State of the Art in 2022 PET/CT in Breast Cancer: A Review
Abstract
1. Introduction
2. FDG PET/CT
2.1. At Initial Staging
2.1.1. Initial Detection of Primary Breast Tumor
2.1.2. Locoregional Nodal Metastases
2.1.3. Initial Detection of Distant Metastases
2.2. Recurrent Disease
2.3. Evaluation of Treatment Response
2.3.1. Metastatic Disease
2.3.2. Neoadjuvant Treatment Response for Primary Tumor
2.4. Drawbacks of FDG PET/CT
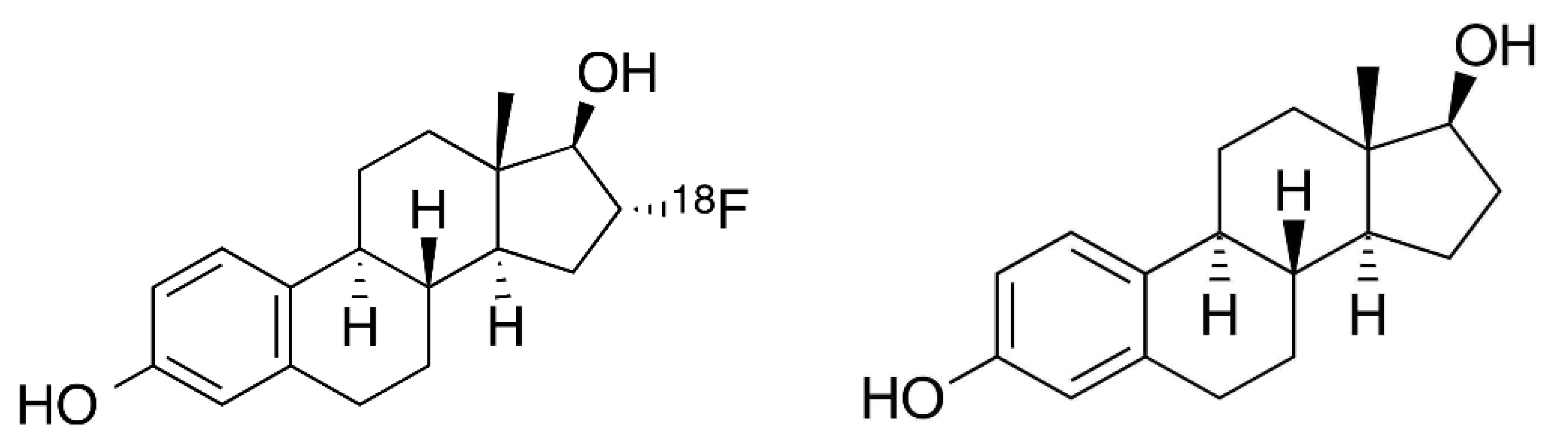
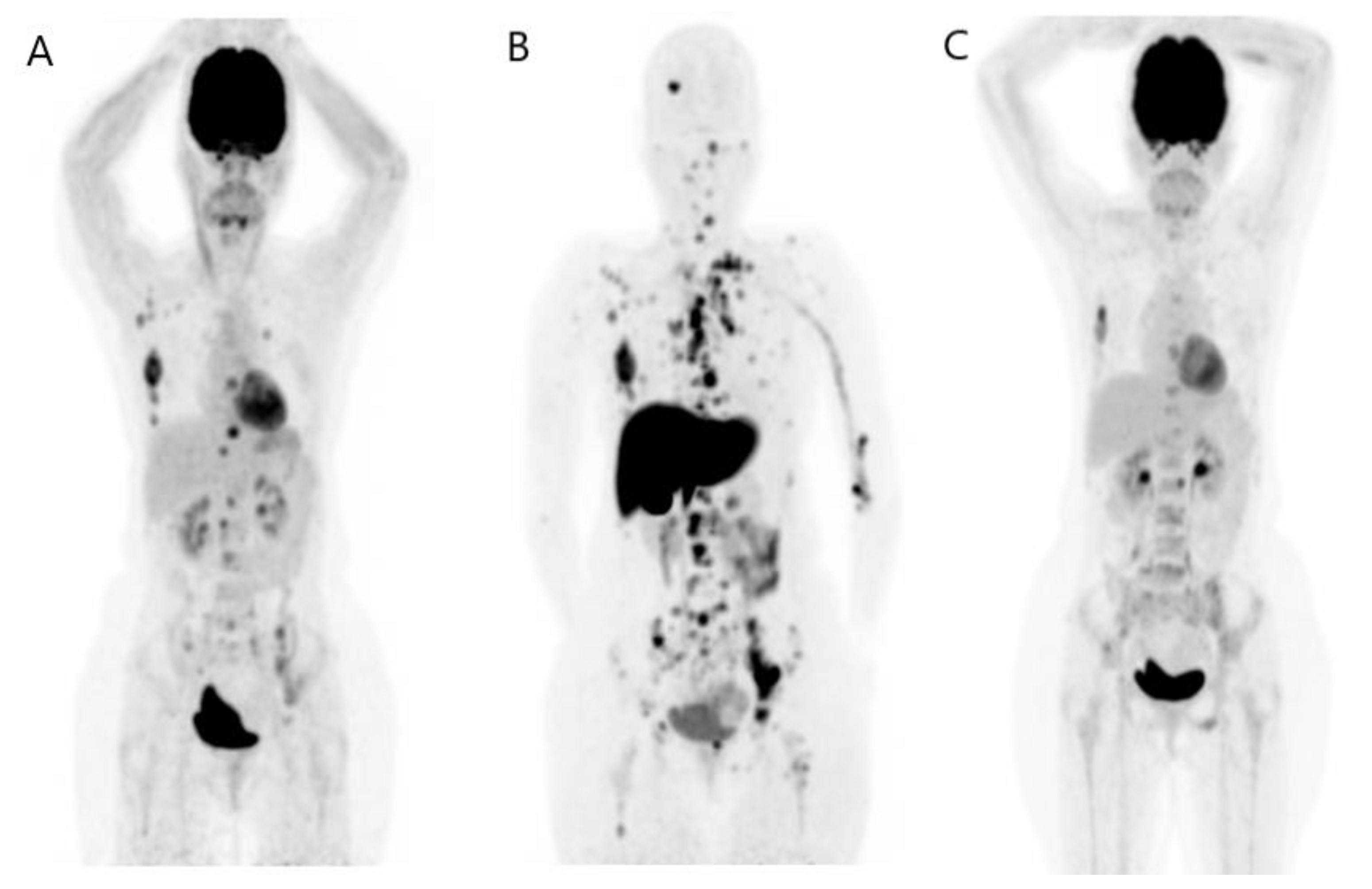
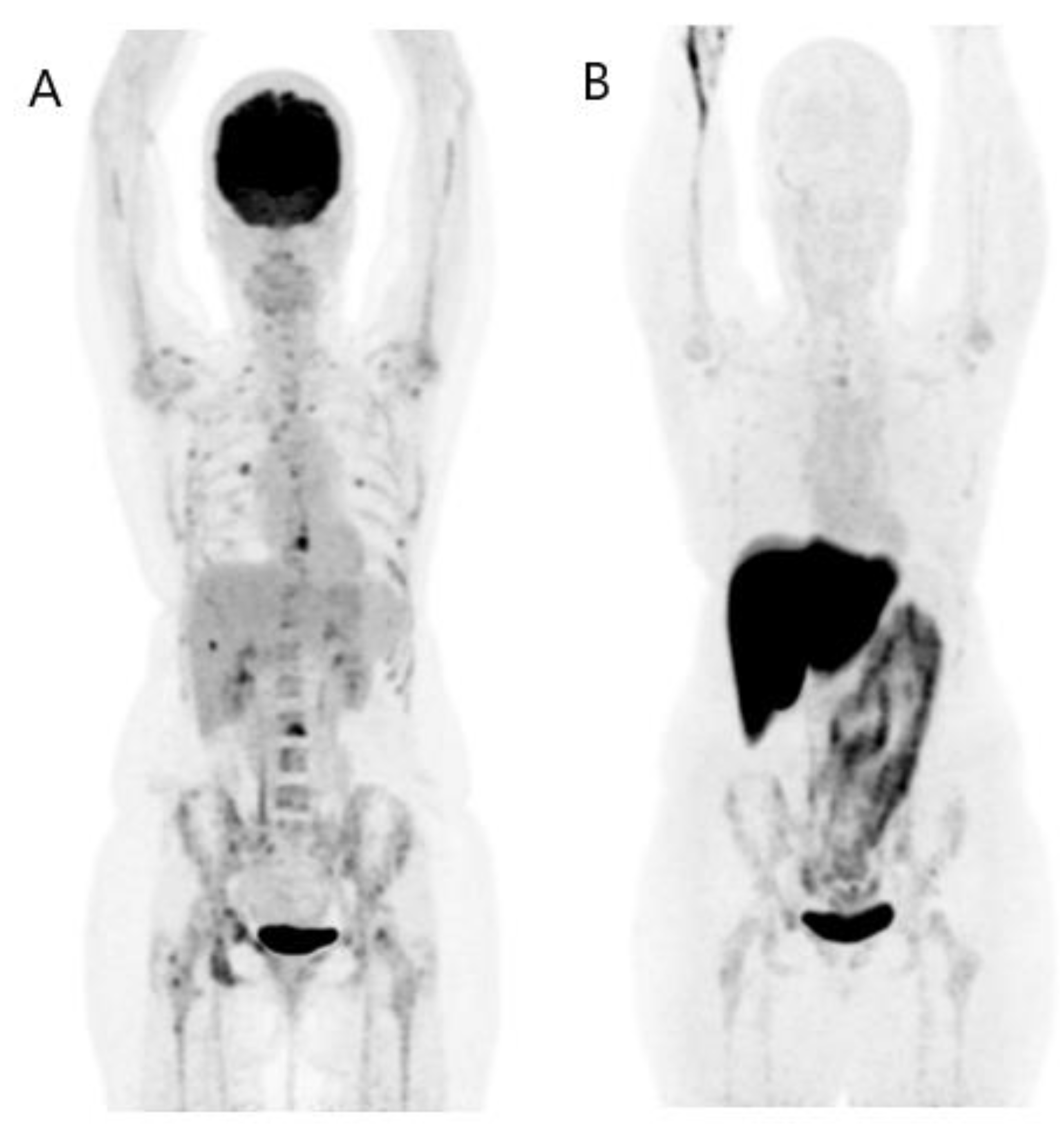
3. FES PET/CT
- -
- Characterization of known or suspected metastatic lesions as expressing ERs; and
- -
- Treatment guidance and monitoring.
4. Other Radio-Pharmaceuticals
4.1. 89Zr-Trastuzumab
4.2. 68Ga- and 18F-Labeled Fibroblast Activation Protein Inhibitor (FAPI)
4.3. Theranostics Application
5. Artificial Intelligence
6. Cost-Effectiveness Considerations
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANN | artificial neural networks |
| AJCC | American Joint Committee on Cancer |
| BC | breast cancer |
| CT | computed tomography |
| ER | Estrogen Receptor |
| ESMO | European Society for Medical Oncology |
| FDG | 18F-fluorodeoxyglucose |
| FES | 16α-18Ffluoro-17β-oestradiol |
| FAPI | fibroblast activation protein inhibitor |
| HER | human epidermal growth factor receptor |
| MRI | magnetic resonance imaging |
| NCCN | National Comprehensive Cancer Network |
| NAC | neoadjuvant chemotherapy |
| PET | positron emission tomography |
| SUV | standardized uptake value |
| TAP | thorax, abdomen, and pelvis |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Breast Cancer Statistics|How Common Is Breast Cancer? 2022. Available online: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html (accessed on 25 December 2022).
- Groheux, D. FDG-PET/CT for Primary Staging and Detection of Recurrence of Breast Cancer. Semin. Nucl. Med. 2022, 52, 508–519. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Edge, S.B.; Hortobagyi, G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018, 25, 1783–1785. [Google Scholar] [CrossRef]
- Goetz, M.P.; Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. NCCN Guidelines Insights: Breast Cancer, Version 3. J. Natl. Compr. Canc. Netw. 2019, 17, 118–126. [Google Scholar] [CrossRef]
- Groheux, D.; Giacchetti, S.; Moretti, J.L.; Porcher, R.; Espié, M.; Lehmann-Che, J.; de Roquancourt, A.; Hamy, A.S.; Cuvier, C.; Vercellino, L.; et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 426–435. [Google Scholar] [CrossRef]
- Groheux, D.; Hindie, E. Breast cancer: Initial workup and staging with FDG PET/CT. Clin. Transl. Imaging 2021, 9, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, T.; Kasami, M.; Yuen, S. Comparison of FDG PET and MRI for evaluating the tumor extent of breast cancer and the impact of FDG PET on the systemic staging and prognosis of patients who are candidates for breast-conserving therapy. Breast Cancer 2009, 16, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Heusner, T.A.; Kuemmel, S.; Umutlu, L.; Koeninger, A.; Freudenberg, L.S.; Hauth, E.A.; Kimmig, K.R.; Forsting, M.; Bockisch, A.; Antoch, G. Breast cancer staging in a single session: Whole-body PET/CT mammography. J. Nucl. Med. 2008, 49, 1215–1222. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Anderson, B.O.; Balassanian, R.; Blair, S.L.; Burstein, H.J.; Cyr, A.; Elias, A.D.; Farrar, W.B.; Forero, A.; Giordano, S.H.; et al. NCCN Guidelines Insights Breast Cancer, Version 1. J. Natl. Compr. Cancer Netw. 2015, 13, 1475–1485. [Google Scholar] [CrossRef]
- Coibion, M.; Olivier, F.; Courtois, A.; Maes, N.; Jossa, V.; Jerusalem, G. A Randomized Prospective Non-Inferiority Trial of Sentinel Lymph Node Biopsy in Early Breast Cancer: Blue Dye Compared with Indocyanine Green Fluorescence Tracer. Cancers 2022, 14, 888. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women with Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Fanizzi, A.; Pomarico, D.; Paradiso, A.; Bove, S.; Diotaiuti, S.; Didonna, V.; Giotta, F.; La Forgia, D.; Latorre, A.; Pastena, M.I.; et al. Predicting of Sentinel Lymph Node Status in Breast Cancer Patients with Clinically Negative Nodes: A Validation Study. Cancers 2021, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Hindié, E.; Groheux, D.; Brenot-Rossi, I.; Rubello, D.; Moretti, J.L.; Espié, M. The sentinel node procedure in breast cancer: Nuclear medicine as the starting point. J. Nucl. Med. 2011, 52, 405–414. [Google Scholar] [CrossRef]
