Safety and Efficacy of Midface Augmentation Using Bio-Oss Bone Powder and Bio-Gide Collagen Membrane in Asians
Abstract
1. Introduction
2. Methods and Materials
2.1. Subject Selection
2.2. Study Design
2.3. Operation Technique
2.4. Assessments
2.4.1. Efficacy Assessments
2.4.2. Safety Assessments
2.4.3. Satisfaction Assessments
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics and Demographics
3.2. Efficacy
3.3. Safety
3.4. Satisfaction and Its Prediction Model
3.5. Typical Cases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, H.; Chun, K.W.; Kye, M.S.; Dhong, E.S.; Yoon, E.S. Midface augmentation using bony segments obtained from sagittal splitting angle ostectomy in asians. Plast. Reconstr. Surg. 2008, 121, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Taub, P.J.; Lampert, J.A.; Silver, L.; Greenberg, A. Vomer bone graft to augment the deficient cleft maxilla. J. Craniofac. Surg. 2009, 20 (Suppl. S2), 1882–1885. [Google Scholar] [CrossRef] [PubMed]
- Unlu, R.E.; Altun, S.; Inozu, E.; Koc, M.N. Diced cartilage grafts in rhinoplasty surgery: Current techniques and applications. Plast. Reconstr. Surg. 2009, 124, 666. [Google Scholar] [CrossRef]
- Schultz, K.P.; Raghuram, A.; Davis, M.J.; Abu-Ghname, A.; Chamata, E.; Rohrich, R.J. Fat Grafting for Facial Rejuvenation. Semin. Plast. Surg. 2020, 34, 30–37. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, S.; Liu, D.; Chai, M.; Wang, J.; Zhao, Y. Preclinical safety studies on autologous cultured human skin fibroblast transplantation. Cell Transplant. 2014, 23, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Solish, N.J. Assessment of recovery time for the collagen products Dermicol-P35 27G and 30G. J. Am. Acad. Dermatol. 2010, 62, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, K.A. Hyaluronic Acid Fillers: Science and Clinical Uses. Clin. Plast. Surg. 2016, 43, 489–496. [Google Scholar] [CrossRef]
