.0 (20.8; 21.1) | 0% | 0.065 |
| HLDL-Z (nm) | 8.3 (8.3; 8.4) | 8.3 (8.3; 8.4) | 0% | 0.331 |
| Variable | Baseline | End | Relative Change | p-Value |
|---|---|---|---|---|
| us-CRP (mg/L) | 3.2 (1.6; 5.0) | 2.2 a (1.0; 3.5) | −21% | 0.0331 |
| GOT (U/L) | 30.0 (23.3; 36.8) | 33.0 a (29.0; 39.8) | 21% | 0.0322 |
| GPT (U/L) | 37.0 (30.0; 51.0) | 36.0 (30.3; 56.0) | 1% | 0.4002 |
| GOT/GPT | 0.8 (0.6; 0.9) | 0.8 (0.6; 1.0) | 8% | 0.0518 |
| GGT (U/L) | 29.5 (23.3; 37.0) | 34.0 (22.8; 49.8) | 3% | 0.1372 |
| Glycemia (mg/dL) | 94.0 (86.0; 100.2) | 96.0 a (90.5; 104.5) | 7% | 0.0232 |
| Insulin (μUI/mL) | 13.0 (8.7; 19.6) | 14.7 (11.5; 22.0) | 16% | 0.0654 |
| HOMA | 3.0 (1.9; 4.4) | 3.4 a (3.0; 5.0) | 21% | 0.0317 |
| HbA1c (%) | 5.6 (5.4; 5.9) | 5.7 (5.4; 5.9) | 1% | 0.0537 |
| BMI (kg/m2) | 32.8 (30.5; 35.0) | 32.4 (30.5; 34.6) | 0% | 0.3748 |
| Waist circumf. (cm) | 103.3 (98.3; 106.8) | 103.0 (97.7; 109.3) | −1% | 0.4576 |
| Body fat (%) | 42.6 (37.8; 46.4) | 43.8 (37.2; 47.5) | 0% | 0.2223 |
| Sistolic BP (mm Hg) | 124.5 (118.0; 129.5) | 126.5 (113.8; 130.8) | −2% | 0.1383 |
| Diastolic BP (mm Hg) | 84.5 (76.5; 89.8) | 79.0 a (72.5; 86.5) | −4% | 0.0290 |
| Vitamin D (ng/mL) | 21.3 (16.3; 26.1) | 23.2 a (18.6; 28.8) | 23% | 0.0471 |
| Carotenes (μg/dL) | 179.5 (133.3; 218.0) | 165.0 (122.3; 206.5) | −7% | 0.0952 |
| Campesterol (μM) | 8.4 (5.0; 11.1) | 9.8 (7.2; 11.6) | 6% | 0.1423 |
| Sitosterol (μM) | 5.6 (4.0; 7.2) | 6.4 a (5.3; 8.7) | 36% | 0.0173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brañes, M.C.; Gillet, R.; Valenzuela, R. Efficacy of Submicron Dispersible Free Phytosterols on Non-Alcoholic Fatty Liver Disease: A Pilot Study. J. Clin. Med. 2023, 12, 979. https://doi.org/10.3390/jcm12030979
Brañes MC, Gillet R, Valenzuela R. Efficacy of Submicron Dispersible Free Phytosterols on Non-Alcoholic Fatty Liver Disease: A Pilot Study. Journal of Clinical Medicine. 2023; 12(3):979. https://doi.org/10.3390/jcm12030979
Chicago/Turabian StyleBrañes, María C., Raimundo Gillet, and Rodrigo Valenzuela. 2023. "Efficacy of Submicron Dispersible Free Phytosterols on Non-Alcoholic Fatty Liver Disease: A Pilot Study" Journal of Clinical Medicine 12, no. 3: 979. https://doi.org/10.3390/jcm12030979
APA StyleBrañes, M. C., Gillet, R., & Valenzuela, R. (2023). Efficacy of Submicron Dispersible Free Phytosterols on Non-Alcoholic Fatty Liver Disease: A Pilot Study. Journal of Clinical Medicine, 12(3), 979. https://doi.org/10.3390/jcm12030979