- Cooper, K.L.; Harnan, S.; Meng, Y.; Ward, S.E.; Fitzgerald, P.; Papaioannou, D.; Wyld, L.; Ingram, C.; Wilkinson, I.D.; Lorenz, E. Positron emission tomography (PET) for assessment of axillary lymph node status in early breast cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2011, 37, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Kasem, J.; Wazir, U.; Mokbel, K. Sensitivity, Specificity and the Diagnostic Accuracy of PET/CT for Axillary Staging in Patients With Stage I-III Cancer: A Systematic Review of The Literature. In Vivo 2021, 35, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Turan, U.; Aygun, M.; Duman, B.B.; Kelle, A.P.; Cavus, Y.; Tas, Z.A.; Dirim, A.B.; Irkorucu, O. Efficacy of, U.S.; MRI, and F-18 FDG-PET/CT for Detecting Axillary Lymph Node Metastasis after Neoadjuvant Chemotherapy in Breast Cancer Patients. Diagnostics 2021, 11, 2361. [Google Scholar] [CrossRef]
- Brown, A.H.; Shah, S.; Groves, A.M.; Wan, S.; Malhotra, A. The Challenge of Staging Breast Cancer With PET/CT in the Era of COVID Vaccination. Clin. Nucl. Med. 2021, 46, 1006–1010. [Google Scholar] [CrossRef]
- Nikpayam, M.; Uzan, C.; Rivera, S.; Delaloge, S.; Cahen-Doidy, L.; Giacchetti, S.; Espié, M.; Groheux, D. Impact of radical surgery on outcome in locally advanced breast cancer patients without metastasis at the time of diagnosis. Anticancer Res. 2015, 35, 1729–1734. [Google Scholar]
- Borm, K.J.; Voppichler, J.; Düsberg, M.; Oechsner, M.; Vag, T.; Weber, W.; Combs, S.E.; Duma, M.N. FDG/PET-CT-Based Lymph Node Atlas in Breast Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 574–582. [Google Scholar] [CrossRef]
- Borm, K.J.; Oechsner, M.; Düsberg, M.; Buschner, G.; Weber, W.; Combs, S.E.; Duma, M.N. Irradiation of regional lymph node areas in breast cancer—Dose evaluation according to the Z0011, AMAROS, EORTC 10981-22023 and MA-20 field design. Radiother. Oncol. 2020, 142, 195–201. [Google Scholar] [CrossRef]
- Groheux, D.; Giacchetti, S.; Delord, M.; Hindié, E.; Vercellino, L.; Cuvier, C.; Toubert, M.E.; Merlet, P.; Hennequin, C.; Espié, M. 18F-FDG PET/CT in staging patients with locally advanced or inflammatory breast cancer: Comparison to conventional staging. J. Nucl. Med. 2013, 54, 5–11. [Google Scholar] [CrossRef]
- Fuster, D.; Duch, J.; Paredes, P.; Velasco, M.; Muñoz, M.; Santamaría, G.; Fontanillas, M.; Pons, F. Preoperative staging of large primary breast cancer with [18F]fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J. Clin. Oncol. 2008, 26, 4746–4751. [Google Scholar] [CrossRef] [PubMed]
- Vogsen, M.; Jensen, J.D.; Christensen, I.Y.; Gerke, O.; Jylling, A.M.B.; Larsen, L.B.; Braad, P.E.; Søe, K.L.; Bille, C.; Ewertz, M. FDG-PET/CT in high-risk primary breast cancer-a prospective study of stage migration and clinical impact. Breast Cancer Res. Treat. 2021, 185, 145–153. [Google Scholar] [CrossRef]
- Morris, P.G.; Lynch, C.; Feeney, J.N.; Patil, S.; Howard, J.; Larson, S.M.; Dickler, M.; Hudis, C.A.; Jochelson, M.; McArthur, H.L. Integrated positron emission tomography/computed tomography may render bone scintigraphy unnecessary to investigate suspected metastatic breast cancer. J. Clin. Oncol. 2010, 28, 3154–3159. [Google Scholar] [CrossRef]
- Groheux, D.; Moretti, J.L.; Baillet, G.; Espie, M.; Giacchetti, S.; Hindie, E.; Hennequin, C.; Vilcoq, J.R.; Cuvier, C.; Toubert, M.E. Effect of (18)F-FDG PET/CT imaging in patients with clinical Stage II and III breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 695–704. [Google Scholar] [PubMed]
- Groheux, D.; Hindié, E.; Espié, M.; Ulaner, G.A. Letter to the Editor: PET/CT in Locally Advanced Breast Cancer: Time for a Guideline Change? J. Natl. Compr. Canc. Netw. 2021, 19, 1. [Google Scholar] [CrossRef]