- Hobar, P.C.; Pantaloni, M.; Byrd, H.S. Porous hydroxyapatite granules for alloplastic enhancement of the facial region. Clin. Plast. Surg. 2000, 27, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Vleggaar, D. Facial volumetric correction with injectable poly-L-lactic acid. Dermatol. Surg. 2005, 31, 1511–1517. [Google Scholar] [CrossRef]
- Waldman, S.R. Gore-Tex for augmentation of the nasal dorsum: A preliminary report. Ann. Plast. Surg. 1991, 26, 520–525. [Google Scholar] [CrossRef]
- Naik, M.N.; Murthy, R.K.; Honavar, S.G. Comparison of vascularization of Medpor and Medpor-Plus orbital implants: A prospective, randomized study. Ophthalmic Plast. Reconstr. Surg. 2007, 23, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Mao, J.; Yang, G.; Wang, H. Influence of different incision designs on bone increment of guided bone regeneration (Bio-Gide collagen membrane +Bio-OSS bone powder) during the same period of maxillary anterior tooth implantation. Bioengineered 2021, 12, 2155–2163. [Google Scholar] [CrossRef]
- Pang, K.; Um, I.; Kim, Y.; Woo, J.; Lee, J. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: A prospective randomized clinical trial in comparison with anorganic bovine bone. Clin. Oral Implants Res. 2017, 28, 809–815. [Google Scholar] [CrossRef]
- Pisoni, L.; Lucchi, A.; Persia, M.; Marchi, O.; Ordesi, P.; Siervo, S. Sinus lift: 3 years follow up comparing autogenous bone block versus autogenous particulated grafts. J. Dent. Sci. 2016, 11, 231–237. [Google Scholar] [CrossRef] [PubMed]
- De Molon, R.S.; De Paula, W.N.; Spin-Neto, R.; Verzola, M.H.A.; Tosoni, G.M.; Lia, R.C.C.; Scaf, G.; Marcantonio, E., Jr. Correlation of fractal dimension with histomorphometry in maxillary sinus lifting using autogenous bone graft. Braz. Dent. J. 2015, 26, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; Apel, C.; Sellhaus, B.; van Neerven, S.; Wessing, B.; Hilgers, R.