- Pritchard, K.I.; Julian, J.A.; Holloway, C.M.; McCready, D.; Gulenchyn, K.Y.; George, R.; Hodgson, N.; Lovrics, P.; Perera, F.; Elavathil, L.; et al. Prospective study of 2-[¹⁸F]fluorodeoxyglucose positron emission tomography in the assessment of regional nodal spread of disease in patients with breast cancer: An Ontario clinical oncology group study. J. Clin. Oncol. 2012, 30, 1274–1279. [Google Scholar] [CrossRef]
- Dong, Y.; Hou, H.; Wang, C.; Li, J.; Yao, Q.; Amer, S.; Tian, M. The diagnostic value of 18F-FDG PET/CT in association with serum tumor marker assays in breast cancer recurrence and metastasis. Biomed. Res. Int. 2015, 2015, 489021. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Harbeck, N.; Fallowfield, L.; Kyriakides, S.; Senkus, E.; ESMO Guidelines Working Group. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. 7), vii11–vii19. [Google Scholar] [CrossRef]
- Di Gioia, D.; Stieber, P.; Schmidt, G.P.; Nagel, D.; Heinemann, V.; Baur-Melnyk, A. Early detection of metastatic disease in asymptomatic breast cancer patients with whole-body imaging and defined tumour marker increase. Br. J. Cancer 2015, 112, 809–818. [Google Scholar] [CrossRef]
- Vogsen, M.; Jensen, J.D.; Gerke, O.; Jylling, A.M.B.; Asmussen, J.T.; Christensen, I.Y.; Braad, P.E.; Thye-Rønn, P.; Søe, K.L.; Ewertz, M. Benefits and harms of implementing [18F]FDG-PET/CT for diagnosing recurrent breast cancer: A prospective clinical study. EJNMMI Res. 2021, 11, 93. [Google Scholar] [CrossRef]
- Chang, H.T.; Hu, C.; Chiu, Y.L.; Peng, N.J.; Liu, R.S. Role of 2-[18F] fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in the post-therapy surveillance of breast cancer. PLoS ONE 2014, 9, e115127. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.G.; Gerke, O.; Baun, C.; Falch, K.; Hansen, J.A.; Petersen, Z.A.F.; Larsen, L.B.; Duvnjak, S.; Buskevica, I.; Bektas, S.; et al. [18F]Fluorodeoxyglucose (FDG)-Positron Emission Tomography (PET)/Computed Tomography (CT) in Suspected Recurrent Breast Cancer: A Prospective Comparative Study of Dual-Time-Point FDG-PET/CT, Contrast-Enhanced CT, and Bone Scintigraphy. J. Clin. Oncol. 2016, 34, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.P.; Baur-Melnyk, A.; Haug, A.; Heinemann, V.; Bauerfeind, I.; Reiser, M.F.; Schoenberg, S.O. Comprehensive imaging of tumor recurrence in breast cancer patients using whole-body MRI at 1.5 and 3 T compared to FDG-PET-CT. Eur. J. Radiol. 2008, 65, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, L.; Jiang, X.; She, W.; He, L.; Hu, G. Diagnostic efficacy of 18F-FDG-PET or PET/CT in breast cancer with suspected recurrence: A systematic review and meta-analysis. Nucl. Med. Commun. 2016, 37, 1180–1188. [Google Scholar] [CrossRef]
- Evangelista, L.; Cervino, A.R.; Ghiotto, C.; Al-Nahhas, A.; Rubello, D.; Muzzio, P.C. Tumor marker-guided PET in breast cancer patients-a recipe for a perfect wedding: A systematic literature review and meta-analysis. Clin. Nucl. Med. 2012, 37, 467–474. [Google Scholar] [CrossRef]
- Gil-Rendo, A.; Martínez-Regueira, F.; Zornoza, G.; García-Velloso, M.J.; Beorlegui, C.; Rodriguez-Spiteri, N. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br. J. Surg. 2009, 96, 166–170. [Google Scholar] [CrossRef]
- Zhang, F.C.; Xu, H.Y.; Liu, J.J.; Xu, Y.F.; Chen, B.; Yang, Y.J.; Yan, N.N.; Song, S.L.; Lin, Y.M.; Xu, Y.C. 18F-FDG PET/CT for the early prediction of the response rate and survival of patients with recurrent or metastatic breast cancer. Oncol. Lett. 2018, 16, 4151–4158. [Google Scholar] [CrossRef]
- Lin, N.U.; Guo, H.; Yap, J.T.; Mayer, I.A.; Falkson, C.I.; Hobday, T.J.; Dees, E.C.; Richardson, A.L.; Nanda, R.; Rimawi, M. Phase II Study of Lapatinib in Combination With Trastuzumab in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer: Clinical Outcomes and Predictive Value of Early [18F]Fluorodeoxyglucose Positron Emission Tomography Imaging (TBCRC 003). J. Clin. Oncol. 2015, 33, 2623–2631. [Google Scholar]
- Edmonds, C.E.; O’Brien, S.R.; Mankoff, D.A.; Pantel, A.R. Novel applications of molecular imaging to guide breast cancer therapy. Cancer Imaging 2022, 22, 31. [Google Scholar] [CrossRef] [PubMed]
- Gombos, A.; Venet, D.; Ameye, L.; Vuylsteke, P.; Neven, P.; Richard, V.; Duhoux, F.P.; Laes, J.F.; Rothe, F.; Sotiriou, C. FDG positron emission tomography imaging and ctDNA detection as an early dynamic biomarker of everolimus efficacy in advanced luminal breast cancer [published correction appears in NPJ Breast Cancer. 2022 Mar 16, 8, 38]. NPJ Breast Cancer 2021, 7, 125. [Google Scholar] [CrossRef]
- Hildebrandt, M.G.; Naghavi-Behzad, M.; Vogsen, M. A role of FDG-PET/CT for response evaluation in metastatic breast cancer? Semin. Nucl. Med. 2022, 52, 520–530. [Google Scholar] [CrossRef]
- Cook, G.J.R. Imaging of Bone Metastases in Breast Cancer. Semin. Nucl. Med. 2022, 52, 531–541. [Google Scholar] [CrossRef]
- Tateishi, U.; Gamez, C.; Dawood, S.; Yeung, H.W.; Cristofanilli, M.; Macapinlac, H.A. Bone metastases in patients with metastatic breast cancer: Morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT. Radiology 2008, 247, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Iagaru, A.; Minamimoto, R. Nuclear Medicine Imaging Techniques for Detection of Skeletal Metastases in Breast Cancer. PET Clin. 2018, 13, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Al-Muqbel, K.M.; Yaghan, R.J. Effectiveness of 18F-FDG-PET/CT vs Bone Scintigraphy in Treatment Response Assessment of Bone Metastases in Breast Cancer. Medicine 2016, 95, e3753. [Google Scholar] [CrossRef]
- Dose Schwarz, J.; Bader, M.; Jenicke, L.; Hemminger, G.; Jänicke, F.; Avril, N. Early prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG PET. J. Nucl. Med. 2005, 46, 1144–1150. [Google Scholar]
- Cachin, F.; Prince, H.M.; Hogg, A.; Ware, R.E.; Hicks, R.J. Powerful prognostic stratification by [18F]fluorodeoxyglucose positron emission tomography in patients with metastatic breast cancer treated with high-dose chemotherapy. J. Clin. Oncol. 2006, 24, 3026–3031. [Google Scholar] [CrossRef]
- Moo, T.A.; Sanford, R.; Dang, C.; Morrow, M. Overview of Breast Cancer Therapy. PET Clin. 2018, 13, 339–354. [Google Scholar] [CrossRef]
- Schwarz-Dose, J.; Untch, M.; Tiling, R.; Sassen, S.; Mahner, S.; Kahlert, S.; Harbeck, N.; Lebeau, A.; Brenner, W.; Schwaiger, M. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J. Clin. Oncol. 2009, 27, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, C.; Devillers, A.; Sagan, C.; Ferrer, L.; Bridji, B.; Campion, L.; Ricaud, M.; Bourbouloux, E.; Doutriaux, I.; Clouet, M.; et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J. Clin. Oncol. 2006, 24, 5366–5372. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Hindié, E.; Giacchetti, S.; Delord, M.; Hamy, A.S.; de Roquancourt, A.; Vercellino, L.; Berenger, N.; Marty, M.; Espié, M.; et al. Triple-negative breast cancer: Early assessment with 18F-FDG PET/CT during neoadjuvant chemotherapy identifies patients who are unlikely to achieve a pathologic complete response and are at a high risk of early relapse. J. Nucl. Med. 2012, 53, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Hatt, M.; Hindié, E.; Giacchetti, S.; de Cremoux, P.; Lehmann-Che, J.; Espié, M. Estrogen receptor-positive/human epidermal growth factor receptor 2-negative breast tumors: Early prediction of chemosensitivity with 18F-fluorodeoxyglucose positron emission tomography/computed tomography during neoadjuvant chemotherapy. Cancer 2013, 119, 1960–1968. [Google Scholar] [CrossRef]
- Groheux, D. Role of Fludeoxyglucose in Breast Cancer: Treatment Response. PET Clin. 2018, 13, 395–414. [Google Scholar] [CrossRef]
- Groheux, D.; Mankoff, D.; Espié, M.; Hindié, E. ¹⁸F-FDG PET/CT in the early prediction of pathological response in aggressive subtypes of breast cancer: Review of the literature and recommendations for use in clinical trials. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 983–993. [Google Scholar] [CrossRef]