-D.; Pallua, N. Differences in degradation behavior of two non-cross-linked collagen barrier membranes: An in vitro and in vivo study. Clin. Oral Implants Res. 2014, 25, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Molla, R.; Radvar, M.; Sohrabi, K.; Najafi, M.H. An evaluation of bovine derived xenograft with and without a bioabsorbable collagen membrane in the treatment of mandibular Class II furcation defects. Aust. Dent. J. 2009, 54, 220–227. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, M.-Y.; Jin, R.; Zhang, Y.; Shi, Y.-M.; Sun, B.-S.; Zhang, Y.-G. Classification of nasolabial folds in Asians and the corresponding surgical approaches: By Shanghai 9th People’s Hospital. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 914–919. [Google Scholar] [CrossRef]
- Day, D.J.; Littler, C.M.; Swift, R.W.; Gottlieb, S. The wrinkle severity rating scale: A validation study. Am. J. Clin. Dermatol. 2004, 5, 49–52. [Google Scholar] [CrossRef]
- Lindqvist, C.; Tveten, S.; Bondevik, B.E.; Fagrell, D. A randomized, evaluator-blind, multicenter comparison of the efficacy and tolerability of Perlane versus Zyplast in the correction of nasolabial folds. Plast. Reconstr. Surg. 2005, 115, 282–289. [Google Scholar] [CrossRef]
- Chen, B.; Song, H. Measuring satisfaction with appearance: Validation of the FACE-Q scales for double-eyelid blepharoplasty with minor incision in young Asians—Retrospective study of 200 cases. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Klassen, A.F.; Cano, S.J.; Schwitzer, J.A.; Scott, A.M.; Pusic, A.L. FACE-Q scales for health-related quality of life, early life impact, satisfaction with outcomes, and decision to have treatment: Development and validation. Plast. Reconstr. Surg. 2015, 135, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, B.C.; Jacobson, S.R.; Lavoipierre, A.M.; Huggins, R.J. The fate of porous hydroxyapatite granules used in facial skeletal augmentation. Aesthet. Plast. Surg. 2010, 34, 455–461. [Google Scholar] [CrossRef]