- Loo, C.E.; Straver, M.E.; Rodenhuis, S.; Muller, S.H.; Wesseling, J.; Vrancken Peeters, M.J.; Gilhuijs, K.G. Magnetic resonance imaging response monitoring of breast cancer during neoadjuvant chemotherapy: Relevance of breast cancer subtype. J. Clin. Oncol. 2011, 29, 660–666. [Google Scholar] [CrossRef]
- Taourel, P.; Pages, E.; Millet, I.; Bourgier, C.; Rouanet, P.; Jacot, W.; Crochet, P.; Azria, D. Magnetic resonance imaging in breast cancer management in the context of neo-adjuvant chemotherapy. Crit. Rev. Oncol. Hematol. 2018, 132, 51–65. [Google Scholar] [CrossRef]
- Jochelson, M.S.; Lampen-Sachar, K.; Gibbons, G.; Dang, C.; Lake, D.; Morris, E.A.; Morrow, M. Do MRI and mammography reliably identify candidates for breast conservation after neoadjuvant chemotherapy? Ann. Surg. Oncol. 2015, 22, 1490–1495. [Google Scholar] [CrossRef]
- Riedl, C.C.; Pinker, K.; Ulaner, G.A.; Ong, L.T.; Baltzer, P.; Jochelson, M.S.; McArthur, H.L.; Gönen, M.; Dickler, M.; Weber, W.A.; et al. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1428–1437. [Google Scholar] [CrossRef]
- Goulon, D.; Necib, H.; Henaff, B.; Rousseau, C.; Carlier, T.; Kraeber-Bodere, F. Quantitative Evaluation of Therapeutic Response by FDG-PET-CT in Metastatic Breast Cancer. Front. Med. 2016, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Taralli, S.; Lorusso, M.; Scolozzi, V.; Masiello, V.; Marazzi, F.; Calcagni, M.L. Response evaluation with 18F-FDG PET/CT in metastatic breast cancer patients treated with Palbociclib: First experience in clinical practice. Ann. Nucl. Med. 2019, 33, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.; Schirrmeister, H.; Kühn, T.; Shen, C.; Kalker, T.; Kotzerke, J.; Dankerl, A.; Glatting, G.; Reske, S.; Mattfeldt, T.; et al. FDG uptake in breast cancer: Correlation with biological and clinical prognostic parameters. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1317–1323. [Google Scholar] [CrossRef]
- Mohamadien, N.R.A.; Sayed, M.H.M. Correlation between semiquantitative and volumetric 18F-FDG PET/computed tomography parameters and Ki-67 expression in breast cancer. Nucl. Med. Commun. 2021, 42, 656–664. [Google Scholar] [CrossRef]
- Mintun, M.A.; Welch, M.J.; Siegel, B.A.; Mathias, C.J.; Brodack, J.W.; McGuire, A.H.; Katzenellenbogen, J.A. Breast cancer: PET imaging of estrogen receptors. Radiology 1988, 169, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, F.; Mortimer, J.E.; Siegel, B.A.; Griffeth, L.K.; Bonasera, T.J.; Fusselman, M.J.; Detert, D.D.; Cutler, P.D.; Katzenellenbogen, J.A.; Welch, M.J.; et al. Positron tomographic assessment of estrogen receptors in breast cancer: Comparison with FDG-PET and in vitro receptor assays. J. Nucl. Med. 1995, 36, 1766–1774. [Google Scholar]
- Gemignani, M.L.; Patil, S.; Seshan, V.E.; Sampson, M.; Humm, J.L.; Lewis, J.S.; Brogi, E.; Larson, S.M.; Morrow, M.; Pandit-Taskar, N.; et al. Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer. J. Nucl. Med. 2013, 54, 1697–1702. [Google Scholar] [CrossRef]
- Katzenellenbogen, J.A. The quest for improving the management of breast cancer by functional imaging: The discovery and development of 16α-[18F]fluoroestradiol (FES), a PET radiotracer for the estrogen receptor, a historical review. Nucl. Med. Biol. 2021, 92, 24–37. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212155s000lbl.pdf (accessed on 25 December 2022).
- Ulaner, G.A. 16α-18F-fluoro-17β-Fluoroestradiol (FES): Clinical Applications for Patients with Breast Cancer. Semin. Nucl. Med. 2022, 52, 574–583. [Google Scholar] [CrossRef]
- Ellis, M.J.; Gao, F.; Dehdashti, F.; Jeffe, D.B.; Marcom, P.K.; Carey, L.A.; Dickler, M.N.; Silverman, P.; Fleming, G.F.; Kommareddy, A.; et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: A phase 2 randomized study. JAMA 2009, 302, 774–780. [Google Scholar] [CrossRef]
- Chung, C.T.; Carlson, R.W. The role of aromatase inhibitors in early breast cancer. Curr. Treat. Options Oncol. 2003, 4, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.has-sante.fr/upload/docs/application/pdf/2020-05/estrotep_summary_ct18010.pdf (accessed on 25 December 2022).
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/212155Orig1s000MultidisciplineR.pdf (accessed on 25 December 2022).