- Fanous, N.; Yoskovitch, A. Premaxillary augmentation: Adjunct to rhinoplasty. Plast. Reconstr. Surg. 2000, 106, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Xu, Y.; Han, R.; Zheng, R.; Fan, F. Paranasal Augmentation Using Diced Costal Cartilage for Midface Concavity: A Retrospective Study of 68 Patients. Aesthet. Plast. Surg. 2022, 46, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Von Buelow, S.; von Heimburg, D.; Pallua, N. Efficacy and safety of polyacrylamide hydrogel for facial soft-tissue augmentation. Plast. Reconstr. Surg. 2005, 116, 1137–1146, discussion 1147–1148. [Google Scholar] [CrossRef]
- Tapety, F.I.; Amizuka, N.; Uoshima, K.; Nomura, S.; Maeda, T. A histological evaluation of the involvement of Bio-Oss in osteoblastic differentiation and matrix synthesis. Clin. Oral Implants Res. 2004, 15, 315–324. [Google Scholar] [CrossRef]
- Carinci, F.; Piattelli, A.; Degidi, M.; Palmieri, A.; Perrotti, V.; Scapoli, L.; Martinelli, M.; Laino, G.; Pezzetti, F. Genetic effects of anorganic bovine bone (Bio-Oss) on osteoblast-like MG63 cells. Arch. Oral Biol. 2006, 51, 154–163. [Google Scholar] [CrossRef]
- Oliveira, R.; El, H.M.; Carrel, J.P.; Lombardi, T.; Bernard, J.P. Rehabilitation of the edentulous posterior maxilla after sinus floor elevation using deproteinized bovine bone: A 9-year clinical study. Implant Dent. 2012, 21, 422–426. [Google Scholar] [CrossRef]
- Lutz, R.; Berger-Fink, S.; Stockmann, P.; Neukam, F.W.; Schlegel, K.A. Sinus floor augmentation with autogenous bone vs. a bovine-derived xenograft—A 5-year retrospective study. Clin. Oral Implants Res. 2015, 26, 644–648. [Google Scholar] [CrossRef]
- Bunyaratavej, P.; Wang, H.L. Collagen membranes: A review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef] [PubMed]

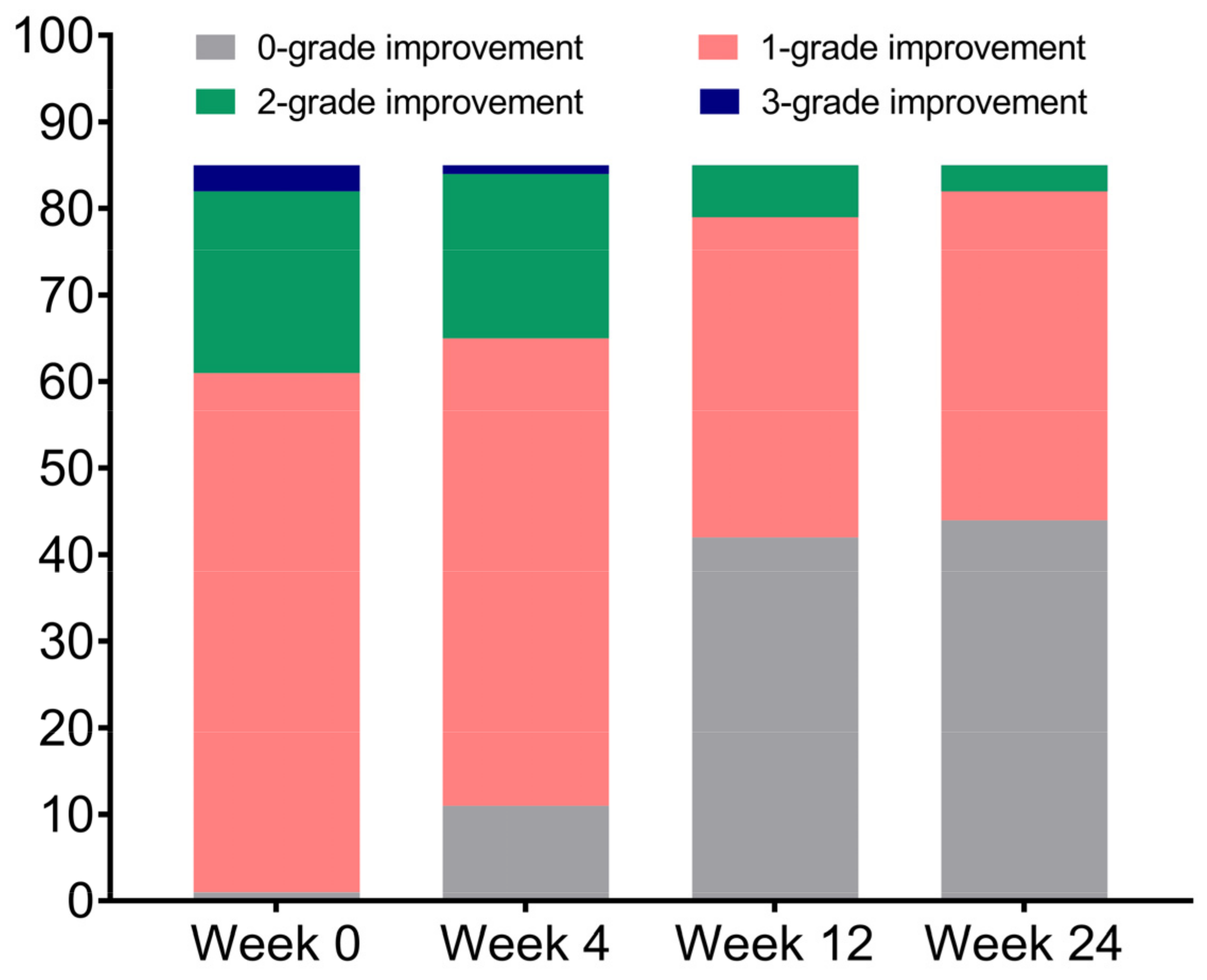
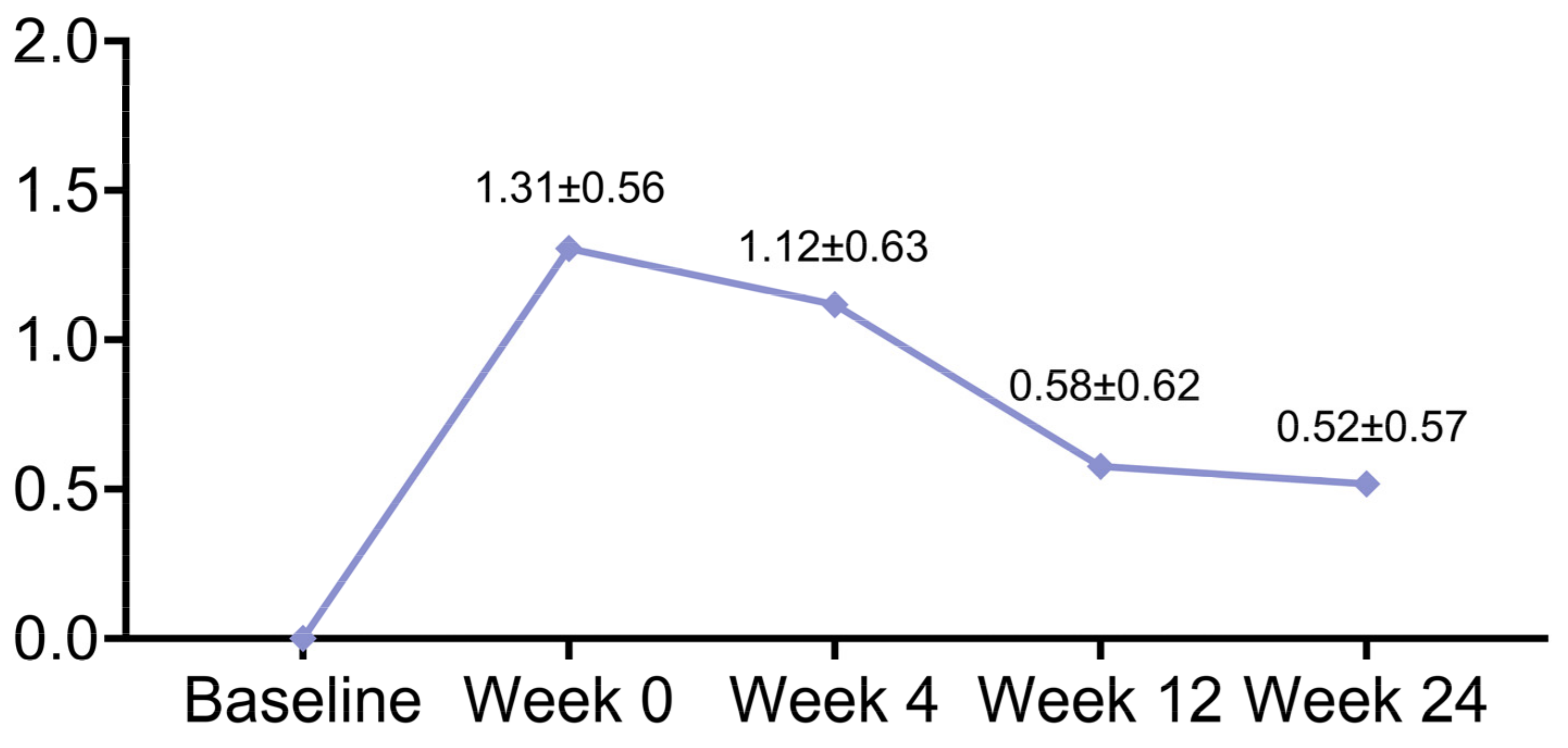
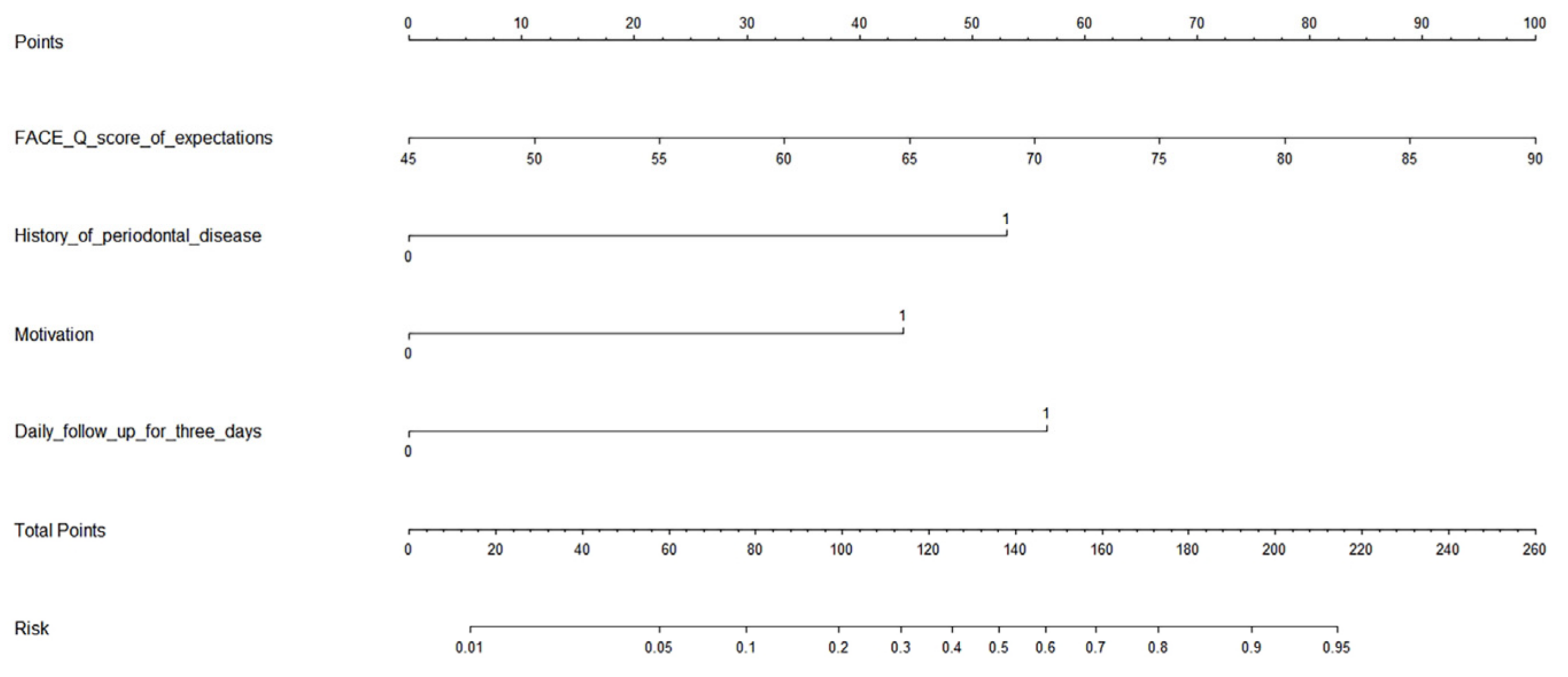
| Follow-Up Duration | WSRS | p Value |
|---|---|---|
| Baseline | 2.56 ± 0.61 | |
| Week 0 | 1.26 ± 0.44 | <0.001 |
| Week 4 | 1.45 ± 0.50 | <0.001 |
| Week 12 | 1.99 ± 0.33 | <0.001 |
| Week 24 | 2.05 ± 0.30 | <0.001 |
| Grade | n (%) | |||
|---|---|---|---|---|