- Peterson, L.M.; Mankoff, D.A.; Lawton, T.; Yagle, K.; Schubert, E.K.; Stekhova, S.; Gown, A.; Link, J.M.; Tewson, T.; Krohn, K.A.; et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J. Nucl. Med. 2008, 49, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Boers, J.; Venema, C.M.; de Vries, E.F.J.; Glaudemans, A.W.J.M.; Kwee, T.C.; Schuuring, E.; Martens, J.W.M.; Elias, S.G.; Hospers, G.A.P.; Schröder, C.P.; et al. Molecular imaging to identify patients with metastatic breast cancer who benefit from endocrine treatment combined with cyclin-dependent kinase inhibition. Eur. J. Cancer 2020, 126, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Currin, E.; Peterson, L.M.; Schubert, E.K.; Link, J.M.; Krohn, K.A.; Livingston, R.B.; Mankoff, D.A.; Linden, H.M. Temporal Heterogeneity of Estrogen Receptor Expression in Bone-Dominant Breast Cancer: 18F-Fluoroestradiol PET Imaging Shows Return of ER Expression. J. Natl. Compr. Canc. Netw. 2016, 14, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Linden, H.M.; Kurland, B.F.; Peterson, L.M.; Schubert, E.K.; Gralow, J.R.; Specht, J.M.; Ellis, G.K.; Lawton, T.J.; Livingston, R.B.; Petra, P.H.; et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin. Cancer Res. 2011, 17, 4799–4805. [Google Scholar] [CrossRef] [PubMed]
- Alexandra, T.; Ahsan, K.S.; Charles, L.; Chen, S.M. Survival by HER2 receptor status in stage IV breast cancer. SEER 2010-J. Clin. Oncol. 2017, 35 (Suppl. 15), 1032. [Google Scholar]
- Boers, J.; de Vries, E.F.J.; Glaudemans, A.W.J.M.; Hospers, G.A.P.; Schröder, C.P. Application of PET Tracers in Molecular Imaging for Breast Cancer. Curr. Oncol. Rep. 2020, 22, 85. [Google Scholar] [CrossRef]
- Dehdashti, F.; Wu, N.; Bose, R.; Naughton, M.J.; Ma, C.X.; Marquez-Nostra, B.V.; Diebolder, P.; Mpoy, C.; Rogers, B.E.; Lapi, S.E.; et al. Evaluation of [89Zr]trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res. Treat. 2018, 169, 523–530. [Google Scholar] [CrossRef]
- Bensch, F.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Jong, J.R.; van der Vegt, B.; Sleijfer, S.; de Vries, E.G.E.; Schröder, C.P. 89Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2300–2306. [Google Scholar] [CrossRef]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef]
- Aertgeerts, K.; Levin, I.; Shi, L.; Snell, G.P.; Jennings, A.; Prasad, G.S.; Zhang, Y.; Kraus, M.L.; Salakian, S.; Sridhar, V.; et al. Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein alpha. J. Biol. Chem. 2005, 280, 19441–19444. [Google Scholar] [CrossRef] [PubMed]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom. Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Puré, E.; Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: Back to the basics. Oncogene 2018, 37, 4343–4357. [Google Scholar] [CrossRef]
- Siveke, J.T. Fibroblast-activating protein: Targeting the roots of the tumor microenvironment. J. Nucl. Med. 2018, 59, 1412–1414. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Kömek, H.; Can, C.; Güzel, Y.; Oruç, Z.; Gündoğan, C.; Yildirim, Ö.A.; Kaplan, İ.; Erdur, E.; Yıldırım, M.S.; Çakabay, B. 68Ga-FAPI-04 PET/CT, a new step in breast cancer imaging: A comparative pilot study with the 18F-FDG PET/CT. Ann. Nucl. Med. 2021, 35, 744–752. [Google Scholar] [CrossRef]
- Hu, K.; Wang, L.; Wu, H.; Huang, S.; Tian, Y.; Wang, Q.; Xiao, C.; Han, Y.; Tang, G. [18F]FAPI-42 PET imaging in cancer patients: Optimal acquisition time, biodistribution, and comparison with [68Ga]Ga-FAPI-Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2833–2843. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Foekens, J.A.; Klijn, J.G.; Lamberts, S.W.; Laissue, J. Somatostatin receptor incidence and distribution in breast cancer using receptor autoradiography: Relationship to EGF receptors. Int. J. Cancer 1990, 46, 416–420. [Google Scholar] [CrossRef]
- Dalm, S.U.; Haeck, J.; Doeswijk, G.N.; de Blois, E.; de Jong, M.; van Deurzen, C.H.M. SSTR-Mediated Imaging in Breast Cancer: Is There a Role for Radiolabeled Somatostatin Receptor Antagonists? J. Nucl. Med. 2017, 58, 1609–1614. [Google Scholar] [CrossRef]
- Available online: https://www.clinicaltrials.gov/ct2/show/NCT04529044 (accessed on 25 December 2022).
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef]
- Sadaghiani, M.S.; Rowe, S.P.; Sheikhbahaei, S. Applications of artificial intelligence in oncologic 18F-FDG PET/CT imaging: A systematic review. Ann. Transl. Med. 2021, 9, 823. [Google Scholar] [CrossRef] [PubMed]