| Week 0 | Week 4 | Week 12 | Week 24 | |
| Very much improved | 81 (95.3%) | 78 (91.8%) | 76 (89.4%) | 54 (63.5%) |
| Much improved | 4 (4.7%) | 6 (7.1%) | 3 (3.5%) | 23 (27.1%) |
| Improved | 0 | 1 (1.2%) | 5 (5.9%) | 2 (2.4%) |
| No change | 0 | 0 | 1 (1.2%) | 4 (4.7%) |
| Worse | 0 | 0 | 0 | 2 (2.4%) |
| Overall Satisfied, n (%) | ||||
|---|---|---|---|---|
| Yes | No | x2 | p Value | |
| Insufficient improvement in concavity | 64.555 | <0.001 | ||
| Yes | 0 | 18 | ||
| No | 64 | 3 | ||
| Visual asymmetry | 2.785 | 0.095 | ||
| Yes | 0 | 2 | ||
| No | 64 | 19 | ||
| Foreign body sensation | 5.746 | 0.017 | ||
| Yes | 0 | 3 | ||
| No | 64 | 18 | ||
| Variable | Satisfaction | |||
|---|---|---|---|---|
| Satisfied (n = 64) | Dissatisfied (n = 21) | x2 | p Value | |
| Gender | 0.672 | 0.412 | ||
| Male | 2 (3.1%) | 0 (0.0%) | ||
| Female | 62 (96.9%) | 21 (100.0%) | ||
| Age (years) | 27.3 ± 4.6 | 27.2 ± 3.1 | 0.956 | |
| BMI (kg/m2) | 19.7 ± 1.5 | 19.4 ± 1.4 | 0.457 | |
| ABO blood type | 1.440 | 0.696 | ||
| A (+) | 17 (26.6%) | 4 (19.0%) | ||
| AB (+) | 11 (17.2%) | 3 (14.3%) | ||
| B (+) | 16 (25.0%) | 8 (38.1%) | ||
| O (+) | 20 (31.1%) | 6 (28.6%) | ||
| Personality | 1.611 | 0.204 | ||
| Extrovert | 39 (60.9%) | 16 (76.2%) | ||
| Introverted | 25 (39.1%) | 5 (23.8%) | ||
| Motivation | 6.256 | 0.012 | ||
| Proactive | 44 (68.8%) | 8 (38.1%) | ||
| Passive | 20 (31.3%) | 13 (61.9%) | ||
| Main Purpose | 1.745 | 0.186 | ||
| Midface depression | 26 (40.6%) | 12 (51.7%) | ||
| Nasolabial folds | 38 (59.4%) | 9(42.9%) | ||
| History of periodontal disease | 10.209 | 0.001 | ||
| Yes | 11(17.2%) | 11(52.4%) | ||
| No | 53(82.8%) | 10(47.6%) | ||
| FACE-Q score of expectations | 59.0 ± 7.5 | 68.1 ± 11.1 | <0.001 | |
| Systolic blood pressure (mmHg) | 105.1 ± 12.1 | 100.9 ± 10.5 | 0.157 | |
| Diastolic blood pressure (mmHg) | 73.2 ± 9.7 | 70.9 ± 9.0 | 0.327 | |
| Blood glucose (mmol/L) | 6.4 ± 0.9 | 6.5 ± 0.8 | 0.595 | |
| Heart rate (bpm) | 85.8 ± 5.8 | 83.7 ± 7.9 | 0.182 | |