- Markus, A.F.; Kors, J.A.; Rijnbeek, P.R. The role of explainability in creating trustworthy artificial intelligence for health care: A comprehensive survey of the terminology, design choices, and evaluation strategies. J. Biomed. Inform. 2021, 113, 103655. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kitajima, K.; Hirata, K.; Togo, R.; Takenaka, J.; Miyoshi, Y.; Kudo, K.; Ogawa, T.; Haseyama, M. Preliminary study of AI-assisted diagnosis using FDG-PET/CT for axillary lymph node metastasis in patients with breast cancer. EJNMMI Res. 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Fujioka, T.; Oyama, J.; Mori, M.; Yamaga, E.; Yashima, Y.; Imokawa, T.; Hayashi, A.; Kujiraoka, Y.; Tsuchiya, J.; et al. Deep Learning Using Multiple Degrees of Maximum-Intensity Projection for PET/CT Image Classification in Breast Cancer. Tomography 2022, 8, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Jager, J.J.; Keymeulen, K.; Beets-Tan, R.G.; Hupperets, P.; van Kroonenburgh, M.; Houben, R.; de Ruysscher, D.; Lambin, P.; Boersma, L.J. FDG-PET-CT for staging of high-risk breast cancer patients reduces the number of further examinations: A pilot study. Acta Oncol. 2010, 49, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Miquel-Cases, A.; Teixeira, S.; Retèl, V.; Steuten, L.; Valdés Olmos, R.; Rutgers, E.; van Harten, W.H. Cost-effectiveness of 18FFDG PET/CT for screening distant metastasis in stage II/III breast cancer patients of the, U.K.; the United States and the Netherlands. Value Health 2015, 18, A337. [Google Scholar] [CrossRef]
- Hyland, C.J.; Varghese, F.; Yau, C.; Beckwith, H.; Khoury, K.; Varnado, W.; Hirst, G.L.; Flavell, R.R.; Chien, A.J.; Yee, D.; et al. Use of 18F-FDG PET/CT as an Initial Staging Procedure for Stage II-III Breast Cancer: A Multicenter Value Analysis. J. Natl. Compr. Canc. Netw. 2020, 18, 1510–1517. [Google Scholar] [CrossRef]
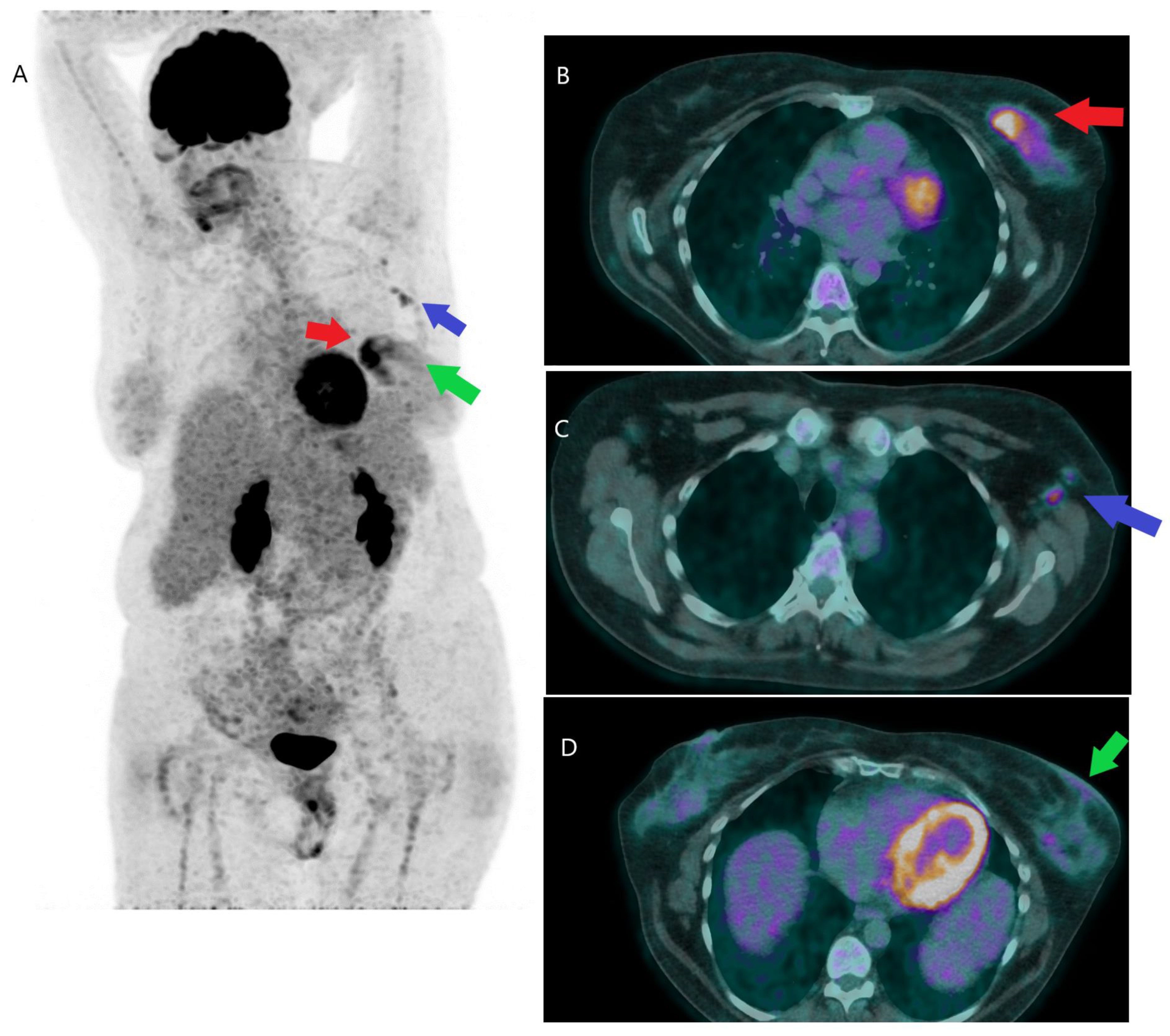
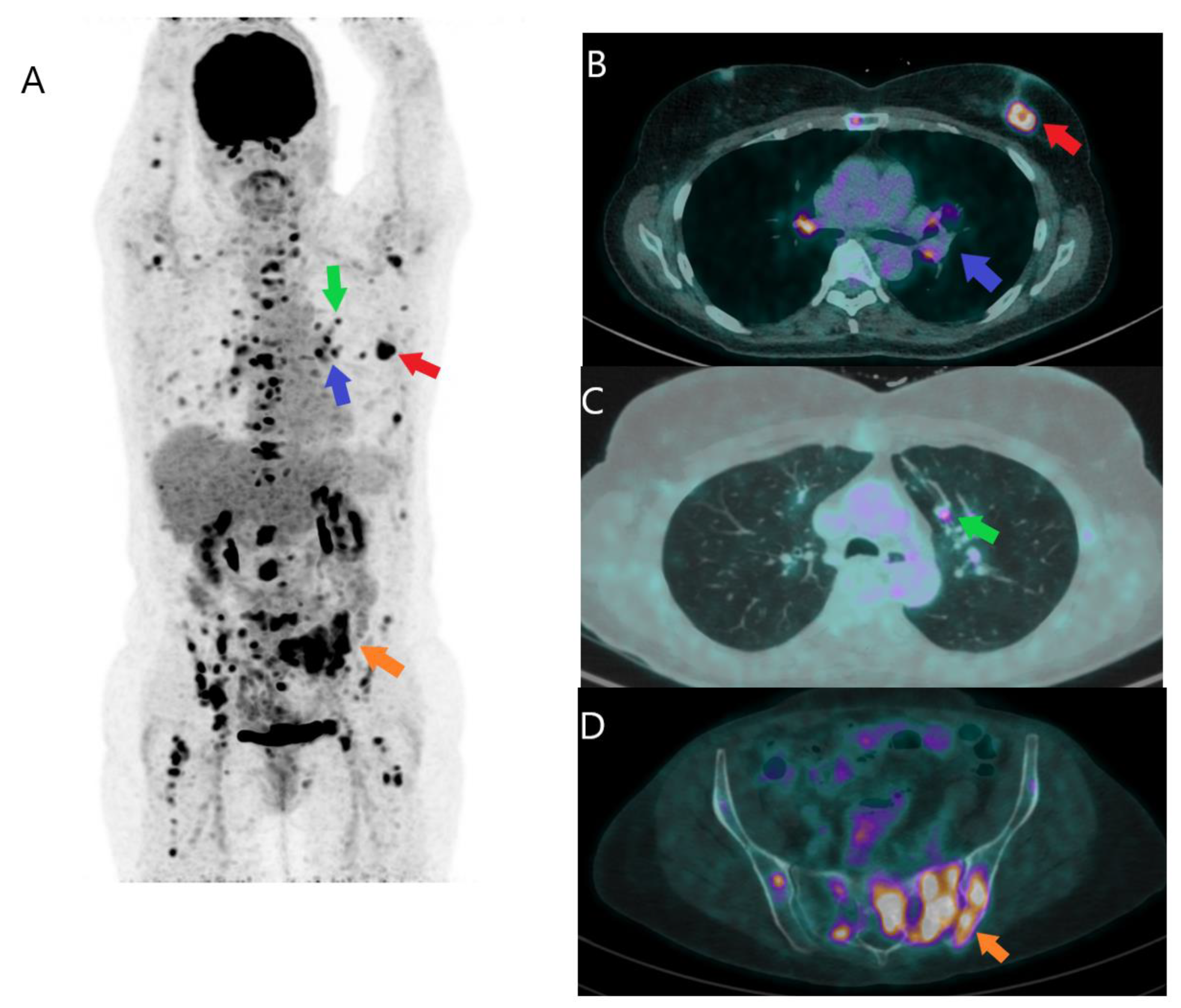
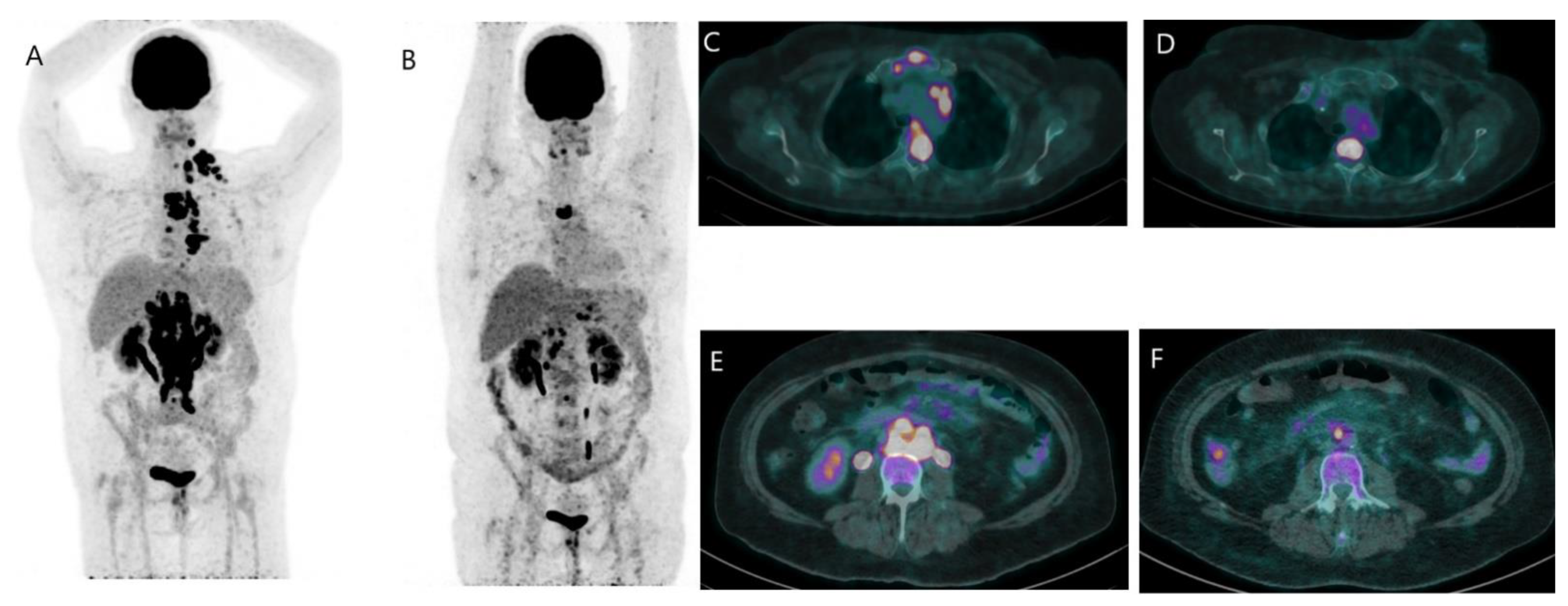
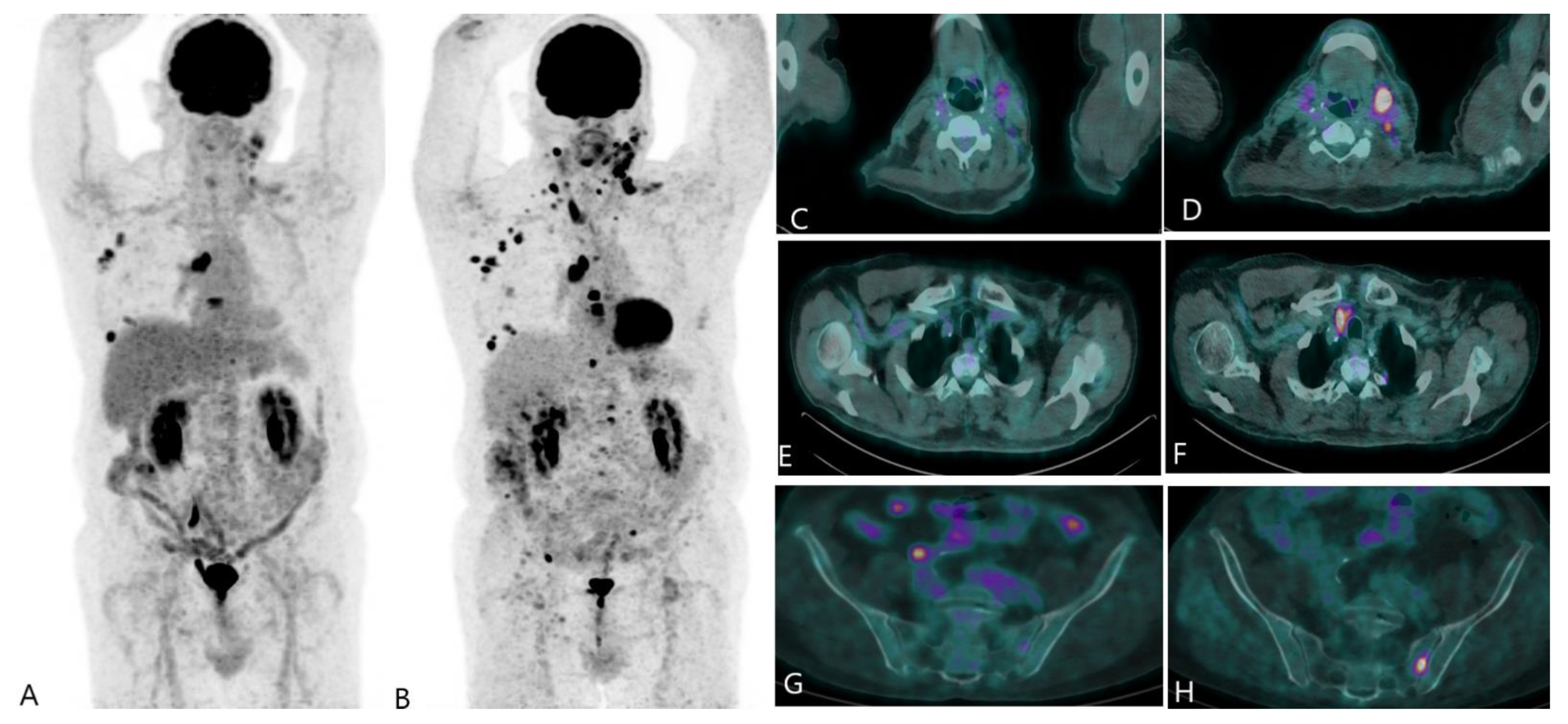
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang-Yin, J. State of the Art in 2022 PET/CT in Breast Cancer: A Review. J. Clin. Med. 2023, 12, 968. https://doi.org/10.3390/jcm12030968
Zhang-Yin J. State of the Art in 2022 PET/CT in Breast Cancer: A Review. Journal of Clinical Medicine. 2023; 12(3):968. https://doi.org/10.3390/jcm12030968
Chicago/Turabian StyleZhang-Yin, Jules. 2023. "State of the Art in 2022 PET/CT in Breast Cancer: A Review" Journal of Clinical Medicine 12, no. 3: 968. https://doi.org/10.3390/jcm12030968
APA StyleZhang-Yin, J. (2023). State of the Art in 2022 PET/CT in Breast Cancer: A Review. Journal of Clinical Medicine, 12(3), 968. https://doi.org/10.3390/jcm12030968