| Operative time (min) | 101.3 ± 10.4 | 101.8 ± 10.5 | 0.842 | |
| Amount of Bio-Oss (g) | 2.2 ± 0.5 | 2.3 ± 0.4 | 0.632 | |
| Number of titanium screws | 11.0 ± 2.5 | 11.3 ± 1.7 | 0.554 | |
| Size of Bio-Gide | 0.005 | 0.943 | ||
| 25 × 25 mm | 28 (43.8%) | 9 (42.9%) | ||
| 30 × 40 mm | 36 (56.3%) | 12 (57.1%) | ||
| Simultaneous orthodontic treatment | 0.008 | 0.930 | ||
| Yes | 22 (34.4%) | 7 (33.3%) | ||
| No | 42 (65.6%) | 14 (66.7%) | ||
| Daily follow-up for 3 days | 2.884 | 0.089 | ||
| Yes | 38 (59.4%) | 8 (38.1%) | ||
| No | 26 (40.6%) | 13 (61.9%) | ||
| Variable | B | SE | Wals | p Value | OR | 95% CI |
|---|---|---|---|---|---|---|
| FACE-Q score of expectations | 0.084 | 0.035 | 5.631 | 0.018 | 1.087 | 1.015–1.165 |
| History of periodontal disease | 1.996 | 0.879 | 5.163 | 0.023 | 7.361 | 1.316–41.186 |
| Motivation | 1.650 | 0.670 | 6.075 | 0.014 | 5.209 | 1.402–19.356 |
| Daily follow-up for 3 days | 2.131 | 0.847 | 6.328 | 0.012 | 8.420 | 1.601–44.281 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-Y.; Liu, K.; Liu, R.-X.; Xu, B.-H. Safety and Efficacy of Midface Augmentation Using Bio-Oss Bone Powder and Bio-Gide Collagen Membrane in Asians. J. Clin. Med. 2023, 12, 959. https://doi.org/10.3390/jcm12030959
Zhang J-Y, Liu K, Liu R-X, Xu B-H. Safety and Efficacy of Midface Augmentation Using Bio-Oss Bone Powder and Bio-Gide Collagen Membrane in Asians. Journal of Clinical Medicine. 2023; 12(3):959. https://doi.org/10.3390/jcm12030959
Chicago/Turabian StyleZhang, Jia-Yu, Ke Liu, Ruo-Xi Liu, and Bao-Hua Xu. 2023. "Safety and Efficacy of Midface Augmentation Using Bio-Oss Bone Powder and Bio-Gide Collagen Membrane in Asians" Journal of Clinical Medicine 12, no. 3: 959. https://doi.org/10.3390/jcm12030959
APA StyleZhang, J.-Y., Liu, K., Liu, R.-X., & Xu, B.-H. (2023). Safety and Efficacy of Midface Augmentation Using Bio-Oss Bone Powder and Bio-Gide Collagen Membrane in Asians. Journal of Clinical Medicine, 12(3), 959. https://doi.org/10.3390/jcm12030959







